Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Honey and Honey Analog (Mixed-Sugar) Diets
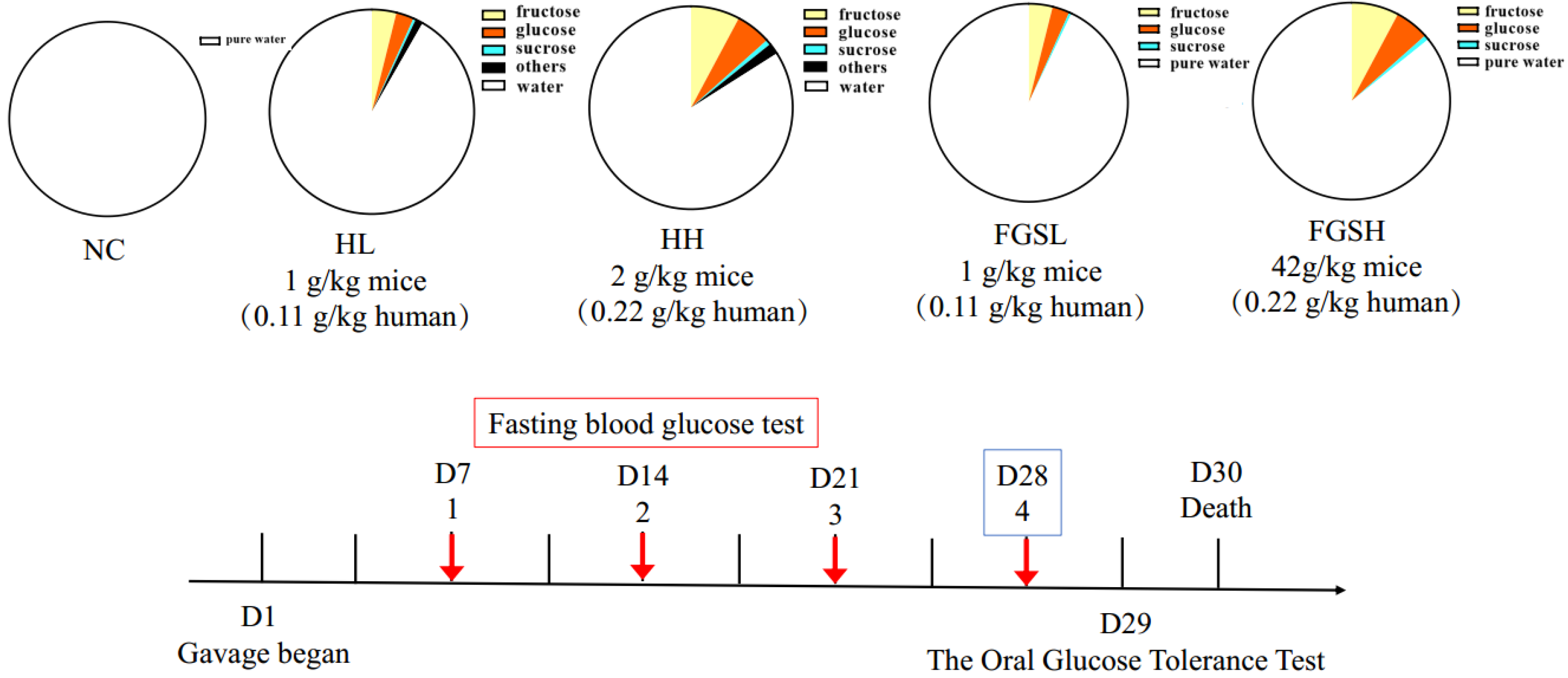
2.3. Animal Experiment
2.4. Oral Glucose Tolerance Test
2.5. Histological Analysis
2.6. Analysis of Lipids, Inflammation, and Antioxidant Factors in Serum
2.7. Gut Microbiota Analysis
2.8. Metabolite Profiling
2.9. Statistical Analyses
3. Results
3.1. Effects of Honey and Mixed-Sugar Exposure on Blood Glucose, Body Weight, Adipocytes, Serum Lipids, Inflammatory Response, and Antioxidant Activity
3.2. Honey and Mixed Sugar Were Associated with Distinct Gut Microbiota Compositions
3.3. Mice Fed Honey and Mixed-Sugar Solutions Had Different Metabolite Profiles
3.4. Systems Biology Approaches to Interpreting the Effects of Honey and Mixed-Sugar Diets on the Organism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Treatment | Dose (g/kg) | Honey Dilution (mg/mL) | Volume (mL) |
|---|---|---|---|---|
| NC | Control diet | 0 | 0 | ~0.2 |
| HL | Honey low-dose | 2 | 200 | ~0.2 |
| HH | Honey high-dose | 4 | 400 | ~0.2 |
| FGSL | Mixed sugar low-dose | 2 | 200 | ~0.2 |
| FGSH | Mixed sugar high-dose | 4 | 400 | ~0.2 |



References
- Iwuozor, K.O.; Anyanwu, V.U.; Olaniyi, B.O.; Mbamalu, P.S.; Adeniyi, A.G. Adulteration of Sugar: A Growing Global Menace. Sugar Tech 2022, 24, 914–991. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Cancer Med. Assoc. J. 2017, 189, E929–E939. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, X.; Bian, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; et al. Association between consumption frequency of honey and non-alcoholic fatty liver disease: Results from a cross-sectional analysis based on the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study. Br. J. Nutr. 2021, 125, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.M.; Petrisko, M.; Hong, M.Y.; Rezende, L.; Clayton, Z.S.; Kern, M. Honey does not adversely impact blood lipids of adult men and women: A randomized cross-over trial. Nutr. Res. 2020, 74, 87–95. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Gerber, P.A. Reply to: “Comments on ‘Fructose- and sucrose- but not glucosesweetened beverages promote hepatic de novo lipogenesis-A randomized controlled trial’”. J. Hepatol. 2021, 75, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J. Nutr. 2002, 132, 3379–3382. [Google Scholar] [CrossRef]
- Guo, N.; Zhao, L.; Zhao, Y.; Li, Q.; Xue, X.; Wu, L.; Escalada, M.G.; Wang, K.; Peng, W. Comparison of the Chemical Composition and Biological Activity of Mature and Immature Honey: An HPLC/QTOF/MS-Based Metabolomic Approach. J. Agric. Food Chem. 2020, 68, 4062–4071. [Google Scholar] [CrossRef]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Zulkawi, N.; Ng, K.H.; Zamberi, N.R.; Yeap, S.K.; Satharasinghe, D.A.; Tan, S.W.; Ho, W.Y.; Abd-Rashid, N.Y.; Md-Lazim, M.I.; Jamaluddin, A.; et al. Antihyperglycemic and anti-inflammatory effects of fermented food paste in high-fat diet and streptozotocin-challenged mice. Drug Des. Dev. Ther. 2018, 12, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Tan, M.; Chen, X. Punicic acid ameliorates obesity and liver steatosis by regulating gut microbiota composition in mice. Food Funct. 2021, 12, 7897–7908. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q.; et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Seijo, M.C. Honey: Chemical Composition, Stability and Authenticity. Foods 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.R.; Nor Aripin, K.N.; Syed Mohideen, F.B.; Baharom, N.; Omar, K.; Md Taujuddin, N.M.S.; Mohd Yusof, H.H.; Addnan, F.H. The Effect of Kelulut Honey on Fasting Blood Glucose and Metabolic Parameters in Patients with Impaired Fasting Glucose. J. Nutr. Metab. 2019, 2019, 3176018. [Google Scholar] [CrossRef]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Galizzi, G.; Caruana, L.; Carlo, M.D.; Lentini, L.; Puleio, R.; Mulè, F. Preventive Impact of Long-Term Ingestion of Chestnut Honey on Glucose Disorders and Neurodegeneration in Obese Mice. Nutrients 2022, 14, 756. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Oligosaccharides Might Contribute to the Antidiabetic Effect of Honey: A Review of the Literature. Molecules 2012, 17, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Gohar, A.; Shakeel, M.; Atkinson, R.L.; Haleem, D.J. Potential mechanisms of improvement in body weight, metabolic profile, and liver metabolism by honey in rats on a high fat diet. PharmaNutrition 2020, 14, 100227. [Google Scholar] [CrossRef]
- Samat, S.; Enchang, F.K.; Hussein, F.N.; Ismail, W.I.W. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1342150. [Google Scholar] [CrossRef]
- Nemoseck, T.M.; Carmody, E.G.; Furchner-Evanson, A.; Gleason, M.; Li, A.; Potter, H.; Rezende, L.M.; Lane, K.J.; Kern, M. Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr. Res. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- Romero-Silva, S.; Miguel, A.M.R.; Romero-Romero, L.P.; Rodriguez, O.; Salas, G.; Morel, N.; López-Muñoz, F.; Lima-Mendoza, L.A.; Bravo, G. Effects of Honey Against the Accumulation of Adipose Tissue and the Increased Blood Pressure on Carbohydrate-Induced Obesity in Rat. Lett. Drug. Des. Discov. 2011, 8, 69–75. [Google Scholar] [CrossRef]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Ferrocino, I.; Liberto, E.; Chiazza, F.; Cento, A.S.; Collotta, D.; Querio, G.; Nigro, D.; Bitonto, V.; Cutrin, J.C.; et al. Fructose liquid and solid formulations differently affect gut integrity, microbiota composition and related liver toxicity: A comparative in vivo study. J. Nutr. Biochem. 2018, 55, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Samat, S.; Nor, N.M.; Hussein, F.N.; Eshak, Z.; Ismail, W.I.W. Short-term consumption of Gelam honey reduces triglyceride level. Int. Food Res. J. 2017, 24, 1519–1524. [Google Scholar]
- Gholami, Z.; Sohrabi, Z.; Zare, M.; Pourrajab, B.; Nasimi, N. The effect of honey on lipid profiles: A systematic review and meta-analysis of controlled clinical trials. Br. J. Nutr. 2022, 127, 1482–1496. [Google Scholar] [CrossRef]
- Mohammadimanesh, A.; Vahidiniya, A.A.; Doaei, S.; Gholamalizadeh, M.; Shahvegharasl, Z.; Salehi, I.; Fayyaz, N.; Khosravi, H.M. The effect of different types of honey on the lipid profile of streptozotocin-induced diabetic rats. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, e113–e118. [Google Scholar] [CrossRef]
- Navaei-Alipour, N.; Mastali, M.; Ferns, G.A.; Saberi-Karimian, M.; Ghayour-Mobarhan, M. The effects of honey on pro- and anti-inflammatory cytokines: A narrative review. Phytother. Res. 2021, 35, 3690–3701. [Google Scholar] [CrossRef]
- Cochet, F.; Peri, F. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [Green Version]
- Hashim, F.J.; Vichitphan, S.; Boonsiri, P.; Vichitphan, K. Neuroprotective Assessment of Moringa oleifera Leaves Extract against Oxidative-Stress-Induced Cytotoxicity in SHSY5Y Neuroblastoma Cells. Plants 2021, 10, 889. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Abbas, A.T.; Al-Hindi, R.R.; El-Shitany, N.A.; Abdel-Dayem, U.A.; Ali, S.S.; Saleh, R.M.; Al Jaouni, S.K.; Kamal, M.A.; Harakeh, S.M. Manuka Honey Exerts Antioxidant and Anti-Inflammatory Activities That Promote Healing of Acetic Acid-Induced Gastric Ulcer in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 5413917. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [Green Version]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; Van de Wiele, T.; De Backer, F.; Cani, P.D.; Larondelle, Y.; Delzenne, N.M. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.M.; Lee, S.A.; Hong, J.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Wang, W.L.; Su, P.J.; Jiang, K.L.; Yuan, Z.W. Decreased enteric fatty acid amide hydrolase activity is associated with colonic inertia in slow transit constipation. J. Gastroenterol. Hepatol. 2014, 29, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, L.; Lin, C.Y.; Lu, L.; Ning, Z.W.; Hu, D.D.; Zhong, L.L.D.; Yang, Z.J.; Bian, Z.X. Chinese Herbal Medicine (MaZiRenWan) Improves Bowel Movement in Functional Constipation Through Down-Regulating Oleamide. Front. Pharmacol. 2020, 10, 1570. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ding, L.; Shi, H.H.; Wang, C.C.; Xue, C.; Zhang, T.; Wang, Y. Eicosapentaenoic acid-enriched phospholipids suppressed lipid accumulation by specific inhibition of lipid droplet-associated protein FSP27 in mice. J. Sci. Food Agric. 2020, 100, 2244–2251. [Google Scholar] [CrossRef]
- Liu, C.; Lu, D.; You, X.; Shi, G.; Deng, J.; Zhou, T. Carbon dots sensitized lanthanide infinite coordination polymer nanoparticles: Towards ratiometric fluorescent sensing of cerebrospinal Aβ monomer as a biomarker for Alzheimer’s disease. Anal. Chim. Acta 2020, 1105, 147–154. [Google Scholar] [CrossRef]




| Group | Treatment | Dose (g/kg) | Honey Dilution (mg/mL) | Volume (mL) |
|---|---|---|---|---|
| NC | Control diet | 0 | 0 | ~0.2 |
| HL | Honey low-dose | 1 | 100 | ~0.2 |
| HH | Honey high-dose | 2 | 200 | ~0.2 |
| FGSL | Mixed sugar low-dose | 1 | 100 | ~0.2 |
| FGSH | Mixed sugar high-dose | 2 | 200 | ~0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhao, Y.; Naumovski, N.; Zhao, W.; Yang, G.; Xue, X.; Wu, L.; Granato, D.; Peng, W.; Wang, K. Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars. Nutrients 2022, 14, 3445. https://doi.org/10.3390/nu14163445
Zheng X, Zhao Y, Naumovski N, Zhao W, Yang G, Xue X, Wu L, Granato D, Peng W, Wang K. Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars. Nutrients. 2022; 14(16):3445. https://doi.org/10.3390/nu14163445
Chicago/Turabian StyleZheng, Xing, Yazhou Zhao, Nenad Naumovski, Wen Zhao, Guan Yang, Xiaofeng Xue, Liming Wu, Daniel Granato, Wenjun Peng, and Kai Wang. 2022. "Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars" Nutrients 14, no. 16: 3445. https://doi.org/10.3390/nu14163445
APA StyleZheng, X., Zhao, Y., Naumovski, N., Zhao, W., Yang, G., Xue, X., Wu, L., Granato, D., Peng, W., & Wang, K. (2022). Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars. Nutrients, 14(16), 3445. https://doi.org/10.3390/nu14163445









