Absent Metabolic Transition from the Early to the Late Period in Non-Survivors Post Cardiac Surgery
Abstract
1. Introduction
2. Methods and Materials
2.1. Ethical Approval
2.2. Study Design and Patients
2.3. Statistical Analysis
2.4. Data Availability
3. Results
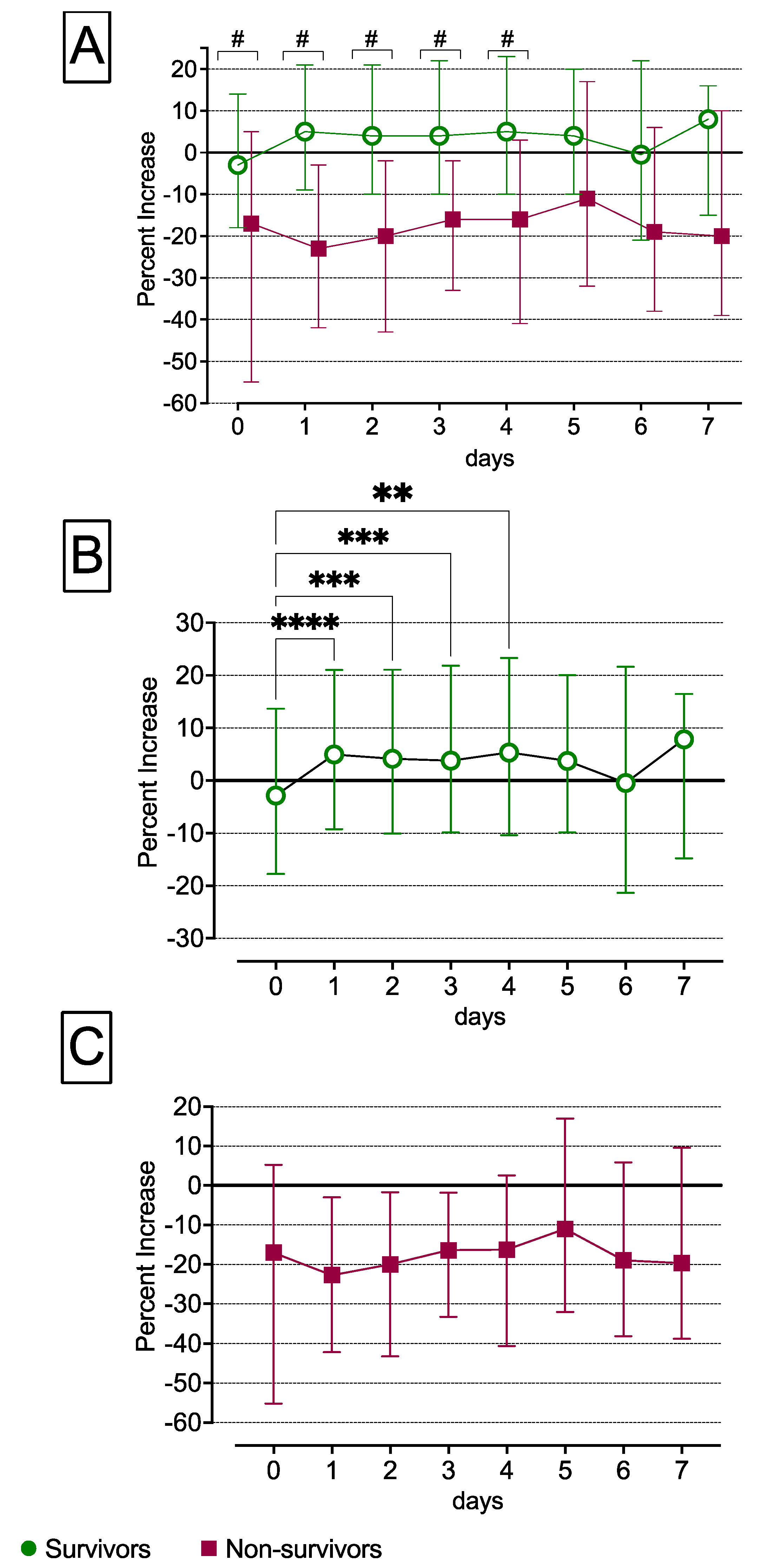
3.1. Absent Metabolic Transition in Non-Survivors from the Early ‘Ebb Phase to the Late ‘Flow’ Phase
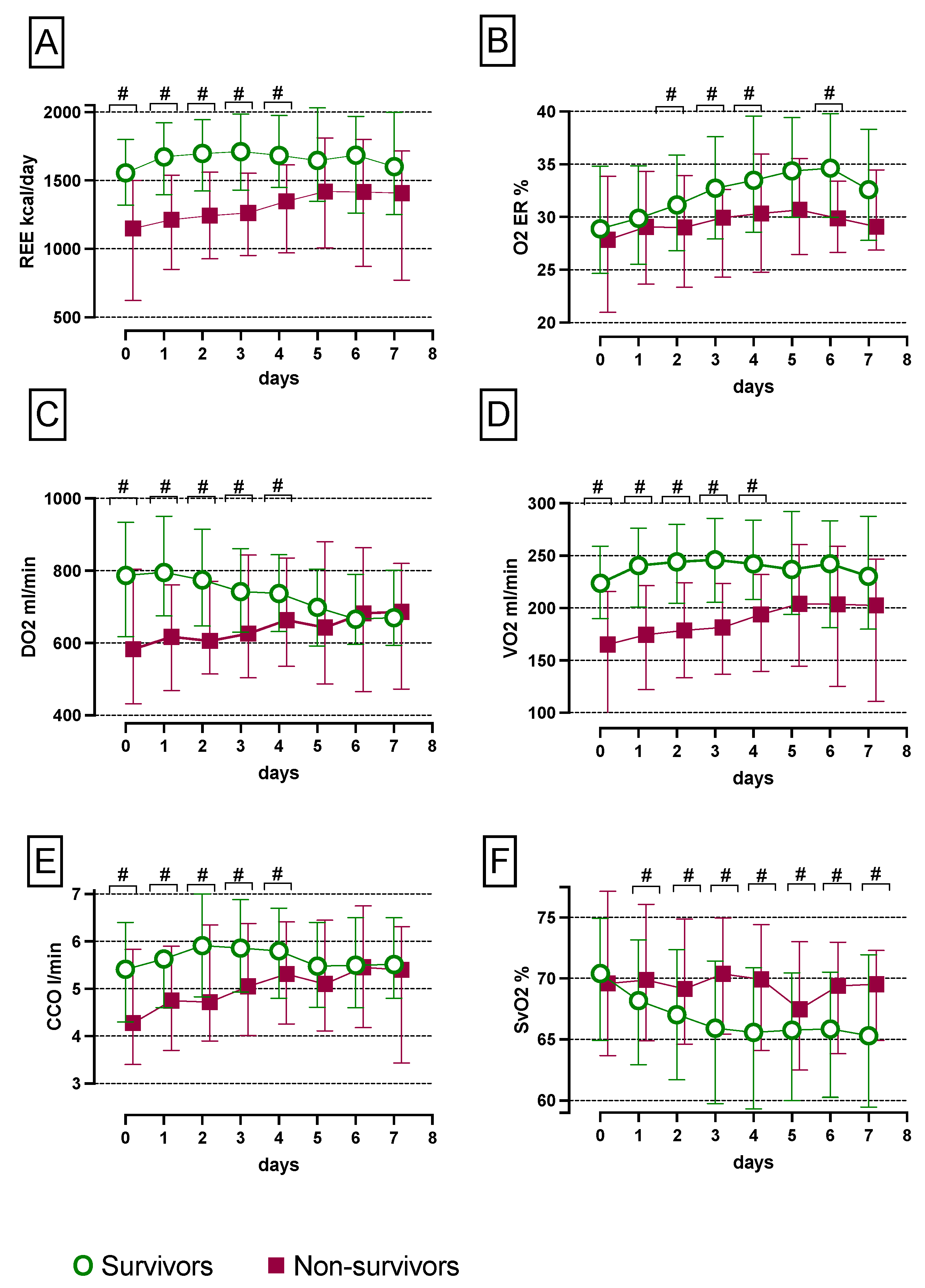
3.2. Increased SvO2 and Reduced CCO, VO2, REE, O2ER and DO2 Levels in Non-Survivors Compared with Survivors over the First Seven Days
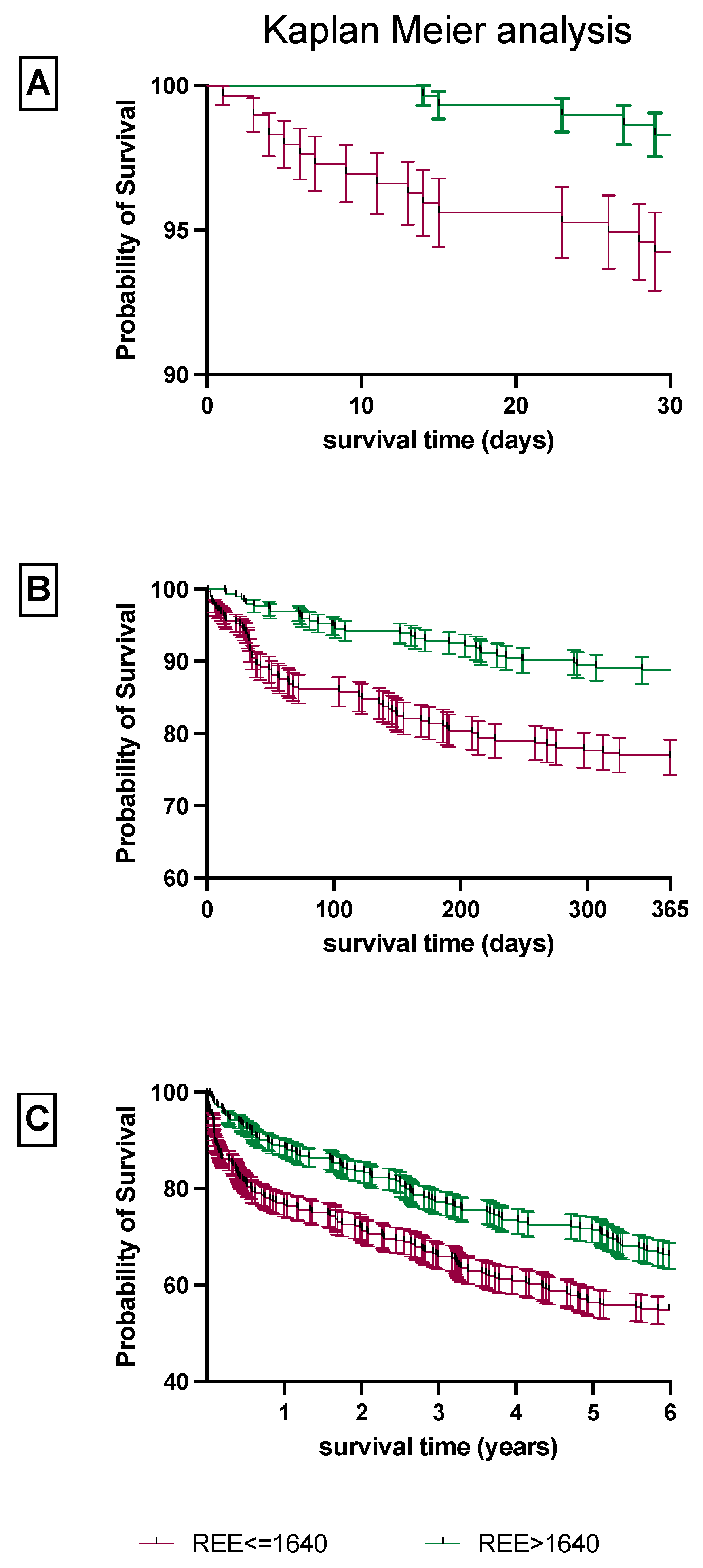
3.3. Increased 30-Day, 1-Year and 6-Year Mortality in Patients with a Reduced REE
3.4. Univariate and Multivariate Cox Regression Analyses for 30 Days, 1 Year and 6 Years after Cardiac Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zuniga, J.D.; Gostel, M.R.; Mulcahy, D.G.; Barker, K.; Hill, A.; Sedaghatpour, M.; Vo, S.Q.; Funk, V.A.; Coddington, J.A. Data Release: DNA barcodes of plant species collected for the Global Genome Initiative for Gardens Program, National Museum of Natural History, Smithsonian Institution. PhytoKeys 2017, 88, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.V.; Jones, A.E.; Gaieski, D.F.; Arnold, R.C.; Trzeciak, S.; Shapiro, N.I.; Emergency Medicine Shock Research Network, I. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann. Emerg. Med. 2010, 55, 40–46.e41. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R.; Payen, D. Functional hemodynamic monitoring. Crit. Care 2005, 9, 566–572. [Google Scholar] [CrossRef][Green Version]
- Uber, A.; Grossestreuer, A.V.; Ross, C.E.; Patel, P.V.; Trehan, A.; Donnino, M.W.; Berg, K.M. Preliminary observations in systemic oxygen consumption during targeted temperature management after cardiac arrest. Resuscitation 2018, 127, 89–94. [Google Scholar] [CrossRef]
- Rivers, E.P.; Rady, M.Y.; Martin, G.B.; Fenn, N.M.; Smithline, H.A.; Alexander, M.E.; Nowak, R.M. Venous hyperoxia after cardiac arrest. Characterization of a defect in systemic oxygen utilization. Chest 1992, 102, 1787–1793. [Google Scholar] [CrossRef]
- Hayes, M.A.; Timmins, A.C.; Yau, E.H.; Palazzo, M.; Hinds, C.J.; Watson, D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N. Engl. J. Med. 1994, 330, 1717–1722. [Google Scholar] [CrossRef]
- Fink, M.P. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit. Care Clin. 2002, 18, 165–175. [Google Scholar] [CrossRef]
- Simsek, T.; Simsek, H.U.; Canturk, N.Z. Response to trauma and metabolic changes: Posttraumatic metabolism. Turk. J. Surg. Ulus. Cerrahi Derg. 2014, 30, 153–159. [Google Scholar] [CrossRef]
- Jeevanandam, M.; Shamos, R.F.; Petersen, S.R. Substrate efficacy in early nutrition support of critically ill multiple trauma victims. JPEN J. Parenter. Enter. Nutr. 1992, 16, 511–520. [Google Scholar] [CrossRef]
- Van den Berghe, G.; de Zegher, F.; Bouillon, R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J. Clin. Endocrinol. Metab. 1998, 83, 1827–1834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, G.; Grosser, S.; Buggisch, P.; Gottschall, C.; Matthaei, S.; Greten, H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit. Care Med. 1993, 21, 1012–1019. [Google Scholar] [CrossRef]
- Uehara, M.; Plank, L.D.; Hill, G.L. Components of energy expenditure in patients with severe sepsis and major trauma: A basis for clinical care. Crit. Care Med. 1999, 27, 1295–1302. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.S.; Oleggini, M.; Lachi, S.; Frediani, M.; Bevilacqua, S.; Mosca, F.; Ferrannini, E. Energy metabolism of surgical patients in the early postoperative period: A reappraisal. Crit. Care Med. 1988, 16, 18–22. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Vehe, K.L.; Mullen, J.L.; Feurer, I.D. Resting energy expenditure in patients with pancreatitis. Crit. Care Med. 1991, 19, 484–490. [Google Scholar] [CrossRef]
- Conti-Patara, A.; de Araujo Caldeira, J.; de Mattos-Junior, E.; de Carvalho Hda, S.; Reinoldes, A.; Pedron, B.G.; Patara, M.; Francisco Talib, M.S.; Faustino, M.; de Oliveira, C.M.; et al. Changes in tissue perfusion parameters in dogs with severe sepsis/septic shock in response to goal-directed hemodynamic optimization at admission to ICU and the relation to outcome. J. Vet. Emerg. Crit. Care 2012, 22, 409–418. [Google Scholar] [CrossRef]
- Rosario, A.L.; Park, M.; Brunialti, M.K.; Mendes, M.; Rapozo, M.; Fernandes, D.; Salomao, R.; Laurindo, F.R.; Schettino, G.P.; Azevedo, L.C. SvO(2)-guided resuscitation for experimental septic shock: Effects of fluid infusion and dobutamine on hemodynamics, inflammatory response, and cardiovascular oxidative stress. Shock 2011, 36, 604–612. [Google Scholar] [CrossRef]
- Rivers, E.P.; Yataco, A.C.; Jaehne, A.K.; Gill, J.; Disselkamp, M. Oxygen extraction and perfusion markers in severe sepsis and septic shock: Diagnostic, therapeutic and outcome implications. Curr. Opin. Crit. Care 2015, 21, 381–387. [Google Scholar] [CrossRef]
- Boulain, T.; Garot, D.; Vignon, P.; Lascarrou, J.B.; Desachy, A.; Botoc, V.; Follin, A.; Frat, J.P.; Bellec, F.; Quenot, J.P.; et al. Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: A prospective multicentre study. Crit. Care 2014, 18, 609. [Google Scholar] [CrossRef] [PubMed]
- Ndahimana, D.; Kim, E.K. Energy Requirements in Critically Ill Patients. Clin. Nutr. Res. 2018, 7, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance in critically ill patients. Crit. Care Med. 2009, 37, S398–S399. [Google Scholar] [CrossRef] [PubMed]
- Fraipont, V.; Preiser, J.C. Energy estimation and measurement in critically ill patients. JPEN J. Parenter. Enter. Nutr. 2013, 37, 705–713. [Google Scholar] [CrossRef]
- Kagan, I.; Zusman, O.; Bendavid, I.; Theilla, M.; Cohen, J.; Singer, P. Validation of carbon dioxide production (VCO2) as a tool to calculate resting energy expenditure (REE) in mechanically ventilated critically ill patients: A retrospective observational study. Crit. Care 2018, 22, 186. [Google Scholar] [CrossRef]
| Determinants | |
|---|---|
| CvO2 | =Hb × 1.37 × SvO2 + 0.003 × PvO2 |
| CaO2 | =Hb × 1.37 × SaO2 + 0.003 × PaO2 |
| DO2 | =CO × CaO2 × 10 |
| VO2 | =CO × (CaO2 − CvO2) × 10 |
| O2ER | =VO2/DO2 |
| REE meas | =VO2 × 4.83 kcal/L × 1.44 |
| REE pred | =20 kcal/kg/day |
| Percent increase | =(REE meas − REE pred)/REE pred × 100 |
| Demographic Data n | Total 566 | Survivors 517 | Non-Survivors 49 | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Male, n (%) | 422 (74) | 389 (75) | 33 (67) | |
| Female, n (%) | 144 (25) | 128 (25) | 16 (33) | 0.266 |
| Age, mean ± SD | 63 ± 12 | 63 ± 12 | 66 ± 9 | 0.118 |
| BMI, mean ± SD | 26 ± 4 | 26 ± 4 | 26 ± 5 | 0.594 |
| Surgical procedure | ||||
| Valve procedure, n (%) | 156 (27) | 148 (28) | 8 (16) | |
| CABG, n (%) | 68 (12) | 64 (12) | 4 (8) | |
| CABG and valve, n (%) | 96 (17) | 89 (17) | 7 (14) | |
| Vascular graft, n (%) | 15 (3) | 15 (3) | 0 (0) | |
| LVAD, n (%) | 93 (16) | 76 (14) | 17 (34) | |
| HTX, n (%) | 114 (20) | 105 (20) | 9 (18) | |
| Others, n (%) | 24 (4) | 20 (4) | 4 (8) | <0.001 |
| Perioperative data | ||||
| Lactate max, mean ± SD | 3.9 ± 1.9 | 3.8 ± 1.7 | 5.2 ± 2.8 | <0.001 |
| HB min, mean ± SD | 8.1 ± 1.3 | 8.2 ± 1.4 | 7.9 ± 1.1 | 0.071 |
| PRBC count, mean ± SD | 3.8 ± 3.0 | 3.5 ± 2.8 | 5.9 ± 3.9 | <0.001 |
| FFP count, mean ± SD | 1124 ± 878 | 5.5 ± 4.3 | 6.4 ± 3.7 | 0.313 |
| Blood loss mL, mean ± SD | 494 ± 601 | 475 ± 586 | 786 ± 826 | 0.045 |
| ECC min, mean ± SD | 177 ± 77 | 173 ± 73 | 232 ± 114 | 0.002 |
| XCT min, mean ± SD | 104 ± 45 | 102 ± 43 | 131 ± 67 | 0.020 |
| Cox Regression Analyses | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | ||||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | ||
| 30-day all-cause mortality | |||||||
| REE | >1640 kcal/d # | 1.0 | |||||
| ≤1640 kcal/d | 3.5 | 1.3–9.5 | 0.013 | 3.2 | 1.1–8.7 | 0.021 | |
| Gender | Male # | 1.0 | |||||
| Female | 1.6 | 0.7–4.0 | 0.235 | ||||
| Age | <55 years # | 1.0 | |||||
| 55–65 years | 1.8 | 0.4–7.3 | 0.354 | ||||
| 66–75 years | 2.0 | 0.5–7.6 | 0.271 | ||||
| >75 years | 1.4 | 0.2–7.1 | 0.652 | ||||
| BMI | <25 kg/m2# | 1.0 | |||||
| 25–30 kg/m2 | 0.9 | 0.3–2.4 | 0.885 | ||||
| >30 kg/m2 | 0.6 | 0.1–2.4 | 0.550 | ||||
| Missing | 1.0 | 0.2–4.7 | 0.984 | ||||
| ECC time | ≤170 min # | 1.0 | |||||
| >170 min | 3.8 | 1.0–13.7 | 0.040 | 3.7 | 1.0–13.3 | 0.043 | |
| Missing | 9.2 | 2.4–34.7 | 0.001 | 8.3 | 2.2–31.5 | 0.002 | |
| Hb min | ≥8 g/dL # | 1.0 | |||||
| <8 g/dL | 1.5 | 0.6–3.6 | 0.332 | ||||
| Missing | 1.2 | 0.1–9.8 | 0.834 | ||||
| Lac max | ≤3.6 mmol/L # | 1.0 | |||||
| >3.6 mmol/L | 2.0 | 0.8–4.9 | 0.124 | ||||
| PRBCs | ≤3 units | 1.0 | |||||
| >3 units | 2.2 | 0.8–5.6 | 0.091 | ||||
| Missing/no PRBCs | 0.5 | 0.1–1.9 | 0.334 | ||||
| FFPs | ≤4 units # | 1.0 | |||||
| >4 units | 3.6 | 0.7–17.8 | 0.103 | ||||
| Missing/no FFPs | 1.1 | 0.2–4.8 | 0.895 | ||||
| 1-year all-cause mortality | |||||||
| REE | >1640 kcal/d # | 1.0 | |||||
| ≤1640 kcal/d | 2.5 | 1.6–3.8 | <0.001 | 2.0 | 1.3–3.1 | 0.001 | |
| Gender | Male # | 1.0 | |||||
| Female | 1.4 | 0.9–2.1 | 0.103 | ||||
| Age | <55 years # | 1.0 | |||||
| 55–65 years | 2.5 | 1.2–5.0 | 0.007 | 2.8 | 1.4–5.6 | 0.003 | |
| 66–75 years | 2.1 | 1.1–4.3 | 0.026 | 2.7 | 1.3–5.4 | 0.005 | |
| >75 years | 2.9 | 1.4–6.1 | 0.003 | 3.1 | 1.4–6.6 | 0.003 | |
| BMI | <25 kg/m2# | 1.0 | |||||
| 25–30 kg/m2 | 0.7 | 0.4–1.1 | 0.207 | ||||
| >30 kg/m2 | 0.8 | 0.5–1.5 | 0.700 | ||||
| Missing | 0.7 | 0.5–1.6 | 0.456 | ||||
| ECC time | ≤170 min # | 1.0 | |||||
| >170 min | 1.7 | 1.1–2.6 | 0.017 | 1.5 | 0.9–2.3 | 0.080 | |
| Missing | 1.8 | 1.0–3.3 | 0.048 | 2.1 | 1.1–4.1 | 0.017 | |
| Hb min | ≥8 g/dL # | 1.0 | |||||
| <8 g/dL | 1.7 | 1.1–2.6 | 0.005 | 1.1 | 0.7–1.8 | 0.624 | |
| Missing | 0.2 | 0.03–1.9 | 0.201 | 0.2 | 0.0–2.0 | 0.017 | |
| Lac max | ≤3.6 mmol/L # | 1.0 | |||||
| >3.6 mmol/L | 1.9 | 1.2–2.9 | 0.002 | 1.4 | 0.9–2.2 | 0.102 | |
| PRBCs | ≤3 units | 1.0 | |||||
| >3 units | 2.6 | 1.6–4.0 | <0.001 | 2.4 | 1.5–3.9 | <0.001 | |
| Missing/no PRBCs | 0.5 | 0.2–1.0 | 0.063 | 0.7 | 0.3–1.3 | 0.298 | |
| FFPs | ≤4 units # | 1.0 | |||||
| >4 units | 1.7 | 0.9–3.4 | 0.099 | ||||
| Missing/no FFPs | 0.7 | 0.4–1.2 | 0.280 | ||||
| 6-year all-cause mortality | |||||||
| REE | >1640 kcal/d # | 1.0 | |||||
| ≤1640 kcal/d | 1.5 | 1.1–2.0 | 0.001 | 1.3 | 1.0–1.8 | 0.031 | |
| Gender | Male # | 1.0 | |||||
| Female | 1.0 | 0.7–1.4 | 0.649 | ||||
| Age | <55 years # | ||||||
| 55–65 years | 1.7 | 1.1–2.6 | 0.014 | 1.9 | 1.2–2.9 | 0.003 | |
| 66–75 years | 2.0 | 1.3–3.1 | <0.001 | 2.4 | 1.6–3.7 | <0.001 | |
| >75 years | 2.3 | 1.5–3.7 | <0.001 | 2.5 | 1.6–4.1 | <0.001 | |
| BMI | <25 kg/m2# | 1.0 | |||||
| 25–30 kg/m2 | 0.7 | 0.5–0.9 | 0.040 | 0.7 | 0.5–1.0 | 0.067 | |
| >30 kg/m2 | 1.0 | 0.7–1.5 | 0.687 | 1.3 | 0.9–1.9 | 0.113 | |
| Missing | 0.6 | 0.3–1.1 | 0.174 | 0.7 | 0.4–1.2 | 0.742 | |
| ECC time | ≤170 min # | 1.0 | |||||
| >170 min | 1.0 | 0.7–1.3 | 0.857 | 2.1 | 1.5–2.8 | <0.001 | |
| Missing | 1.8 | 1.2–2.6 | 0.001 | 0.8 | 0.5–1.1 | 0.297 | |
| Hb min | ≥8 g/dL # | 1.0 | |||||
| <8 g/dL | 1.3 | 1.0–1.8 | 0.014 | 0.9 | 0.7–1.2 | 0.792 | |
| Missing | 0.7 | 0.3–1.5 | 0.377 | 0.5 | 0.2–1.2 | 0.142 | |
| Lac max | ≤3.6 mmol/L # | 1.0 | |||||
| >3.6 mmol/L | 1.2 | 0.9–1.6 | 0.116 | ||||
| PRBCs | ≤3 units | 1.0 | |||||
| >3 units | 1.8 | 1.3–2.5 | <0.001 | 2.1 | 1.5–2.8 | <0.001 | |
| Missing/no PRBCs | 0.7 | 0.5–1.0 | 0.148 | 0.8 | 0.5–1.1 | 0.297 | |
| FFPs | ≤4 units # | 1.0 | |||||
| >4 units | 1.5 | 0.9–2.6 | 0.087 | ||||
| Missing/no FFPs | 0.7 | 0.6–1.6 | 0.784 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veraar, C.; Fischer, A.; Bernardi, M.H.; Sulz, I.; Mouhieddine, M.; Dworschak, M.; Tschernko, E.; Lassnigg, A.; Hiesmayr, M. Absent Metabolic Transition from the Early to the Late Period in Non-Survivors Post Cardiac Surgery. Nutrients 2022, 14, 3366. https://doi.org/10.3390/nu14163366
Veraar C, Fischer A, Bernardi MH, Sulz I, Mouhieddine M, Dworschak M, Tschernko E, Lassnigg A, Hiesmayr M. Absent Metabolic Transition from the Early to the Late Period in Non-Survivors Post Cardiac Surgery. Nutrients. 2022; 14(16):3366. https://doi.org/10.3390/nu14163366
Chicago/Turabian StyleVeraar, Cecilia, Arabella Fischer, Martin H. Bernardi, Isabella Sulz, Mohamed Mouhieddine, Martin Dworschak, Edda Tschernko, Andrea Lassnigg, and Michael Hiesmayr. 2022. "Absent Metabolic Transition from the Early to the Late Period in Non-Survivors Post Cardiac Surgery" Nutrients 14, no. 16: 3366. https://doi.org/10.3390/nu14163366
APA StyleVeraar, C., Fischer, A., Bernardi, M. H., Sulz, I., Mouhieddine, M., Dworschak, M., Tschernko, E., Lassnigg, A., & Hiesmayr, M. (2022). Absent Metabolic Transition from the Early to the Late Period in Non-Survivors Post Cardiac Surgery. Nutrients, 14(16), 3366. https://doi.org/10.3390/nu14163366








