A High-Fat Diet Modifies Brain Neurotransmitter Profile and Hippocampal Proteome and Morphology in an IUGR Pig Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Procedure
2.3. Quantification of Neurotransmitters
2.4. Immunohistochemical Analysis of the Hippocampus
2.5. Image Processing and Analysis
2.6. Statistical Analyses
2.7. Proteomic Analysis by Isobaric Mass Tag Labeling with TMT10plex™
2.8. Nanoliquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (nanoLC-ESI-MS/MS)
2.9. Database Searching
2.10. Quantitative Analysis
2.11. Gene Ontology and Bioinformatic Analysis
3. Results
3.1. Effects of High-Fat Diet and the Influence of IUGR on the Neurotransmitter Profile in Several Brain Areas
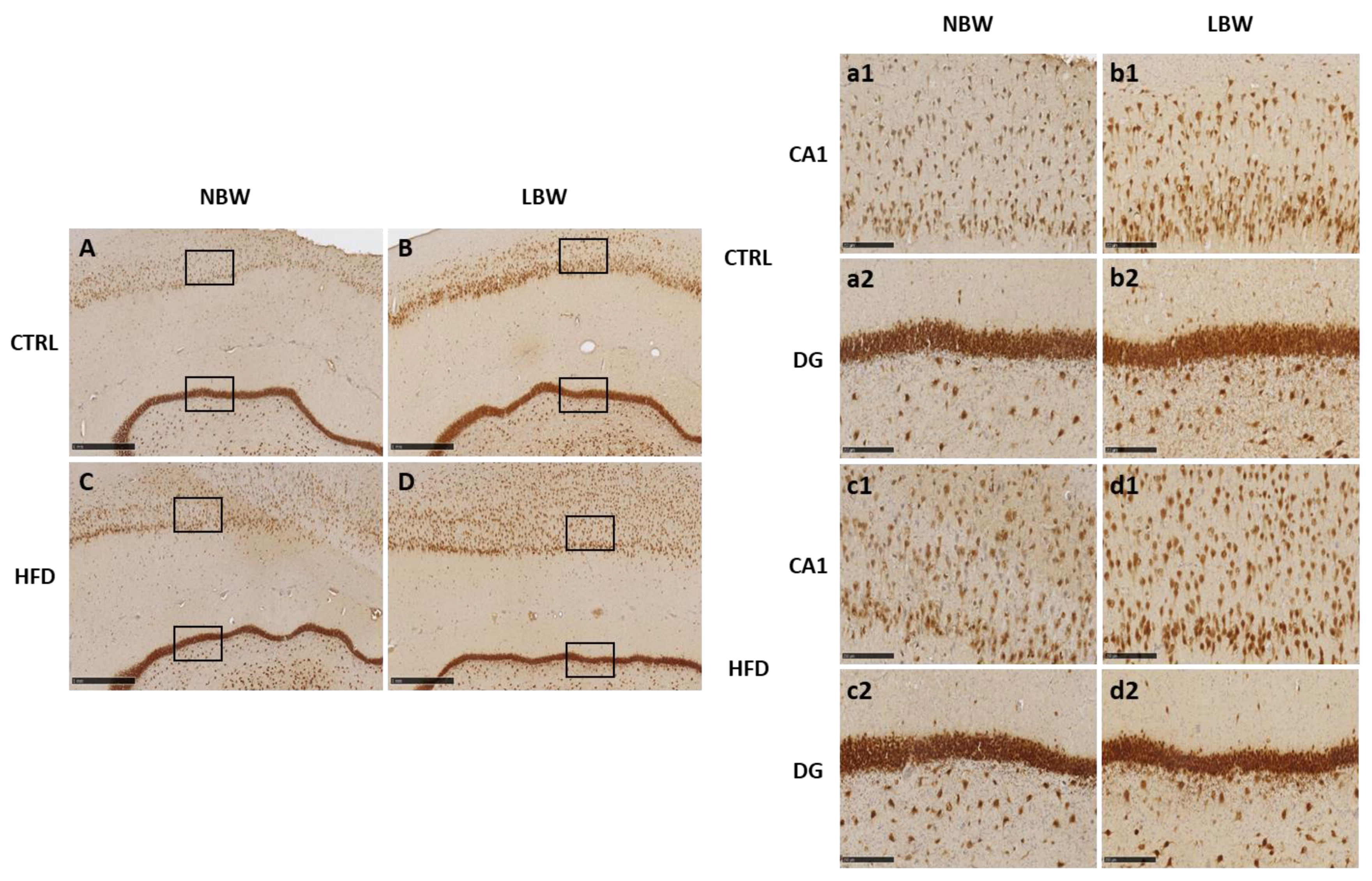
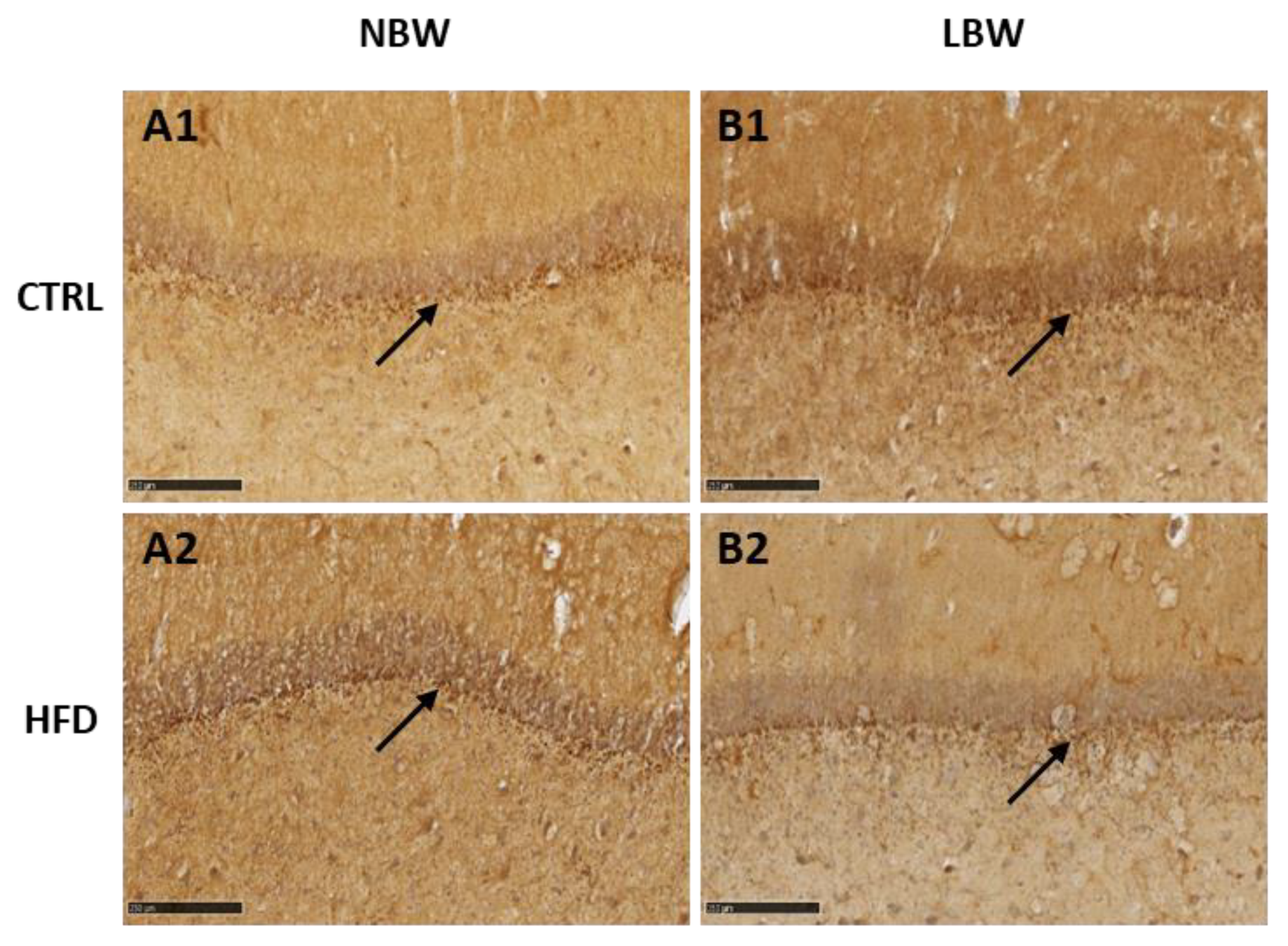
3.2. Effects of High-Fat Diet and the Influence of IUGR on the Morphology of the Hippocampus
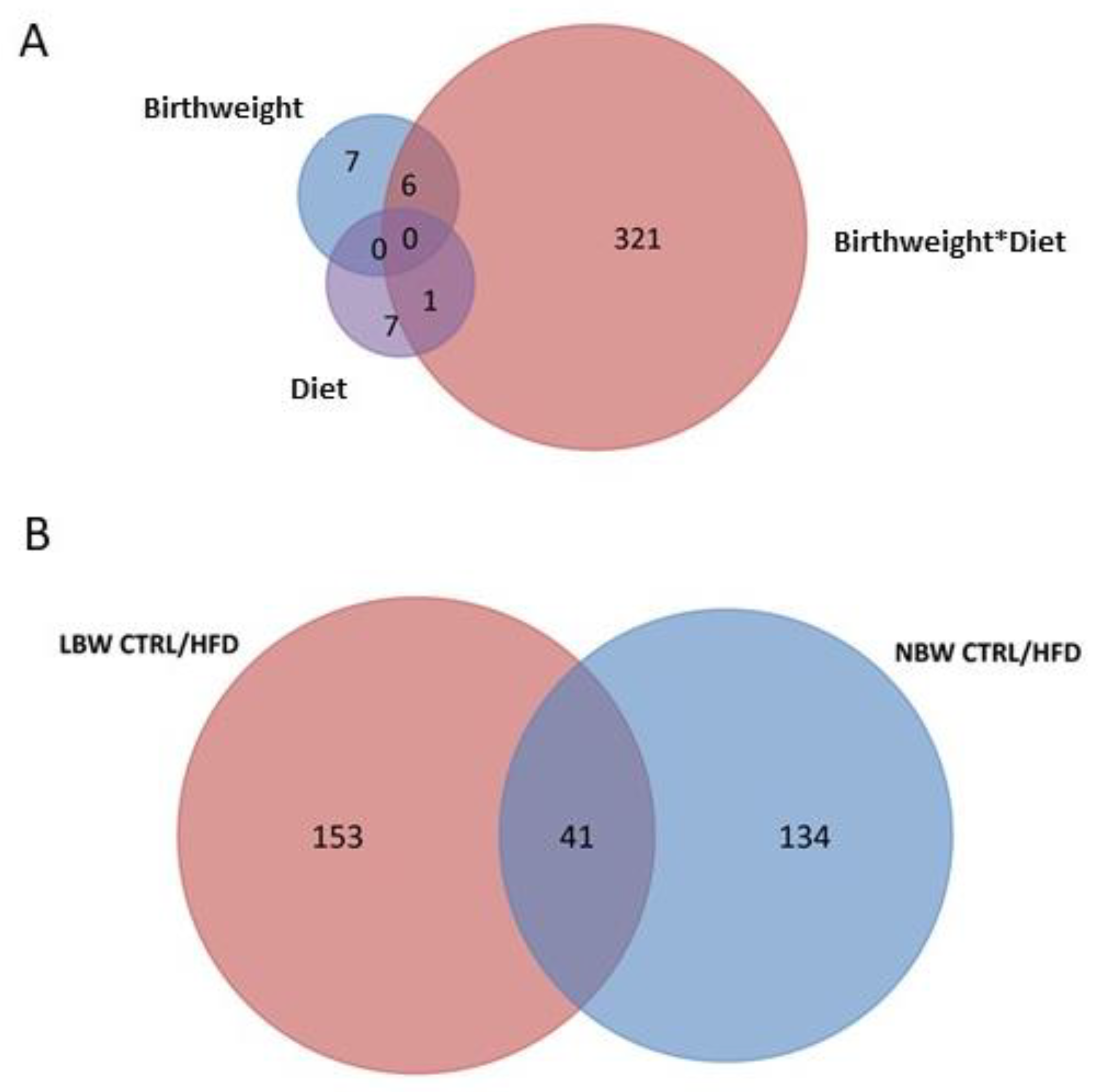
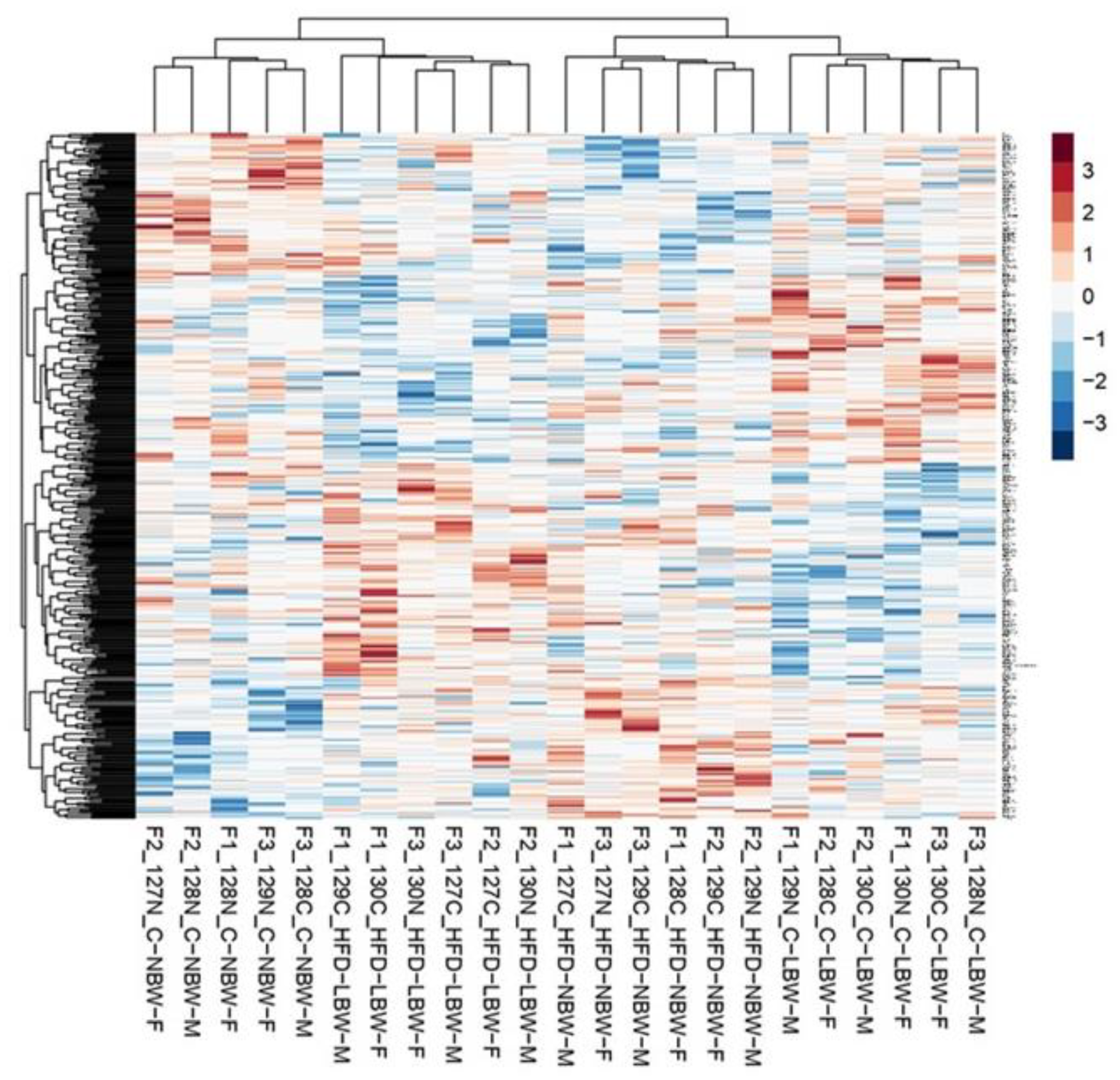
3.3. Effects of High-Fat Diet and the Influence of IUGR on the Proteome of the Hippocampus
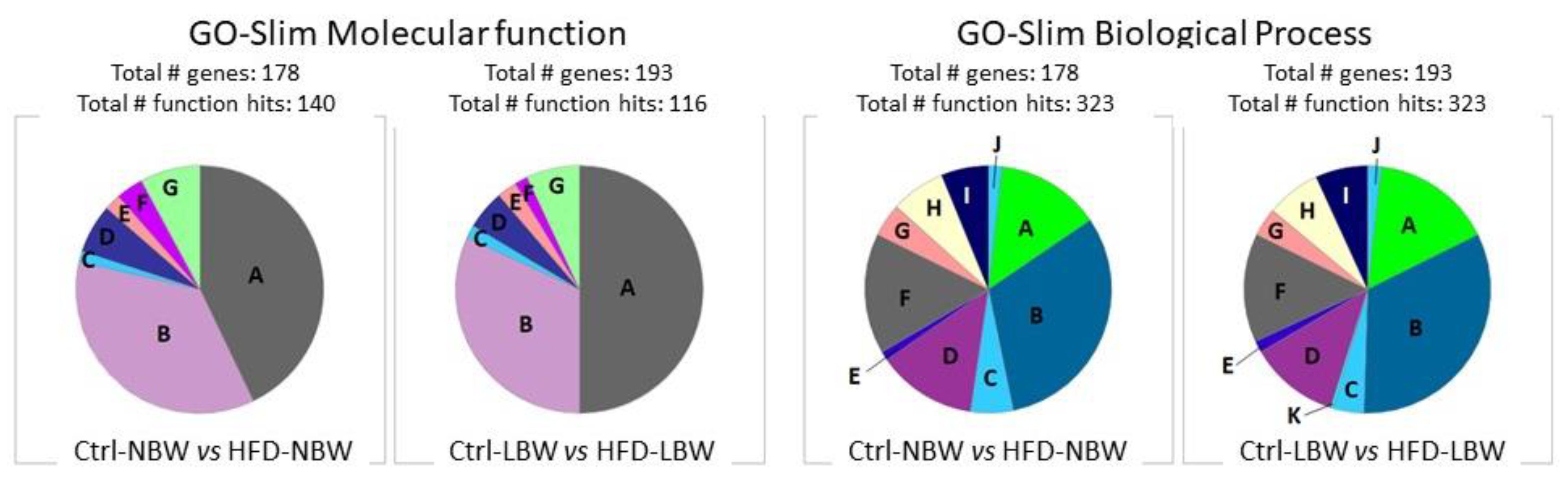
3.3.1. Gene Ontology Analysis
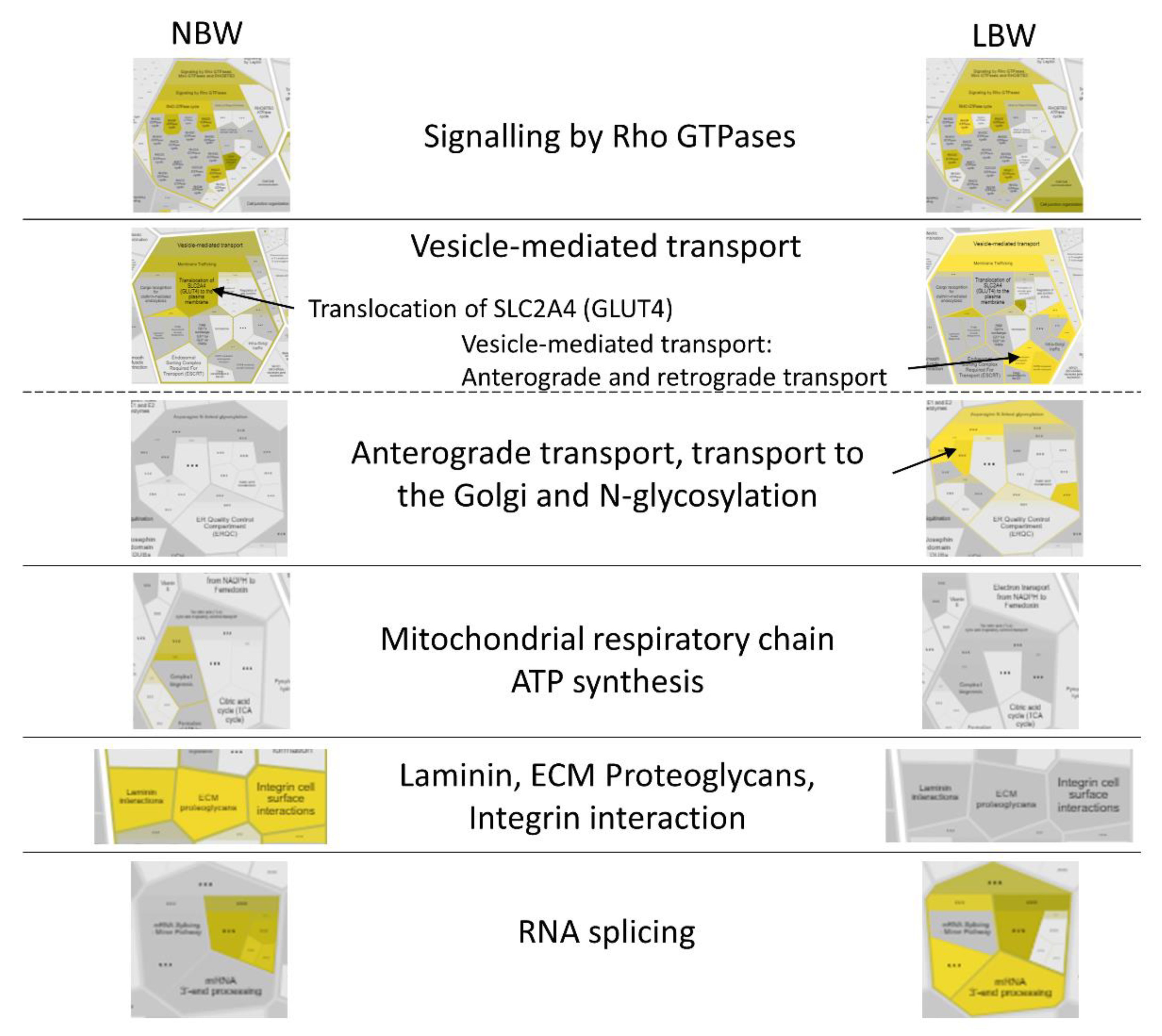
3.3.2. KEGG Pathway Analysis
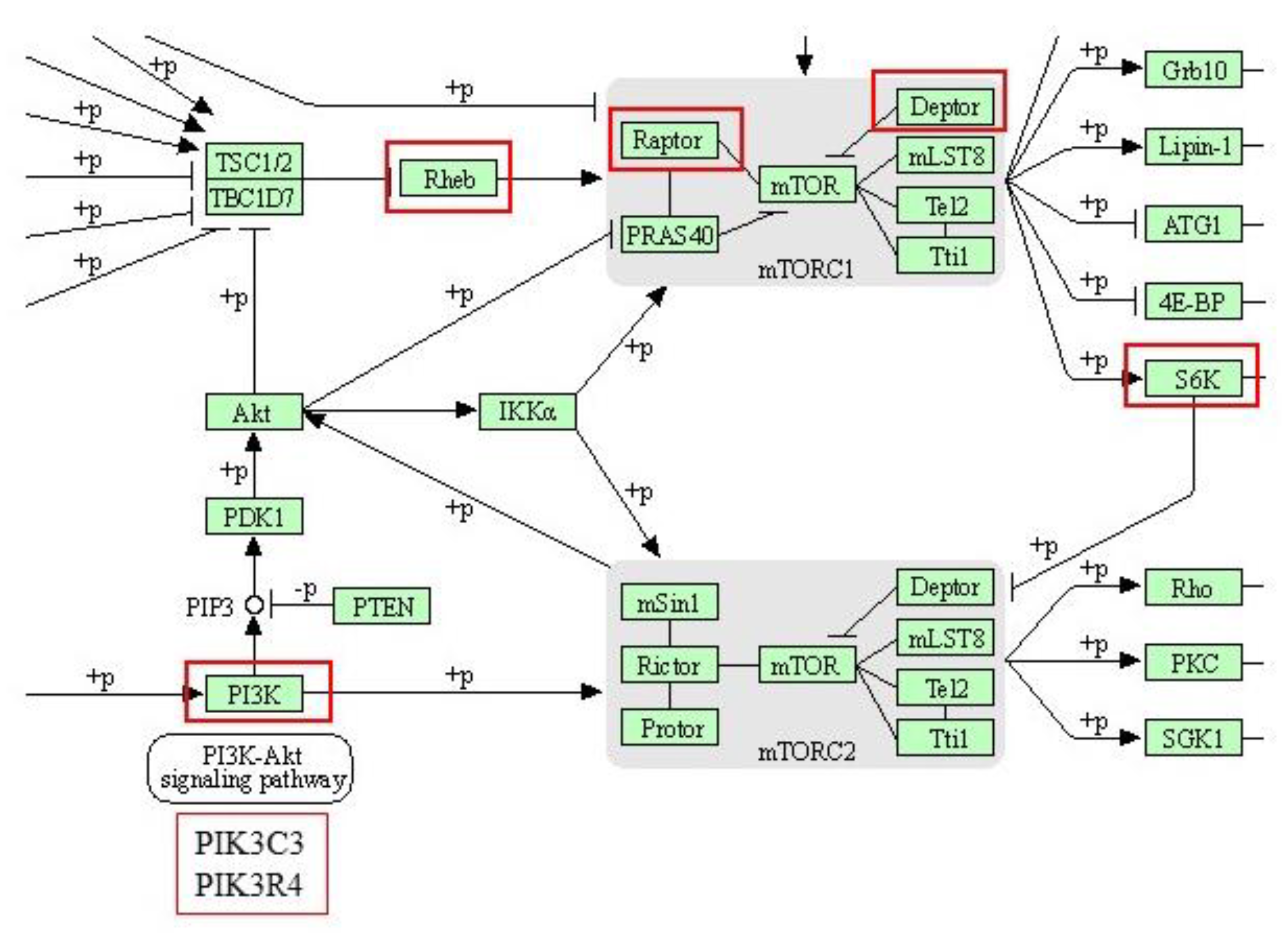
3.3.3. Pathway Analysis with Reactome
3.3.4. Upregulated and Downregulated Proteins
4. Discussion
4.1. Effect of an HFD Diet in NBW and LBW Pigs in the NT Profile and in the Morphology of the Hippocampus
4.2. Effect of an HFD Diet in NBW and LBW Pigs in the Proteome of the Hippocampus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMillen, I.C.; Adams, M.B.; Ross, J.T.; Coulter, C.L.; Simonetta, G.; Owens, J.A.; Robinson, J.S.; Edwards, L.J. Fetal Growth Restriction: Adaptations and Consequences. Reproduction 2001, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ergaz, Z.; Avgil, M.; Ornoy, A. Intrauterine Growth Restriction-Etiology and Consequences: What Do We Know about the Human Situation and Experimental Animal Models? Reprod. Toxicol. 2005, 20, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-Based Interventions for Improvement of Maternal and Child Nutrition: What Can Be Done and at What Cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. The Developmental Origins of the Metabolic Syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Developmental Origins of Health and Disease: New Insights. Basic Clin. Pharmacol. Toxicol. 2008, 102, 90–93. [Google Scholar] [CrossRef]
- Bruce, K.D.; Hanson, M.A. The Developmental Origins, Mechanisms, and Implications of Metabolic Syndrome. J. Nutr. 2010, 140, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Wang, E. Fetal Programming and Metabolic Syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef]
- Barker, D. The Developmental Origins of Chronic Adult Disease. Acta Paediatr. 2004, 446, 26–33. [Google Scholar] [CrossRef]
- Stöger, R. The Thrifty Epigenotype: An Acquired and Heritable Predisposition for Obesity and Diabetes? Bioessays 2008, 30, 156–166. [Google Scholar] [CrossRef]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental Environment Signalling to the Epigenome, Critical Time Windows and Sculpting the Adult Phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Walter, B.; Brust, P.; Füchtner, F.; Zwiener, U. Impact of Asymmetric Intrauterine Growth Restriction on Organ Function in Newborn Piglets. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S40–S49. [Google Scholar] [CrossRef]
- Miller, S.S.L.; Huppi, P.S.P.; Mallard, C. The Consequences of Fetal Growth Restriction on Brain Structure and Neurodevelopmental Outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, W.; Liu, J. Neurodevelopment in Children with Intrauterine Growth Restriction: Adverse Effects and Interventions. J. Matern. Neonatal Med. 2016, 29, 660–668. [Google Scholar] [CrossRef]
- Beukers, F.; Aarnoudse-Moens, C.S.H.; van Weissenbruch, M.M.; Ganzevoort, W.; van Goudoever, J.B.; van Wassenaer-Leemhuis, A.G. Fetal Growth Restriction with Brain Sparing: Neurocognitive and Behavioral Outcomes at 12 Years of Age. J. Pediatr. 2017, 188, 103–109.e2. [Google Scholar] [CrossRef]
- Camprubí, M.C.; Caballé, R.B.; Cano, J.A.O.; Torre, M.D.L.A.O.D.L.; Fernández-Feijoo, C.D.; Girabent-Farrés, M.; Figueras-Aloy, J.; Krauel, X.; Alcántara, S. Learning and Memory Disabilities in IUGR Babies: Functional and Molecular Analysis in a Rat Model. Brain Behav. 2017, 7, e00631. [Google Scholar] [CrossRef]
- McDougall, A.R.A.; Wiradjaja, V.; Azhan, A.; Li, A.; Hale, N.; Wlodek, M.E.; Hooper, S.B.; Wallace, M.J.; Tolcos, M. Intrauterine Growth Restriction Alters the Postnatal Development of the Rat Cerebellum. Dev. Neurosci. 2017, 39, 215–227. [Google Scholar] [CrossRef]
- Pedroso, A.P.; Souza, A.P.; Dornellas, A.P.S.; Oyama, L.M.; Nascimento, C.M.O.; Santos, G.M.S.; Rosa, J.C.; Bertolla, R.P.; Klawitter, J.; Christians, U.; et al. Intrauterine Growth Restriction Programs the Hypothalamus of Adult Male Rats: Integrated Analysis of Proteomic and Metabolomic Data. J. Proteome Res. 2017, 16, 1515–1525. [Google Scholar] [CrossRef]
- Tolcos, M.; McDougall, A.; Shields, A.; Chung, Y.; O’Dowd, R.; Turnley, A.; Wallace, M.; Rees, S. Intrauterine Growth Restriction Affects Cerebellar Granule Cells in the Developing Guinea Pig Brain. Dev. Neurosci. 2018, 40, 162–174. [Google Scholar] [CrossRef]
- Hamernik, D.L. Farm Animals Are Important Biomedical Models. Anim. Front. Rev. Mag. Anim. Agric. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The Pig as an Animal Model for Human Pathologies: A Proteomics Perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rovira, L.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Diet-Induced Swine Model with Obesity/Leptin Resistance for the Study of Metabolic Syndrome and Type 2 Diabetes. Sci. World J. 2012, 2012, 510149. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rovira, L.; Gonzalez-Anover, P.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Effect of an Obesogenic Diet during the Juvenile Period on Growth Pattern, Fatness and Metabolic, Cardiovascular and Reproductive Features of Swine with Obesity/Leptin Resistance. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Óvilo, C.; González-Bulnes, A.; Benítez, R.; Ayuso, M.; Barbero, A.; Pérez-Solana, M.L.; Barragán, C.; Astiz, S.; Fernández, A.; López-Bote, C. Prenatal Programming in an Obese Swine Model: Sex-Related Effects of Maternal Energy Restriction on Morphology, Metabolism and Hypothalamic Gene Expression. Br. J. Nutr. 2014, 111, 735–746. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Torres-Rovira, L.; Astiz, S.; Ovilo, C.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Perez-Solana, M.; Martin-Lluch, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Fetal Sex Modulates Developmental Response to Maternal Malnutrition. PLoS ONE 2015, 10, e0142158. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in Swine: Implications for Animal Production and Biomedical Research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Vázquez-Gómez, M.; Valent, D.; García-Contreras, C.; Arroyo, L.; Óvilo, C.; Isabel, B.; Bassols, A.; González-Bulnes, A. Sex and Intrauterine Growth Restriction Modify Brain Neurotransmitters Profile of Newborn Piglets. Int. J. Dev. Neurosci. 2016, 55, 9–14. [Google Scholar] [CrossRef]
- García-Contreras, C.; Valent, D.; Vázquez-Gómez, M.; Arroyo, L.; Isabel, B.; Astiz, S.; Bassols, A.; Gonzalez-Bulnes, A. Fetal Growth-Retardation and Brain-Sparing by Malnutrition Are Associated to Changes in Neurotransmitters Profile. Int. J. Dev. Neurosci. 2017, 57, 72–76. [Google Scholar] [CrossRef]
- Valent, D.; Yeste, N.; Hernández-Castellano, L.E.; Arroyo, L.; Wu, W.; García-Contreras, C.; Vázquez-Gómez, M.; González-Bulnes, A.; Bendixen, E.; Bassols, A. SWATH-MS Quantitative Proteomic Investigation of Intrauterine Growth Restriction in a Porcine Model Reveals Sex Differences in Hippocampus Development. J. Proteom. 2019, 204, 103391. [Google Scholar] [CrossRef]
- Yeste, N.; Gómez, N.; Vázquez-Gómez, M.; García-Contreras, C.; Pumarola, M.; González-Bulnes, A.; Bassols, A. Polyphenols and IUGR Pregnancies: Intrauterine Growth Restriction and Hydroxytyrosol Affect the Development and Neurotransmitter Profile of the Hippocampus in a Pig Model. Antioxidants 2021, 10, 1505. [Google Scholar] [CrossRef]
- Yeste, N.; Valent, D.; Arroyo, L.; Vázquez-Gómez, M.; García-Contreras, C.; Pumarola, M.; González-Bulnes, A.; Bassols, A. Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model. Antioxidants 2021, 10, 884. [Google Scholar] [CrossRef]
- Yeste, N.; Pérez-Valle, J.; Vázquez-Gómez, M.; García-Contreras, C.; González-Bulnes, A.; Bassols, A. Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on the Hippocampus Proteome in a Porcine Model. Antioxidants 2022, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Stevenson, R. The Longer-Term Impacts of Western Diet on Human Cognition and the Brain. Appetite 2013, 63, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Z.B.; Stevenson, R.J.; Ehrenfeld, L.; Francis, H.M. The Impact of Saturated Fat, Added Sugar and Their Combination on Human Hippocampal Integrity and Function: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 130, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernández-Alfonso, M.S.; Merino, B.; Gil-Ortega, M.; Fole, A.; Morales, L.; Ruiz-Gayo, M.; Olmo, N. Del High-Fat Diets Impair Spatial Learning in the Radial-Arm Maze in Mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef]
- Abbott, K.N.; Arnott, C.K.; Westbrook, R.F.; Tran, D.M.D. The Effect of High Fat, High Sugar, and Combined High Fat-High Sugar Diets on Spatial Learning and Memory in Rodents: A Meta-Analysis. Neurosci. Biobehav. Rev. 2019, 107, 399–421. [Google Scholar] [CrossRef]
- Arroyo, L.; Carreras, R.; Valent, D.; Peña, R.; Mainau, E.; Velarde, A.; Sabrià, J.; Bassols, A. Effect of Handling on Neurotransmitter Profile in Pig Brain According to Fear Related Behaviour. Physiol. Behav. 2016, 167, 374–381. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y. KEGG Mapper for Inferring Cellular Functions from Protein Sequences. Protein Sci. 2019, 29, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Faa, G.; Manchia, M.; Pintus, R.; Gerosa, C.; Marcialis, M.A.; Fanos, V. Fetal Programming of Neuropsychiatric Disorders. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Gerosa, C.; Rais, M.; Ravarino, A.; Van Eyken, P.; Fanos, V.; Faa, G. The Role of Neuropathological Markers in the Interpretation of Neuropsychiatric Disorders: Focus on Fetal and Perinatal Programming. Neurosci. Lett. 2018, 669, 75–82. [Google Scholar] [CrossRef]
- Besser, L.M.; Gill, D.P.; Monsell, S.E.; Brenowitz, W.; Meranus, D.H.; Kukull, W.; Gustafson, D.R. Body Mass Index, Weight Change, and Clinical Progression in Mild Cognitive Impairment and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2014, 28, 36–43. [Google Scholar] [CrossRef]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef]
- Underwood, E.L.; Thompson, L.T. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast. 2016, 2016, 7385314. [Google Scholar] [CrossRef][Green Version]
- Dharavath, R.N.; Arora, S.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. High Fat-Low Protein Diet Induces Metabolic Alterations and Cognitive Dysfunction in Female Rats. Metab. Brain Dis. 2019, 34, 1531–1546. [Google Scholar] [CrossRef]
- Leigh, S.J.; Morris, M.J. Diet, Inflammation and the Gut Microbiome: Mechanisms for Obesity-Associated Cognitive Impairment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165767. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L.K. Brain Serotonin System in the Coordination of Food Intake and Body Weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.R.; Labouèbe, G.; Liu, S.; Nieh, E.H.; Routh, V.H.; Xu, S.; O’Connor, E.C. Homeostasis Meets Motivation in the Battle to Control Food Intake. J. Neurosci. 2016, 36, 11469–11481. [Google Scholar] [CrossRef]
- Pineda, R.; Torres, E.; Tena-Sempere, M. Metabolites H OH OH Extrahypothalamic Control of Energy Balance and Its Connection with Reproduction: Roles of the Amygdala. Metabolites 2021, 11, 837. [Google Scholar] [CrossRef]
- Palmiter, R.D. Is Dopamine a Physiologically Relevant Mediator of Feeding Behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef]
- Kesner, R.P.; Lee, I.; Gilbert, P. A Behavioral Assessment of Hippocampal Function Based on a Subregional Analysis. Rev. Neurosci. 2004, 15, 333–351. [Google Scholar] [CrossRef]
- Lodygensky, G.A.; Seghier, M.L.; Warfield, S.K.; Tolsa, C.B.; Sizonenko, S.; Lazeyras, F.; Hüppi, P.S. Intrauterine Growth Restriction Affects the Preterm Infant’s Hippocampus. Pediatr. Res. 2008, 63, 438–443. [Google Scholar] [CrossRef]
- Benard, O.; Lim, J.; Apontes, P.; Jing, X.; Angeletti, R.H.; Chi, Y. Impact of High-Fat Diet on the Proteome of Mouse Liver. J. Nutr. Biochem. 2016, 31, 10–19. [Google Scholar] [CrossRef]
- Plubell, D.L.; Wilmarth, P.A.; Zhao, Y.; Fenton, A.M.; Minnier, J.; Reddy, A.P.; Klimek, J.; Yang, X.; David, L.L.; Pamir, N. Extended Multiplexing of Tandem Mass Tags (TMT) Labeling Reveals Age and High Fat Diet Specific Proteome Changes in Mouse Epididymal Adipose Tissue. Mol. Cell. Proteom. 2017, 16, 873–890. [Google Scholar] [CrossRef]
- Moriggi, M.; Belloli, S.; Barbacini, P.; Murtaj, V.; Torretta, E.; Chaabane, L.; Canu, T.; Penati, S.; Malosio, M.L.; Esposito, A.; et al. Skeletal Muscle Proteomic Profile Revealed Gender-Related Metabolic Responses in a Diet-Induced Obesity Animal Model. Int. J. Mol. Sci. 2021, 22, 4680. [Google Scholar] [CrossRef]
- McLean, F.H.; Campbell, F.M.; Langston, R.F.; Sergi, D.; Resch, C.; Grant, C.; Morris, A.C.; Mayer, C.D.; Williams, L.M. A High-Fat Diet Induces Rapid Changes in the Mouse Hypothalamic Proteome. Nutr. Metab. 2019, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Siino, V.; Amato, A.; Di Salvo, F.; Caldara, G.F.; Filogamo, M.; James, P.; Vasto, S. Impact of Diet-Induced Obesity on the Mouse Brain Phosphoproteome. J. Nutr. Biochem. 2018, 58, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.M.; Mirzaei, M.; Pardey, M.C.; Haynes, P.A.; Cornish, J.L. A Proteomic Analysis of the Dorsal and Ventral Hippocampus of Rats Maintained on a High Fat and Refined Sugar Diet. Proteomics 2013, 13, 3076–3091. [Google Scholar] [CrossRef] [PubMed]
- Kerrisk, M.E.; Cingolani, L.A.; Koleske, A.J. ECM Receptors in Neuronal Structure, Synaptic Plasticity, and Behavior. Prog. Brain Res. 2014, 214, 101–131. [Google Scholar] [CrossRef]
- Lilja, J.; Ivaska, J. Integrin Activity in Neuronal Connectivity. J. Cell Sci. 2018, 131, jcs212803. [Google Scholar] [CrossRef]
- Jaudon, F.; Thalhammer, A.; Cingolani, L.A. Integrin Adhesion in Brain Assembly: From Molecular Structure to Neuropsychiatric Disorders. Eur. J. Neurosci. 2021, 53, 3831–3850. [Google Scholar] [CrossRef]
- Brutman, J.N.; Zhou, X.; Zhang, Y.; Michal, J.; Stark, B.; Jiang, Z.; Davis, J.F. Mapping Diet-Induced Alternative Polyadenylation of Hypothalamic Transcripts in the Obese Rat. Physiol. Behav. 2018, 188, 173–180. [Google Scholar] [CrossRef]
- Walker, J.M.; Harrison, F.E. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer’s Disease: Impacts on Cognitive Decline. Nutrients 2015, 7, 7332–7357. [Google Scholar] [CrossRef]
- Takalo, M.; Haapasalo, A.; Martiskainen, H.; Kurkinen, K.M.A.; Koivisto, H.; Miettinen, P.; Khandelwal, V.K.M.; Kemppainen, S.; Kaminska, D.; Mäkinen, P.; et al. High-Fat Diet Increases Tau Expression in the Brain of T2DM and AD Mice Independently of Peripheral Metabolic Status. J. Nutr. Biochem. 2014, 25, 634–641. [Google Scholar] [CrossRef]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L.F. Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef]
- Guillaud, L.; El-Agamy, S.E.; Otsuki, M.; Terenzio, M. Anterograde Axonal Transport in Neuronal Homeostasis and Disease. Front. Mol. Neurosci. 2020, 13, 556175. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.B.; Jansson, T. Novel Roles of Mechanistic Target of Rapamycin Signaling in Regulating Fetal Growth. Biol. Reprod. 2019, 100, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.; Morgan, E.; Alejandro, E.U. Nutrient Sensor Signaling Pathways and Cellular Stress in Fetal Growth Restriction. J. Mol. Endocrinol. 2019, 62, R155–R165. [Google Scholar] [CrossRef] [PubMed]
- Kumer, S.C.; Vrana, K.E. Intricate Regulation of Tyrosine Hydroxylase Activity and Gene Expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef]
- Kuhn, D.M. Tryptophan Hydroxylase Regulation. Drug-Induced Modifications That Alter Serotonin Neuronal Function. Adv. Exp. Med. Biol. 1999, 467, 19–27. [Google Scholar] [CrossRef]
- Millan, M.J.; Marin, P.; Bockaert, J.; la Cour, C.M. Signaling at G-Protein-Coupled Serotonin Receptors: Recent Advances and Future Research Directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef]
- Ledonne, A.; Mercuri, N.B. Current Concepts on the Physiopathological Relevance of Dopaminergic Receptors. Front. Cell. Neurosci. 2017, 11, 27. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Ovilo, C.; Lopez-Bote, C.J.; Astiz, S.; Ayuso, M.; Perez-Solana, M.; Sanchez-Sanchez, R.; Torres-Rovira, L. Gender-Specific Early Postnatal Catch-up Growth after Intrauterine Growth Retardation by Food Restriction in Swine with Obesity/Leptin Resistance. Reproduction 2012, 144, 269–278. [Google Scholar] [CrossRef]
| NT | NBW | LBW | p-Value | |||
|---|---|---|---|---|---|---|
| Diet | Weight | Interaction | ||||
| Hippocampus | ||||||
| DOPAC | CTRL | 63.87 ± 4.59 | 74.40 ± 4.13 | 0.024 | 0.287 | 0.370 |
| HFD | 81.10 ± 4.79 | 82.01 ± 6.62 | ||||
| HVA | CTRL | 87.12 ± 4.33 | 94.12 ± 5.20 | 0.836 | 0.998 | 0.182 |
| HFD | 93.06 ± 4.47 | 86.04 ± 6.27 | ||||
| DOPtotal | CTRL | 145.90 ± 7.95 | 168.52 ± 8.08 | 0.253 | 0.264 | 0.150 |
| HFD | 168.77 ± 6.30 | 165.85 ± 11.71 | ||||
| 5-HT | CTRL | 336.03 ± 14.03 | 367.69 ± 13.88 | <0.001 | 0.371 | 0.551 |
| HFD | 434.76 ± 23.53 | 441.15 ± 25.38 | ||||
| 5-HIAA | CTRL | 191.93 ± 8.13 | 213.85 ± 8.08 | 0.209 | 0.187 | 0.326 |
| HFD | 213.22 ± 7.27 | 216.49 ± 13.56 | ||||
| INDtotal | CTRL | 533.40 ± 18.72 | 581.54 ± 19.35 | 0.001 | 0.331 | 0.543 |
| HFD | 655.59 ± 31.81 | 666.77 ± 39.48 | ||||
| Amygdala | ||||||
| DA | CTRL | 705.45 ± 33.80 | 682.81 ± 32.18 | 0.947 | 0.734 | 0.387 |
| HFD | 665.36 ± 39.76 | 717.17 ± 60.81 | ||||
| DOPAC | CTRL | 447.20 ± 41.74 | 543.63 ± 51.70 | 0.828 | 0.150 | 0.447 |
| HFD | 470.87 ± 36.03 | 501.09 ± 43.99 | ||||
| HVA | CTRL | 814.35 ± 19.52 | 861.67 ± 63.50 | 0.285 | 0.809 | 0.543 |
| HFD | 908.06 ± 52.11 | 887.69 ± 71.62 | ||||
| DOPtotal | CTRL | 1966.99 ± 68.37 | 2088.12 ± 121.17 | 0.928 | 0.343 | 0.862 |
| HFD | 1995.30 ± 90.14 | 2079.17 ± 143.26 | ||||
| 5-HT | CTRL | 1501.57 ± 95.32 | 1567.02 ± 99.18 | 0.012 | 0.171 | 0.482 |
| HFD | 1685.72 ± 80.52 | 1886.99 ± 107.41 | ||||
| 5-HIAA | CTRL | 618.86 ± 27.66 | 678.11 ± 27.91 | 0.575 | 0.082 | 0.786 |
| HFD | 643.03 ± 20.20 | 686.51 ± 38.39 | ||||
| INDtotal | CTRL | 2120.43 ± 100.44 | 2245.12 ± 102.41 | 0.013 | 0.061 | 0.452 |
| HFD | 2315.06 ± 89.82 | 2601.64 ± 129.96 | ||||
| Prefrontal cortex | ||||||
| DOPAC | CTRL | 71.95 ± 8.07 | 70.87 ± 5.75 | 0.818 | 0.910 | 0.945 |
| HFD | 70.17 ± 4.05 | 69.91 ± 6.01 | ||||
| HVA | CTRL | 150.58 ± 5.95 | 158.25 ± 6.52 | 0.307 | 0.697 | 0.386 |
| HFD | 149.63 ± 5.82 | 146.70 ± 5.50 | ||||
| DOPtotal | CTRL | 222.53 ± 7.88 | 229.12 ± 9.81 | 0.361 | 0.838 | 0.557 |
| HFD | 219.80 ± 8.40 | 216.60 ± 6.40 | ||||
| 5-HT | CTRL | 325.93 ± 13.14 | 362.59 ± 26.87 | 0.046 | 0.189 | 0.740 |
| HFD | 378.39 ± 17.81 | 400.40 ± 26.18 | ||||
| 5-HIAA | CTRL | 163.75 ± 9.58 | 170.88 ± 9.90 | 0.385 | 0.326 | 0.884 |
| HFD | 169.89 ± 3.98 | 179.48 ± 9.77 | ||||
| INDtotal | CTRL | 489.68 ± 21.47 | 533.47 ± 34.18 | 0.073 | 0.193 | 0.832 |
| HFD | 548.29 ± 20.52 | 579.88 ± 34.62 | ||||
| Hypothalamus | ||||||
| DA | CTRL | 361.95 ± 30.94 | 348.53 ± 27.16 | 0.039 | 0.569 | 0.905 |
| HFD | 428.97 ± 20.97 | 408.44 ± 31.99 | ||||
| L-DOPA | CTRL | 652.69 ± 54.48 | 600.27 ± 104.22 | 0.13 | 0.212 | 0.653 |
| HFD | 781.39 ± 51.58 | 670.69 ± 55.58 | ||||
| DOPAC | CTRL | 511.69 ± 28.01 | 585.81 ± 60.98 | 0.817 | 0.813 | 0.163 |
| HFD | 564.78 ± 51.55 | 511.92 ± 24.00 | ||||
| HVA | CTRL | 616.73 ± 57.76 | 606.47 ± 50.58 | 0.876 | 0.715 | 0.876 |
| HFD | 616.73 ± 39.71 | 591.17 ± 38.61 | ||||
| DOPtotal | CTRL | 2225.07 ± 143.97 | 2057.62 ± 255.38 | 0.293 | 0.176 | 0.878 |
| HFD | 2391.87 ± 91.40 | 2182.21 ± 98.13 | ||||
| 5-HT | CTRL | 1250.61 ± 78.97 | 1146.07 ± 151.71 | 0.023 | 0.732 | 0.180 |
| HFD | 1352.55 ± 88.09 | 1527.81 ± 97.94 | ||||
| 5-HIAA | CTRL | 645.40 ± 37.09 | 622.28 ± 48.06 | 0.297 | 0.943 | 0.601 |
| HFD | 665.78 ± 35.64 | 683.32 ± 29.96 | ||||
| INDtotal | CTRL | 1907.75 ± 100.41 | 1768.35 ± 162.49 | 0.033 | 0.832 | 0.191 |
| HFD | 2018.33 ± 110.94 | 2211.14 ± 120.42 | ||||
| Striatum | ||||||
| DA | CTRL | 21,834.51 ± 2607.80 | 25,637.38 ± 2479.18 | 0.373 | 0.007 | 0.280 |
| HFD | 21,412.39 ± 1348.85 | 29,970.72 ± 2353.49 | ||||
| L-DOPA | CTRL | 2275.64 ± 248.13 | 1962.68 ± 242.11 | 0.528 | 0.061 | 0.636 |
| HFD | 2516.53 ± 221.93 | 1997.44 ± 122.63 | ||||
| DOPAC | CTRL | 4260.22 ± 217.81 | 4320.28 ± 189.50 | 0.011 | 0.437 | 0.653 |
| HFD | 3693.90 ± 116.83 | 3918.48 ± 201.54 | ||||
| HVA | CTRL | 11,262.32 ± 795.05 | 12,643.72 ± 689.32 | 0.175 | 0.113 | 0.655 |
| HFD | 10,641.91 ± 602.61 | 11,422.03 ± 578.05 | ||||
| 3-MT | CTRL | 1210.46 ± 96.21 | 1472.53 ± 56.29 | 0.151 | 0.046 | 0.272 |
| HFD | 1181.48 ± 71.50 | 1259.28 ± 89.19 | ||||
| DOPtotal | CTRL | 38,533.33 ± 1575.54 | 46,228.27 ± 2016.19 | 0.721 | 0.002 | 0.401 |
| HFD | 39,446.20 ± 1443.33 | 43,976.56 ± 2328.97 | ||||
| 5-HT | CTRL | 705.05 ± 29.61 | 720.23 ± 23.70 | 0.024 | 0.119 | 0.309 |
| HFD | 740.54 ± 24.01 | 811.32 ± 29.54 | ||||
| 5-HIAA | CTRL | 533.18 ± 33.11 | 540.28 ± 29.01 | 0.230 | 0.459 | 0.629 |
| HFD | 553.12 ± 23.37 | 586.66 ± 22.67 | ||||
| INDtotal | CTRL | 1270.54 ± 50.59 | 1260.51 ± 45.79 | 0.057 | 0.239 | 0.161 |
| HFD | 1293.66 ± 31.07 | 1406.73 ± 46.71 | ||||
| NBW | LBW | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Diet | Weight | Interaction | |||||
| CA1 | Number of neurons | CTRL | 352.81 ± 17.18 | 481.00 ± 161.83 | 0.897 | <0.001 | 0.539 |
| HFD | 336.84 ± 12.63 | 505.50 ± 22.00 | |||||
| Mean size (µm2) | CTRL | 650.90 ± 52.89 | 626.75 ± 145.43 | 0.521 | 0.175 | 0.309 | |
| HFD | 767.67 ± 30.49 | 600.20 ± 34.96 | |||||
| Area (%) | CTRL | 12.60 ± 0.61 | 15.00 ± 1.94 | 0.037 | 0.010 | 0.759 | |
| HFD | 14.45 ± 0.46 | 17.47 ± 0.98 | |||||
| DG | Number of neurons | CTRL | 208.56 ± 10.39 | 217.33 ± 15.34 | 0.084 | 0.333 | 0.825 |
| HFD | 226.42 ± 4.74 | 240.38 ± 5.55 | |||||
| Mean size (µm2) | CTRL | 1345.26 ± 45.64 | 1131.78 ± 164.07 | 0.575 | 0.050 | 0.260 | |
| HFD | 1307.23 ± 29.95 | 1244.58 ± 26.85 | |||||
| Area (%) | CTRL | 15.92 ± 0.55 | 13.96 ± 1.20 | 0.010 | 0.322 | 0.180 | |
| HFD | 17.02 ± 0.39 | 17.32 ± 0.48 | |||||
| Width (µm) | CTRL | 92.44 ± 2.36 | 92.72 ± 3.29 | 0.008 | 0.781 | 0.857 | |
| HFD | 84.08 ± 1.75 | 85.39 ± 4.57 | |||||
| NBW | Enrichment | LBW | Enrichment |
|---|---|---|---|
| Biological Process | |||
| negative regulation of synaptic vesicle exocytosis (GO:2000301) | 69.81 | negative regulation of mRNA splicing, via spliceosome (GO:0048025) | 24.25 |
| oxidative phosphorylation (GO:0006119) | 7.21 | regulation of alternative mRNA splicing, via spliceosome (GO:0000381) | 16.01 |
| ATP metabolic process (GO:0046034) | 5.18 | post-Golgi vesicle-mediated transport (GO:0006892) | 9.90 |
| regulation of vesicle-mediated transport (GO:0060627) | 3.96 | ||
| Molecular Function | |||
| cadherin binding (GO:0045296) | 3.96 | pre-mRNA binding (GO:0036002) | 16.42 |
| cytoskeletal protein binding (GO:0008092) | 2.90 | structural constituent of cytoskeleton (GO:0005200) | 7.18 |
| oxidoreductase activity (GO:0016491) | 2.82 | cadherin binding (GO:0045296) | 4.96 |
| RNA binding (GO:0003723) | 2.07 | actin binding (GO:0003779) | 4.48 |
| Cellular Component | |||
| platelet alpha granule membrane (GO:0031092) | 20.53 | UFD1-NPL4 complex (GO:0036501) | >100 |
| cytochrome complex (GO:0070069) | 11.35 | endoplasmic reticulum-Golgi intermediate compartment membrane (GO:0033116) | 6.93 |
| lysosomal lumen (GO:0043202) | 7.20 | ruffle membrane (GO:0032587) | 6.47 |
| basement membrane (GO:0005604) | 7.20 | exocytic vesicle (GO:0070382) | 4.66 |
| lipid droplet (GO:0005811) | 7.20 | postsynaptic density (GO:0014069) | 4.46 |
| Schaffer collateral-CA1 synapse (GO:0098685) | 7.01 | asymmetric synapse (GO:0032279) | 4.39 |
| respiratory chain complex (GO:0098803) | 6.54 | postsynaptic specialization (GO:0099572) | 4.14 |
| glutamatergic synapse (GO:0098978) | 5.32 | ||
| secretory granule membrane (GO:0030667) | 3.74 | ||
| secretory granule lumen (GO:0034774) | 3.62 |
| Pathways | n | Proteins | |
|---|---|---|---|
| NBW | Metabolic pathways | 25 | COQ7; ATP5PD; CKMT2; COX6B1; COX7A2; COX15; STT3B; AK2; ALDH1A3; PLCH1; GLUD2; GNS; GSTP1; APRT; ARSA; ND1; MVD; NDUFS5; ACOX1; PKM; UQCRC2; CA4; DGKE; AGPS; KYAT1 |
| Thermogenesis | 10 | ATP5PD; COX6B1; COX7A2; COX15; ND1; NDUFS5; COA1; MAP2K3; RHEB; UQCRC2 | |
| Oxidative phosphorylation | 7 | ATP5PD; COX6B1; COX7A2; COX15; ND1; NDUFS5; UQCRC2 | |
| Autophagy | 7 | AKT2; ATG4B; ITPR1; LAMP2; ZFYVE1; RHEB; ATG3 | |
| Focal adhesion and ECM–receptor interaction | 7 | COL4A1; AKT2; PARVB; ITGA7; PPP1CA; RAP1B; BRAF; HSPG2; AGRIN | |
| Insulin signaling pathway | 6 | AKT2; PPP1CA; PRKAR1B; PTPRF; RHEB; BRAF | |
| cAMP signaling pathway | 6 | AKT2; ATP2A1; ACOX1; PPP1CA; RAP1B; BRAF | |
| Lysosome | 5 | NPC2; GNS; LAMP2; ARSA; AP3B1 | |
| MAPK signaling pathway | 5 | AKT2; NF1; MAP2K3; RAP1B; BRAF | |
| Protein processing in the ER | 4 | SEC63; STT3B; NPLOC4; PDIA4 | |
| LBW | Metabolic pathways | 30 | CBS; CDIPT; PAICS; DGKB; FASN; PLCH1; SIRT5; TKFC; GCSH; GFPT1; ACAD8; MAT2B; GMPPA; HADHA; ARSB; ND1; NDUFS5; ALDH7A1; PDE4B; COQ3; PIK3C3; PMM1; CYCS; UCKL1; PPT1; ECHDC1; CHPT1; CBR1; KYAT1; PIGS |
| Thermogenesis | 7 | ND1; NDUFS5; COA1; RPTOR; RHEB; RPS6KA3; SMARCC1 | |
| Spliceosome | 7 | U2AF2; DDX5; HNRNPK; SRSF4; SRSF6; TRA2B; RBM8A | |
| Autophagy | 6 | PIK3R4; PIK3C3; MAPK10; RPTOR; RHEB; DEPTOR | |
| Protein processing in endoplasmic reticulum | 6 | SEC61B; DNAJB2; NPLOC4; MAPK10; SEC62; UFD1 | |
| cAMP signaling pathway | 5 | GRIA4; AFDN; ATP2A1; PDE4B; MAPK10 | |
| Lysosome | 5 | AP3S1; FUCA1; ARSB; PPT1; SORT1 | |
| mTOR signaling pathway | 4 | RPTOR; RHEB; RPS6KA3; DEPTOR | |
| Focal adhesion | 4 | DIAPH1; ITGB8; PARVA; MAPK10 | |
| Insulin signaling pathway | 4 | FASN; MAPK10; RPTOR; RHEB |
| Access UniProt | Gene | Identification | |
|---|---|---|---|
| NBW | Oxidative phosphorylation/Respiratory chain | ||
| F1RPD2 | UQCRC2 | Cytochrome b-c1 complex subunit 2, mitochondrial | |
| F1S8W1 | COX15 | Cytochrome c oxidase assembly protein COX15 homolog isoform 1 | |
| F1S4V0 | COX7A2 | Cytochrome c oxidase subunit 7A2, mitochondrial | |
| I3LR62 | COA1 | Cytochrome c oxidase assembly factor 1 homolog | |
| F1SV23 | NDUFS5 | Complex I-15 kDa | |
| O79874 | NADH1 | NADH-ubiquinone oxidoreductase chain 1 | |
| A0A5G2QL31 | COX6B1 | Cytochrome c oxidase subunit 6B1 | |
| F1SMF9 | ATP5PD | ATP synthase subunit d, mitochondrial | |
| Focal adhesion and ECM–receptor interaction | |||
| I3LGD9 | AGRN | Agrin | |
| F1SJU4 | PARVB | Parvin beta | |
| A0A481B0D0 | ITGA7 | Integrin alpha-2 | |
| M3V819 | COL4A1 | Collagen alpha-1 (IV) chain isoform 1 preproprotein | |
| F1RW32 | SPARCL1 | SPARC like 1 | |
| F1SU03 | HSPG2 | Heparan sulfate proteoglycan 2 | |
| LBW | mRNA splicing | ||
| I3LFJ5 | SUGP2 | SURP and G-patch domain containing 2 | |
| F1S6R7 | PTBP1 | Polypyrimidine tract-binding protein 1 | |
| F1RZV6 | KHDRBS2 | KH RNA binding domain containing, signal transduction associated 2 | |
| P80230 | ERH | Enhancer of rudimentary homolog | |
| Q06AA7 | TRA2B | Transformer 2 beta homolog | |
| I3LDY1 | EML1 | Echinoderm microtubule-associated protein-like 1 | |
| F6QB00 | SRSF4 | Serine/arginine-rich splicing factor 4 isoform X1 | |
| I3W8V7 | U2AF2 | Splicing factor U2AF 65 kDa subunit | |
| I3LI59 | RBM8A | RNA-binding protein 8A | |
| A0A286ZM27 | DDX5 | DEAD box protein 5 | |
| I3LQS0 | HNRNPK | Heterogeneous nuclear ribonucleoprotein K | |
| K7GNF4 | FMR1 | Synaptic functional regulator FMR1 | |
| F1RUN0 | CELF2 | CUGBP Elav-like family member 2 | |
| A0A4X1TTZ9 | SRSF6 | Serine/arginine-rich splicing factor 6 | |
| K7GNY3 | KHDRBS3 | KH domain-containing, RNA-binding, signal transduction-associated protein 3 | |
| mTOR signaling pathway | |||
| Q5D891 | PIK3C3 | Phosphatidylinositol 3-kinase catalytic subunit type 3 | |
| K9IWD2 | PIK3R4 | Phosphoinositide 3-kinase regulatory subunit 4 | |
| A0A480DP04 | DEPTOR | DEP domain-containing mTOR-interacting protein | |
| I3L942 | RPTOR | Regulatory associated protein of MTOR complex 1 | |
| F1SQN4 | RPS6KA3 | Ribosomal protein S6 kinase alpha-3 | |
| F2Z5R2 | RHEB | GTP-binding protein Rheb | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeste, N.; Pérez-Valle, J.; Heras-Molina, A.; Pesántez-Pacheco, J.L.; Porrini, E.; González-Bulnes, A.; Bassols, A. A High-Fat Diet Modifies Brain Neurotransmitter Profile and Hippocampal Proteome and Morphology in an IUGR Pig Model. Nutrients 2022, 14, 3440. https://doi.org/10.3390/nu14163440
Yeste N, Pérez-Valle J, Heras-Molina A, Pesántez-Pacheco JL, Porrini E, González-Bulnes A, Bassols A. A High-Fat Diet Modifies Brain Neurotransmitter Profile and Hippocampal Proteome and Morphology in an IUGR Pig Model. Nutrients. 2022; 14(16):3440. https://doi.org/10.3390/nu14163440
Chicago/Turabian StyleYeste, Natalia, Jorge Pérez-Valle, Ana Heras-Molina, José Luis Pesántez-Pacheco, Esteban Porrini, Antonio González-Bulnes, and Anna Bassols. 2022. "A High-Fat Diet Modifies Brain Neurotransmitter Profile and Hippocampal Proteome and Morphology in an IUGR Pig Model" Nutrients 14, no. 16: 3440. https://doi.org/10.3390/nu14163440
APA StyleYeste, N., Pérez-Valle, J., Heras-Molina, A., Pesántez-Pacheco, J. L., Porrini, E., González-Bulnes, A., & Bassols, A. (2022). A High-Fat Diet Modifies Brain Neurotransmitter Profile and Hippocampal Proteome and Morphology in an IUGR Pig Model. Nutrients, 14(16), 3440. https://doi.org/10.3390/nu14163440







