Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatments
2.3. Cell Viability Assays
2.4. Apoptosis Detection
2.5. Real-Time qPCR Analysis
2.6. Thiobarbituric Acid Reactive Substances Assay
2.7. Mitochondrial Depolarization Detection
2.8. Statistical Analysis
3. Results
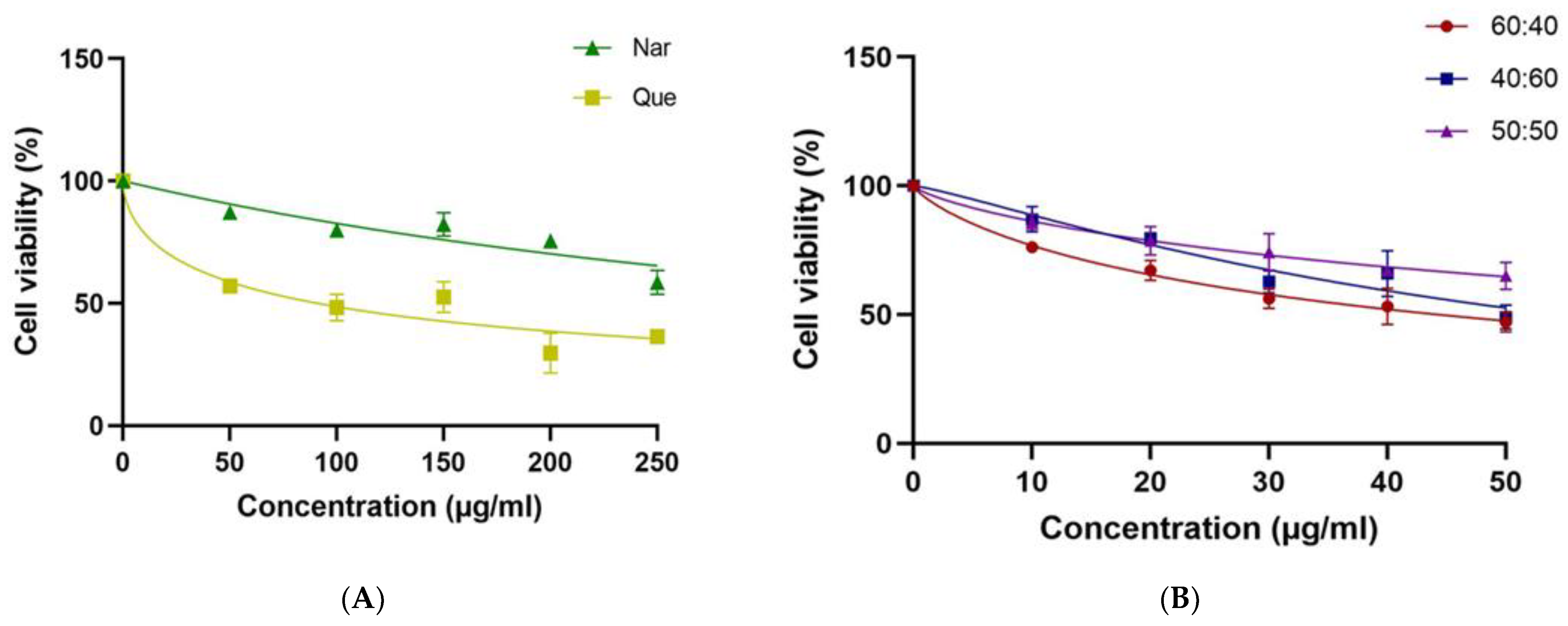
3.1. MCF-7 Cell Viability
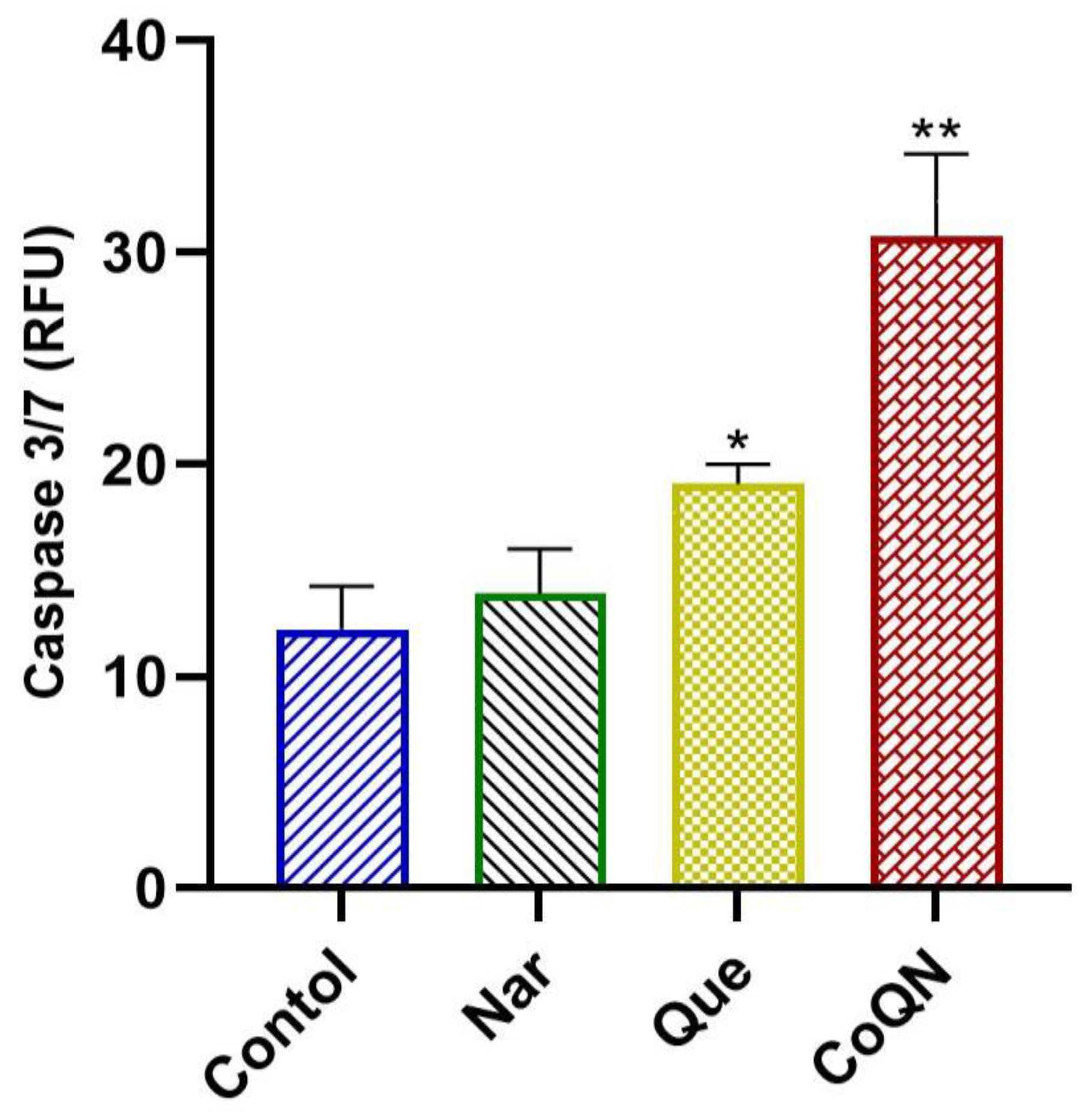
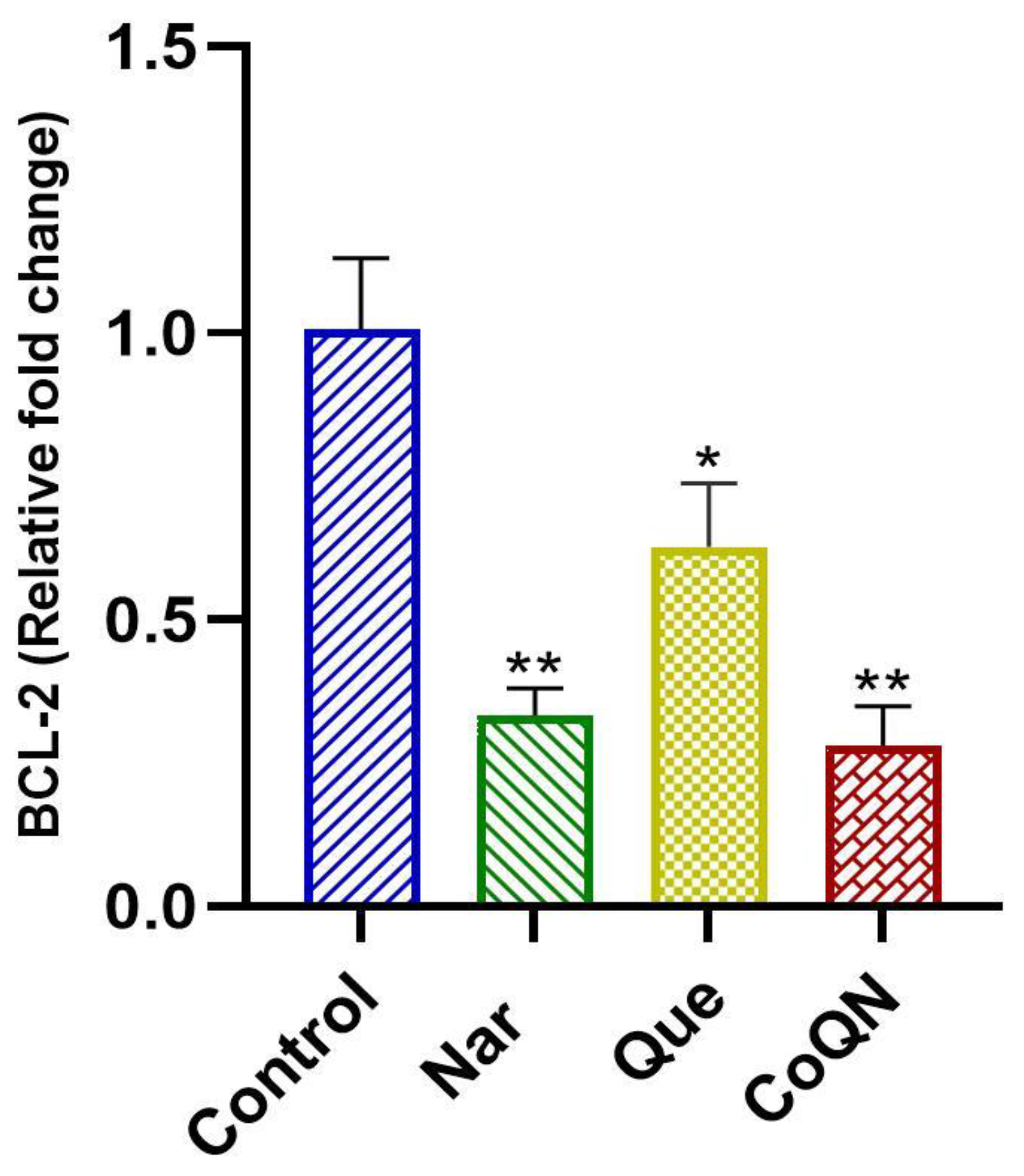
3.2. Apoptosis Induction
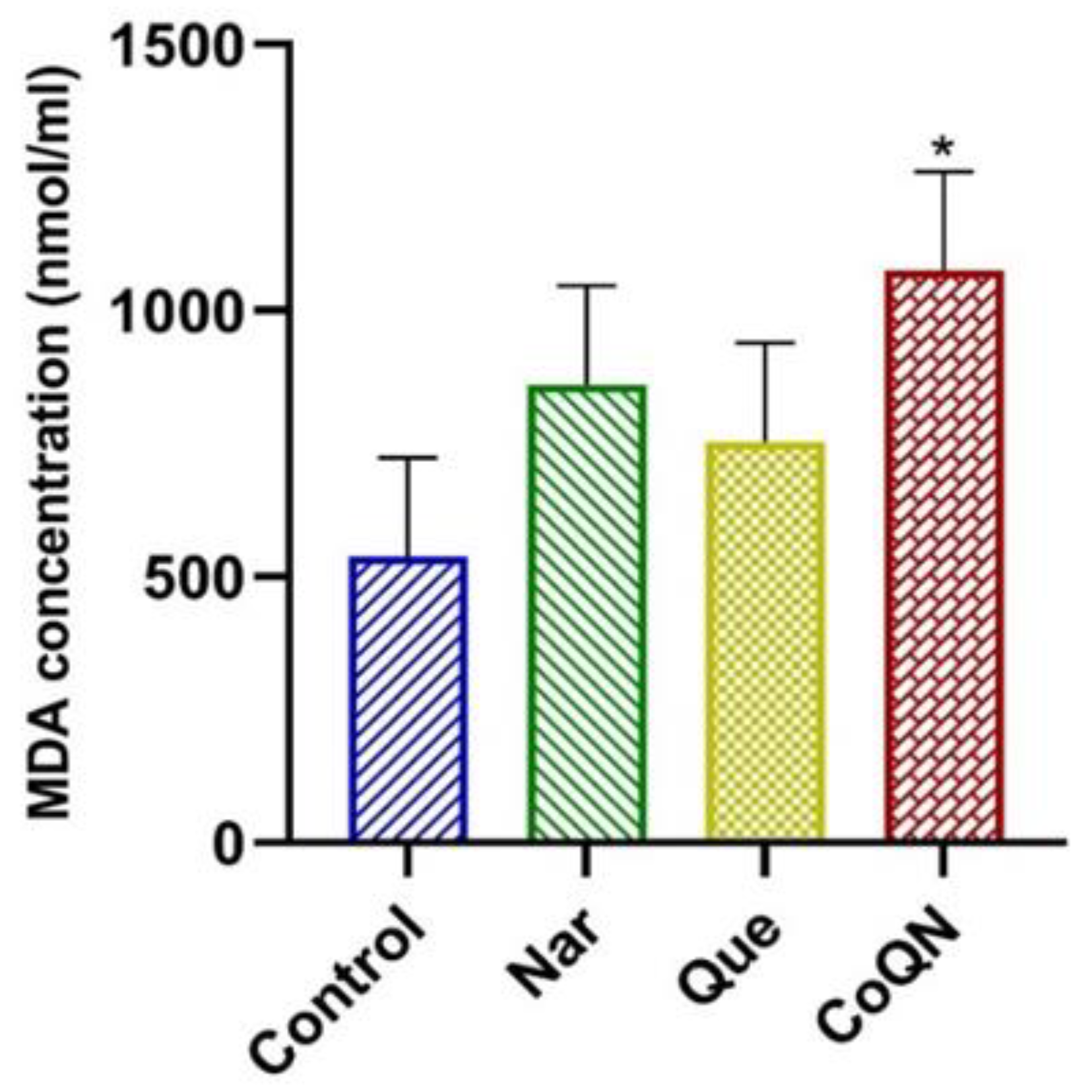
3.3. Lipid Peroxidation
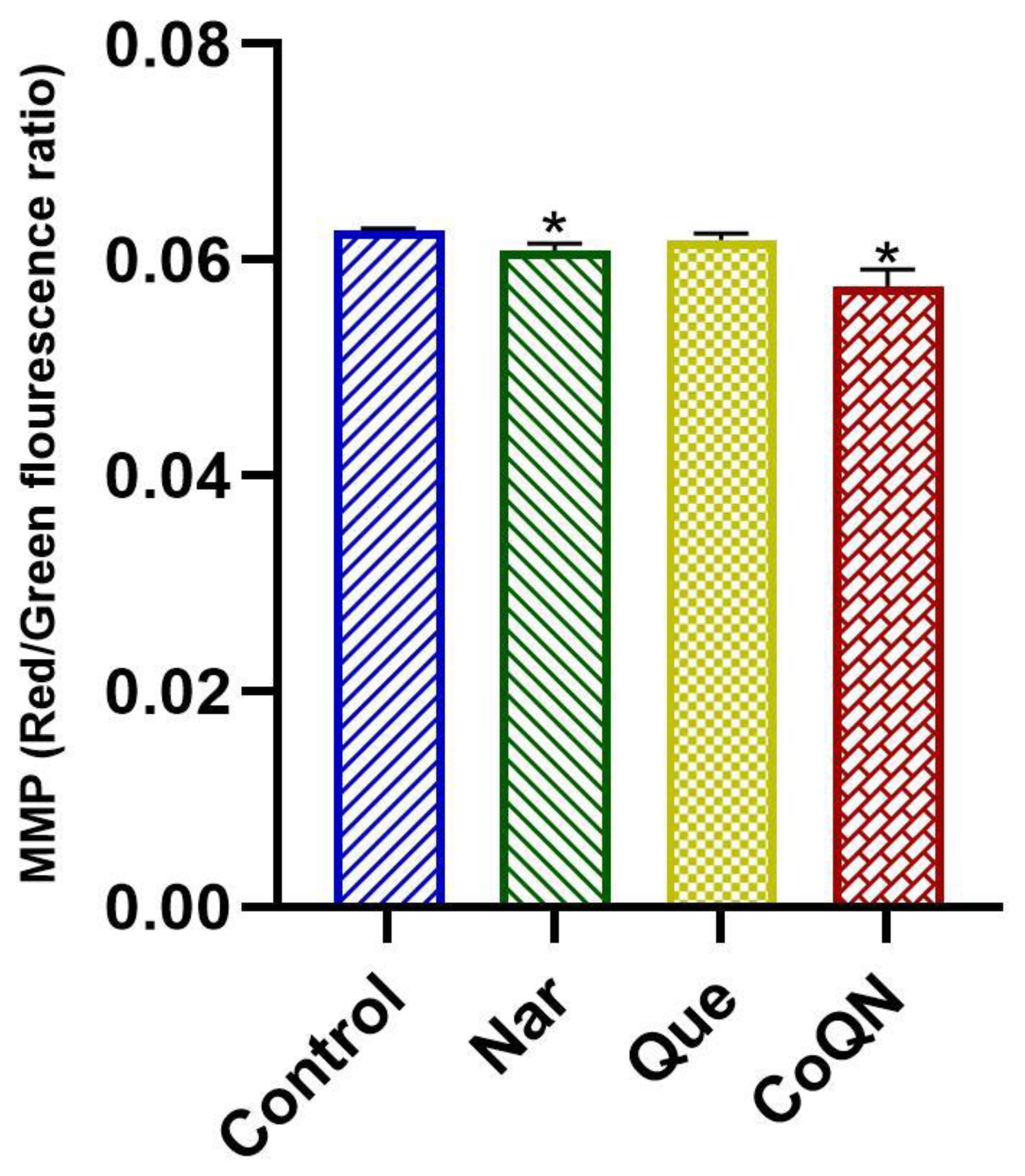
3.4. Mitochondrial Depolarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.R. The epidemiologic transition: A theory of the epidemiology of population change. Milbank Q. 2005, 83, 731–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, E.J. Overview of breast cancer. J. Am. Acad. Physician Assist. 2019, 32, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [Green Version]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anticancer properties of quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented anticancer activity of naringenin-loaded TPGS polymeric nanosuspension for drug resistive MCF-7 human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Gawande, S.; Kotwal, S. Cancer phytotherapeutics: Role for flavonoids at the cellular level. Phytother. Res. 2008, 22, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; King, J.L.; Harmston, M.; Lila, M.A.; Erdman, J.W., Jr. Synergistic effects of flavonoids on cell proliferation in Hepa-1c1c7 and LNCaP cancer cell lines. J. Food Sci. 2006, 71, S358–S363. [Google Scholar] [CrossRef]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Ackland, M.L.; van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. Vivo 2005, 19, 69–76. [Google Scholar]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [Green Version]
- Bruckheimer, E.M.; Cho, S.H.; Sarkiss, M.; Herrmann, J.; McDonnell, T.J. The Bcl-2 gene family and apoptosis. Adv. Biochem. Eng. Biotechnol. 1998, 62, 75–105. [Google Scholar] [CrossRef]
- Makin, G.; Hickman, J.A. Apoptosis and cancer chemotherapy. Cell Tissue Res. 2000, 301, 143–152. [Google Scholar] [CrossRef]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Um, H.D. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 2016, 7, 5193–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Knezevic, C.E.; Clarke, W. Cancer Chemotherapy: The Case for Therapeutic Drug Monitoring. Ther. Drug Monit. 2020, 42, 6–19. [Google Scholar] [CrossRef]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Med. J. 2017, 118, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Aghapour, F.; Moghadamnia, A.A.; Nicolini, A.; Kani, S.N.M.; Barari, L.; Morakabati, P.; Rezazadeh, L.; Kazemi, S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018, 500, 860–865. [Google Scholar] [CrossRef]
- Helle, J.; Kräker, K.; Bader, M.I.; Keiler, A.M.; Zierau, O.; Vollmer, G.; Welsh, J.; Kretzschmar, G. Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol. Cell. Endocrinol. 2014, 392, 125–135. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koyuncu, I.; Dikilitas, M.; Bahadori, F.; Turkkan, B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. et Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, F.; Guo, H.-B.; Li, Y.; Tan, B.-B.; Zhang, W.-X.; Peng, Y.-H. naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef]
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharmacal Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Mutlu Altundag, E.; Kasacı, T.; Yılmaz, A.M.; Karademir, B.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Quercetin-Induced Cell Death in Human Papillary Thyroid Cancer (B-CPAP) Cells. J. Thyroid Res. 2016, 2016, 9843675. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, M.K.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.L.; Yu, C.R.; Lien, H.M.; Sheu, G.T.; Cherng, S.H. Cytotoxicity of naringenin induces Bax-mediated mitochondrial apoptosis in human lung adenocarcinoma A549 cells. Environ. Toxicol. 2020, 35, 1386–1394. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-induced Apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-Y.; Park, C.; Lee, J.-H.; Chung, K.T.; Kwon, T.K.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H. Naringenin-induced apoptosis is attenuated by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human leukemia U937 cells. Toxicol. Vitr. 2009, 23, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Rezaei Tavirani, M.; Deravi, N.; Mahboobi Rabbani, M.I.; Zarghi, A. Naringenin enhances the anti-cancer effect of cyclophosphamide against MDA-MB-231 breast cancer cells via targeting the STAT3 signaling Ppathway. Iran. J. Pharm. Res. 2020, 19, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, S.; Becer, E.; Kabadayı, H.; Vatansever, H.S.; Yücecan, S. Quercetin-mediated apoptosis and cellular senescence in human colon cancer. Anti-Cancer Agents Med. Chem. 2020, 20, 1387–1396. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Dharmarajan, A.; Arunakaran, J. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: A possible mediation by IGFBP-3. Oncol. Res. 2006, 16, 67–74. [Google Scholar] [CrossRef]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep. 2012, 5, 1453–1456. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and Bbenefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin suppresses growth of human placental choriocarcinoma via reactive oxygen species-mediated P38 and JNK MAPK pathways. Phytomedicine 2018, 50, 238–246. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Chi, C.-W.; Wang, J.-J. Reactive Oxygen Species production is involved in quercetin-induced apoptosis in human hepatoma cells. Nutr. Cancer 2006, 55, 201–209. [Google Scholar] [CrossRef]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef] [Green Version]
- Askar, M.A.; El Shawi, O.E.; Abou Zaid, O.A.R.; Mansour, N.A.; Hanafy, A.M. Breast cancer suppression by curcumin-naringenin-magnetic-nano-particles: In vitro and in vivo studies. Tumour Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef]
- Hildeman, D.A.; Mitchell, T.; Aronow, B.; Wojciechowski, S.; Kappler, J.; Marrack, P. Control of Bcl-2 expression by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2003, 100, 15035–15040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugazhenthi, S.; Nesterova, A.; Jambal, P.; Audesirk, G.; Kern, M.; Cabell, L.; Eves, E.; Rosner, M.R.; Boxer, L.M.; Reusch, J.E.-B. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J. Neurochem. 2003, 84, 982–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Maheshwari, U.; Muthukrishnan, A.; Muthuswamy, R.; Anand, K.; Ravindran, B.; Dhanaraj, P.; Balamuralikrishnan, B.; Chang, S.W.; Chung, W.J. Myricetin: Versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS-reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol. Cell Biochem. 2021, 476, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Geng, B.; Yi, Z. Pelargonidin induces antitumor effects in human osteosarcoma cells via autophagy induction, loss of mitochondrial membrane potential, G2/M cell cycle arrest and downregulation of PI3K/AKT signalling pathway. J. BUON 2018, 23, 735–740. [Google Scholar]
- Zhang, J.-Y.; Lin, M.-T.; Zhou, M.-J.; Yi, T.; Tang, Y.-N.; Tang, S.-L.; Yang, Z.-J.; Zhao, Z.-Z.; Chen, H.-B. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 Cells in vitro. Molecules 2015, 20, 11524–11534. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.A.; Ziska, D.S.; Zhao, K.; Elsohly, M.A. Variance of common flavonoids by brand of grapefruit juice. Fitoterapia 2000, 71, 154–156. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
 = Overproduction;
= Overproduction;  = Down regulation;
= Down regulation;  = Activation.
= Activation.
 = Overproduction;
= Overproduction;  = Down regulation;
= Down regulation;  = Activation.
= Activation.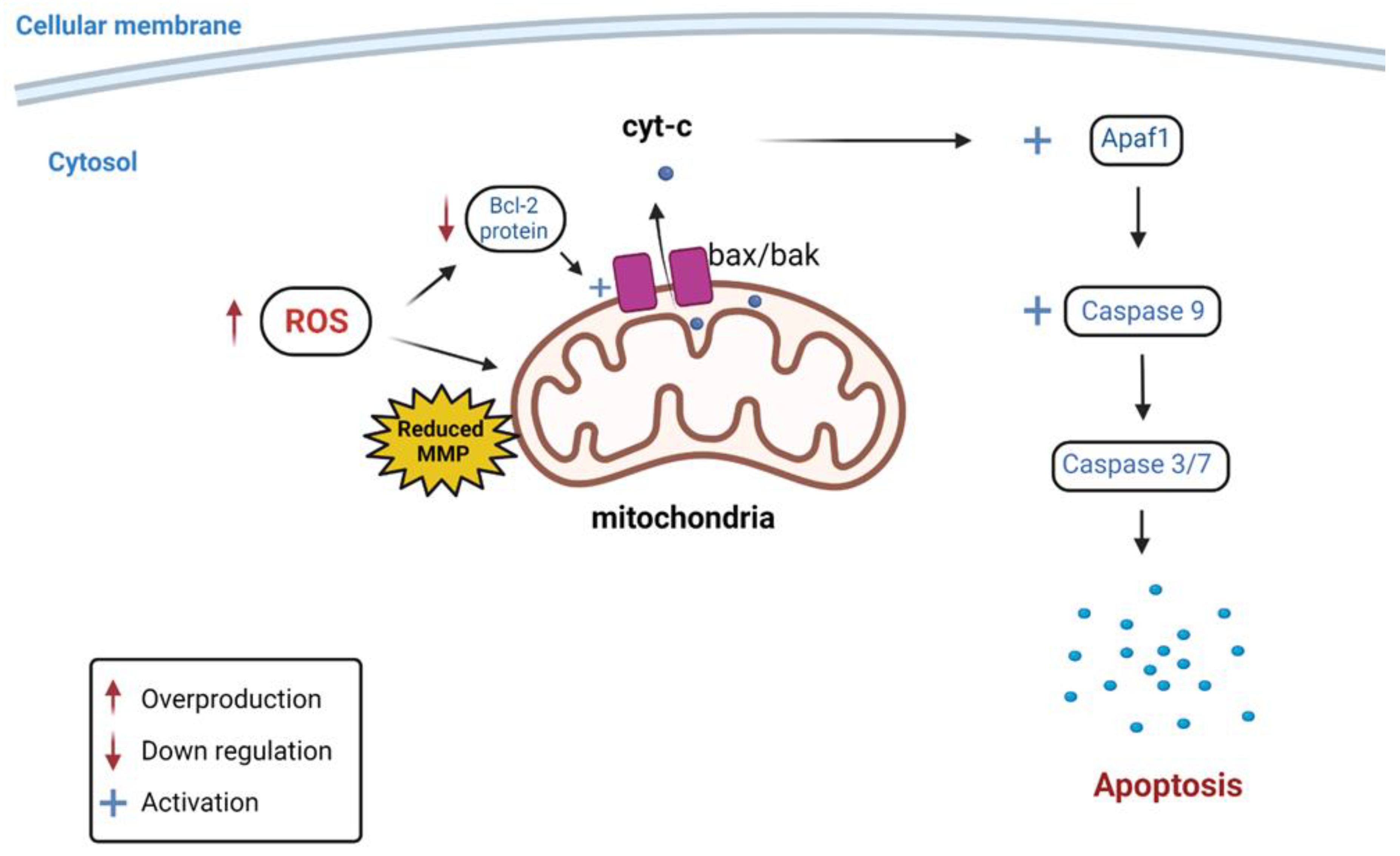
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhman, M.A.; Devnarain, N.; Khan, R.; Owira, P.M.O. Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 3437. https://doi.org/10.3390/nu14163437
Rhman MA, Devnarain N, Khan R, Owira PMO. Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells. Nutrients. 2022; 14(16):3437. https://doi.org/10.3390/nu14163437
Chicago/Turabian StyleRhman, Mahasin Abdel, Nikita Devnarain, Rene Khan, and Peter M. O. Owira. 2022. "Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells" Nutrients 14, no. 16: 3437. https://doi.org/10.3390/nu14163437
APA StyleRhman, M. A., Devnarain, N., Khan, R., & Owira, P. M. O. (2022). Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells. Nutrients, 14(16), 3437. https://doi.org/10.3390/nu14163437





