Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study
Abstract
:1. Introduction
- Pharmaceutical companies might fund studies with weaker comparators.
- Industry may have conducted low-quality research.
- Higher doses of the drug may be administered to subjects.
- Manufacturers tend to prevent the publication of studies unfavorable to their products.
- Sponsored research is more likely to appear in symposiums and use publication platforms with a lack of peer review.
2. Methods
2.1. Eligible Meta-Analyses
- Existence of both trials sponsored by industry and trials by non-industrial institutions;
- At least one qualitative continuous outcome related to oral health;
- At least three trials included in the meta-analysis.
- Inaccessible trials included;
- Studies other than randomized control trials included.
2.2. Data Extraction and Assessment of Sponsorship Status
- Trials reporting that they did not receive sponsorship or only received support from universities or other academic institutions were judged as low-risk.
- Trials reporting that they received sponsorship from industry and academic institutions and declaring the sponsor was not involved in the trial conduct, data management/analysis, or co-authorship were judged as low-risk.
- Trials reporting that they received sponsorship from industry and academic institutions without declaring the sponsor was not involved in the trial conduct, data management/analysis, or co-authorship were judged as high-risk.
- Trials reporting that they received sponsorship from the industry and declaring that the sponsor was not involved in the trial conduct, data management/analysis, or co-authorship were judged as low-risk.
- Trials reporting that they received sponsorship from the industry without declaring that the sponsor was not involved in the trial conduct, data management/analysis, or co-authorship were considered high-risk.
3. Data Synthesis and Analysis
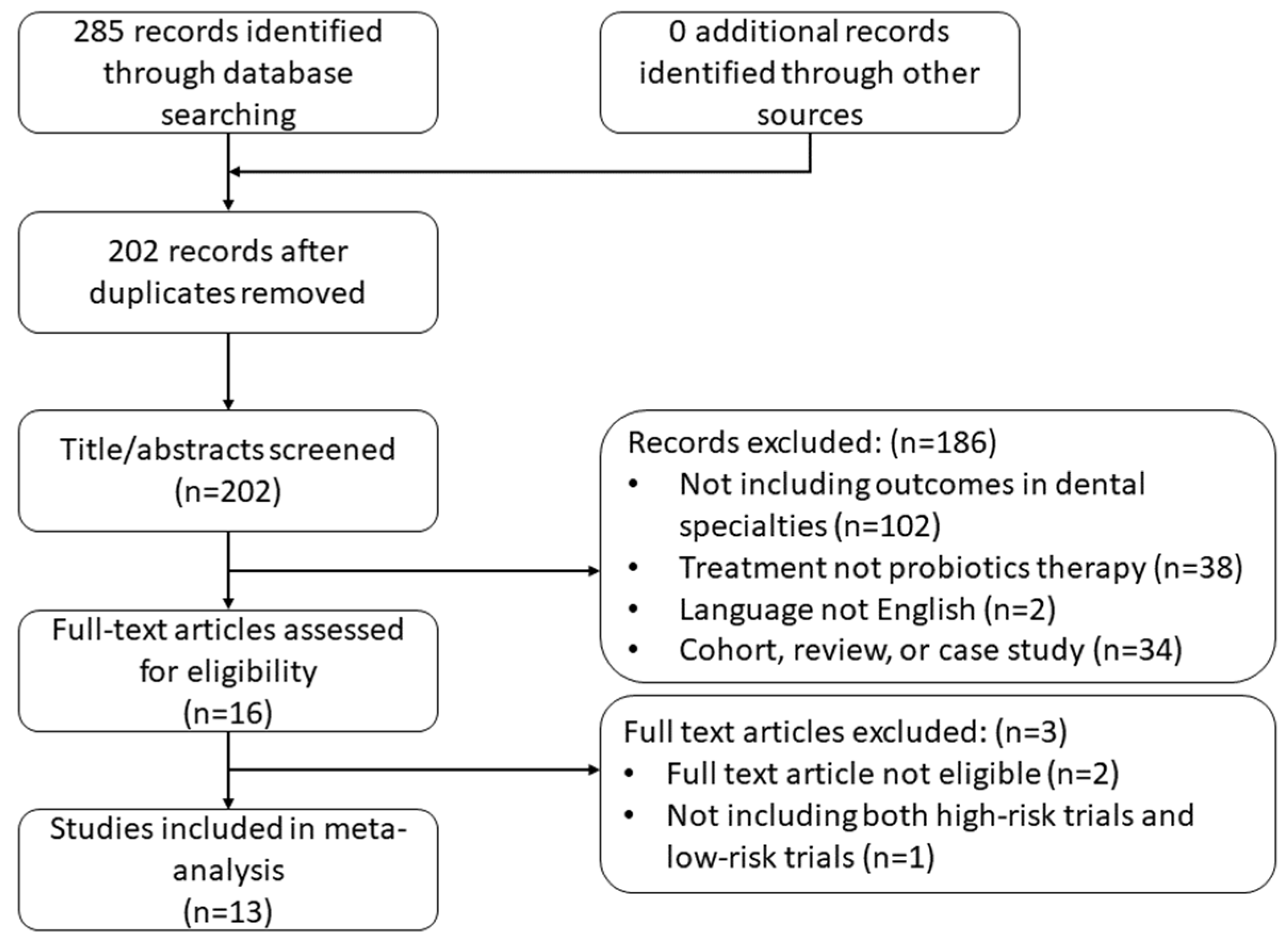
4. Results
4.1. Eligible Meta-Analyses for Continuous Outcomes
| Condition | Experimental Intervention | ControlIntervention | Outcome | Trials in High Risk, N | Trials in Low Risk, N | All Trials, N | |
| Cheng 2020 [32] | Recurrent aphthous stomatitis | Treatment with probiotics, either alone or combined with other drugs | Treatment with placebo or other drugs alone | Visual Analogue Pain Scale | 2 | 1 | 3 |
| Donos 2020 [33] | Periodontitis | Probiotics | Placebo | Periodontal probing depth reduction | 1 | 4 | 5 |
| Gao 2020 [34] | Peri-implant diseases | Lactobacillus agent | Placebo agent or blank control | Periodontal probing depth reduction | 3 | 1 | 4 |
| Gheisary 2022 [35] | Periodontal diseases/health | Probiotics in any form | Without probiotics, with a placebo, or with antibiotics | Plaque index | 6 | 10 | 16 |
| Hao 2021 [36] | Caries | Products containing Bifidobacterium | Products without Bifidobacterium | Streptococcus mutans counts | 3 | 1 | 4 |
| Hu 2021 [37] | Periodontitis | Scaling and root planning + probiotics | Scaling and root planning | Periodontal probing depth reduction | 4 | 4 | 8 |
| Ikram 2018 [38] | Periodontitis | Scaling and root planning + probiotics | Scaling and root planning alone or with a placebo | Periodontal probing depth reduction | 1 | 2 | 3 |
| Martin-Cabezas 2016 [39] | Periodontitis | Scaling and root planning + probiotics | Scaling and root planning alone or with a placebo | Periodontal probing depth reduction | 1 | 2 | 3 |
| Mishra 2021 [40] | Periodontitis | Scaling and root planning + probiotics | Scaling and root planning + placebo | Periodontal probing depth reduction | 1 | 2 | 3 |
| Nadelman 2018 [41] | Oral health establishment | Consumption of probiotic-containing dairy products | Consumption of dairy products without probiotics, other interventions/products, or no intervention | Streptococcus mutans counts | 5 | 4 | 9 |
| Sang-Ngoen 2021 [42] | Caries | Orally administered probiotics | Placebo or no orally administered probiotics | Aggregatibacter actinomycetemcomitans counts | 1 | 2 | 3 |
| Yoo 2019 [43] | Halitosis | Probiotics | Placebo | Volatile sulfur compounds and organoleptic scores | 1 | 2 | 3 |
| Zhao 2020 [44] | Peri-implant mucositis | Mechanical debridement + probiotics | Mechanical debridement + placebo or alone | Periodontal probing depth reduction | 2 | 2 | 4 |
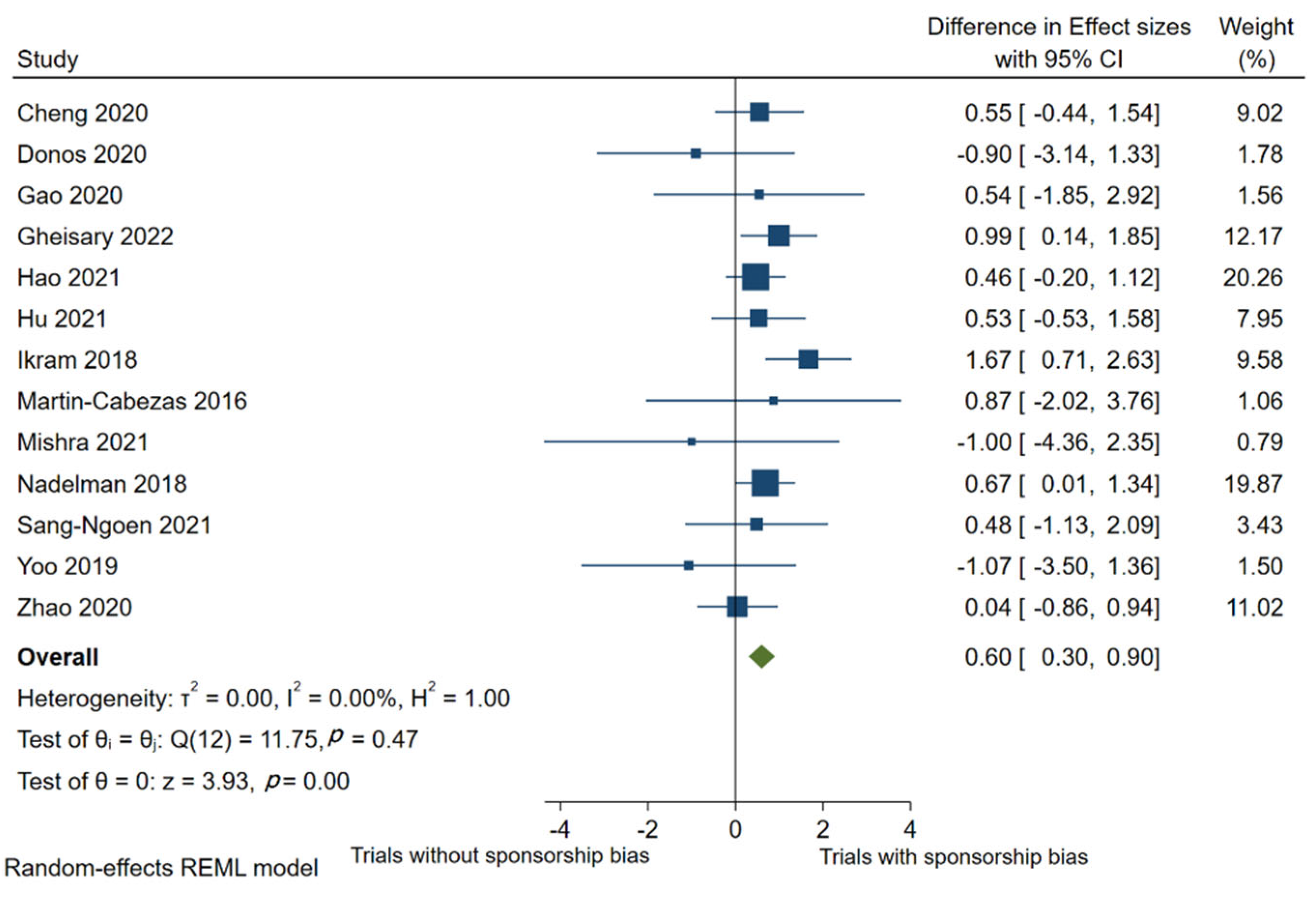
4.2. Estimates of Treatment Effect Differences between High-Risk and Low-Risk Trials
4.3. Reporting Quality Comparison
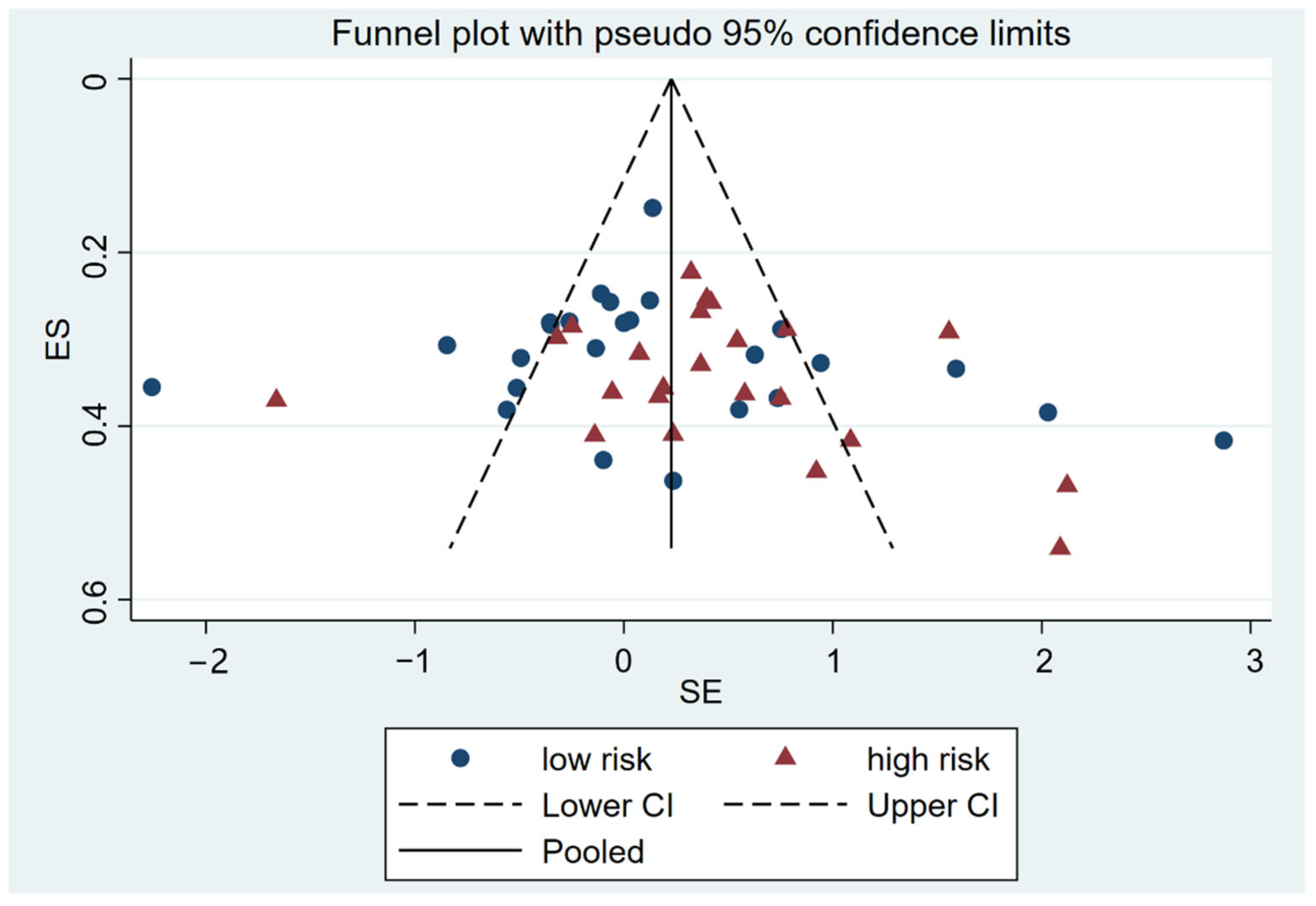
4.4. Risk of Publication Bias
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed]
- Sawas, T.; Al Halabi, S.; Hernaez, R.; Carey, W.D.; Cho, W.K. Patients Receiving Prebiotics and Probiotics Before Liver Transplantation Develop Fewer Infections Than Controls: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1567–1574 e3. [Google Scholar] [CrossRef]
- Bo, L.; Li, J.; Tao, T.; Bai, Y.; Ye, X.; Hotchkiss, R.S.; Kollef, M.H.; Crooks, N.H.; Deng, X. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD009066. [Google Scholar] [CrossRef]
- Hao, Q.; Lu, Z.; Dong, B.R.; Huang, C.Q.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2011, 2, CD006895. [Google Scholar]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, L.; Xi, Y.; Li, Y.; Xie, X.; Fan, C.; Yang, L.; Yang, S.; Chen, X.; Zhang, J.; et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 1670–1688. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Lv, T.; Ye, M.; Luo, F.; Hu, B.; Wang, A.; Chen, J.; Yan, J.; He, Z.; Chen, F.; Qian, C.; et al. Probiotics treatment improves cognitive impairment in patients and animals: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 159–172. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J.; et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Zuccotti, G.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Verma, P. Probiotics Market by Ingredient (Bacteria, and Yeast), Function (Regular, Preventative Healthcare, and Therapeutic), Application (Food & Beverages, Dietary Supplements, and Animal Feed), and End Use (Human Probiotics, and Animal Probiotics)-Global Opportunity Analysis and Industry Forecast, 2014–2022. Retrieved April 2016, 30, 2020. [Google Scholar]
- Ho, S.N.; Acharya, A.; Sidharthan, S.; Li, K.Y.; Leung, W.K.; Mcgrath, C.; Pelekos, G. A Systematic Review and Meta-Analysis of Clinical, Immunological, and Microbiological Shift in Periodontitis after Nonsurgical Periodontal Therapy with Adjunctive Use of Probiotics. J. Evid.-Based Dent. Pract. 2020, 20, 101397. [Google Scholar] [CrossRef]
- Pelekos, G.; Ho, S.N.; Acharya, A.; Leung, W.K.; McGrath, C. A double-blind, paralleled-arm, placebo-controlled and randomized clinical trial of the effectiveness of probiotics as an adjunct in periodontal care. J. Clin. Periodontol. 2019, 46, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Gruner, D.; Paris, S.; Schwendicke, F. Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J. Dent. 2016, 48, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Laleman, I.; Detailleur, V.; Slot, D.E.; Slomka, V.; Quirynen, M.; Teughels, W. Probiotics reduce mutans streptococci counts in humans: A systematic review and meta-analysis. Clin. Oral Investig. 2014, 18, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Macedo de Sousa, B.; Rodríguez, C.; Suárez, A.; Aragoneses, J.M. Role of Probiotics in Halitosis of Oral Origin: A Systematic Review and Meta-Analysis of Randomized Clinical Studies. Front. Nutr. 2021, 8, 787908. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants Methodological Consultants; Merete Aass, A.; et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Lundh, A.; Lexchin, J.; Mintzes, B.; Schroll, J.B.; Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst. Rev. 2017, 2, MR000033. [Google Scholar] [CrossRef]
- Lexchin, J.; Bero, L.A.; Djulbegovic, B.; Clark, O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ 2003, 326, 1167–1170. [Google Scholar] [CrossRef]
- Schwendicke, F.; Tu, Y.K.; Blunck, U.; Paris, S.; Gostemeyer, G. Effect of Industry Sponsorship on Dental Restorative Trials. J. Dent. Res. 2016, 95, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.B.F.; Agostini, B.A.; de Moraes, R.R.; Schwendicke, F.; Sarkis-Onofre, R. Industry sponsorship bias in clinical trials in implant dentistry: Systematic review and meta-regression. J. Clin. Periodontol. 2019, 46, 510–519. [Google Scholar] [CrossRef]
- Saltaji, H.; Armijo-Olivo, S.; Cummings, G.G.; Amin, M.; Major, P.W.; da Costa, B.R.; Flores-Mir, C. Influence of Sponsorship Bias on Treatment Effect Size Estimates in Randomized Trials of Oral Health Interventions: A Meta-epidemiological Study. J. Evid.-Based Dent. Pract. 2021, 21, 101544. [Google Scholar] [CrossRef]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D.; et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]
- Als-Nielsen, B.; Chen, W.; Gluud, C.; Kjaergard, L.L. Association of funding and conclusions in randomized drug trials: A reflection of treatment effect or adverse events? JAMA 2003, 290, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Pustejovsky, J.E.; Shadish, W.R. A standardized mean difference effect size for single case designs. Res. Synth. Methods 2012, 3, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Bafeta, A.; Dechartres, A.; Trinquart, L.; Yavchitz, A.; Boutron, I.; Ravaud, P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: Meta-epidemiological study. BMJ 2012, 344, e813. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Zeng, X.Y.; Liu, S.Y.; Zou, J.; Wang, Y. The efficacy of probiotics in management of recurrent aphthous stomatitis: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 21181. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 199–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.X.; Yu, S.C.; Zhu, X.F.; Yan, Y.Z.; Zhang, Y.C.; Pei, D.D. Does probiotic Lactobacillus have an adjunctive effect in the nonsurgical treatment of peri-implant diseases? A systematic review and meta-analysis. J. Evid.-Based Dent. Pract. 2020, 20, 101398. [Google Scholar] [CrossRef]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.R.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef]
- Hao, S.Y.; Wang, J.H.; Wang, Y. Effectiveness and safety of Bifidobacterium in preventing dental caries: A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 613–622. [Google Scholar] [CrossRef]
- Hu, D.Y.; Zhong, T.; Dai, Q. Clinical Efficacy of Probiotics as an Adjunctive Therapy to Scaling and Root Planning in the Management of Periodontitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trails. J. Evid.-Based Dent. Pract. 2021, 21, 101547. [Google Scholar] [CrossRef]
- Ikram, S.; Hassan, N.; Raffat, M.A.; Mirza, S.; Akram, Z. Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Investig. Clin. Dent. 2018, 9, e12338. [Google Scholar] [CrossRef]
- Martin-Cabezas, R.; Davideau, J.L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef]
- Mishra, S.; Misra, S.R.; Panda, S.; Mohanty, N.; Manfredi, B.; Parrini, M.; Giacomello, M.S.; Mortellaro, C.; Lucchina, A.G.; Annunziata, M.; et al. Role of probiotics in adjunct to non-surgical periodontal therapy in patients with chronic periodontitis: A systematic review and meta-analysis. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. 1), 67–78. [Google Scholar]
- Nadelman, P.; Magno, M.B.; Masterson, D.; da Cruz, A.G.; Maia, L.C. Are dairy products containing probiotics beneficial for oral health? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2763–2785. [Google Scholar] [CrossRef] [PubMed]
- Sang-Ngoen, T.; Czumbel, L.M.; Sadaeng, W.; Mikó, A.; Németh, D.I.; Mátrai, P.; Hegyi, P.; Tóth, B.; Csupor, D.; Kiss, I.; et al. Orally Administered Probiotics Decrease Aggregatibacter actinomycetemcomitans but Not Other Periodontal Pathogenic Bacteria Counts in the Oral Cavity: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 682656. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Shin, I.S.; Jeon, J.G.; Yang, Y.M.; Kim, J.G.; Lee, D.W. The Effect of Probiotics on Halitosis: A Systematic Review and Meta-analysis. Probiotics Antimicro. Proteins 2019, 11, 150–157. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, H.M.; Wang, Y.; Lai, W.L.; Jian, F. Efficacy of Probiotics as Adjunctive Therapy to Nonsurgical Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 11, 541752. [Google Scholar] [CrossRef]
- Tsagris, M.; Fragkos, K.C. Umbrella reviews, overviews of reviews, and meta-epidemiologic studies: Similarities and differences. In Umbrella Reviews; Springer: Cham, Switzerland, 2016; pp. 43–54. [Google Scholar]
- López-López, J.A.; Rubio-Aparicio, M.; Sánchez-Meca, J. Overviews of Reviews: Concept and Development. Psicothema 2022, 34, 175–181. [Google Scholar]
- Cristea, I.A.; Gentili, C.; Pietrini, P.; Cuijpers, P. Sponsorship bias in the comparative efficacy of psychotherapy and pharmacotherapy for adult depression: Meta-analysis. Br. J. Psychiatry 2017, 210, 16–23. [Google Scholar] [CrossRef] [PubMed]
| Risk of Sponsorship Bias | p-Value | ||
|---|---|---|---|
| Low | High | ||
| Randomization | 0.01 * | ||
| Adequate | 18 (69.2%) | 8 (31.8%) | |
| Inadequate/unclear | 7 (30.8%) | 15 (68.2%) | |
| Allocation concealment | 0.414 | ||
| Adequate | 18 (72%) | 14 (60.9%) | |
| Inadequate/unclear | 7 (28%) | 9 (30.1%) | |
| Blinding of participants | 0.02 * | ||
| Adequate | 23 (65.7%) | 12 (52.2%) | |
| Inadequate/unclear | 2 (15.4%) | 11 (47.8%) | |
| Blinding of outcome assessment | |||
| Adequate | 21 (92%) | 21 (91.3%) | 0.445 |
| Inadequate/unclear | 4 (8%) | 2 (8.7%) | |
| Incomplete outcome data | 0.331 | ||
| Adequate | 19 (76%) | 20 (87%) | |
| Inadequate/unclear | 6 (24%) | 3 (13%) | |
| Selective reporting | 0.606 | ||
| Adequate | 17 (68%) | 14 (60.9%) | |
| Inadequate/unclear | 8 (32%) | 9 (39.1%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Acharya, A.; Leung, W.K.; Pelekos, G. Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study. Nutrients 2022, 14, 3409. https://doi.org/10.3390/nu14163409
Hu Q, Acharya A, Leung WK, Pelekos G. Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study. Nutrients. 2022; 14(16):3409. https://doi.org/10.3390/nu14163409
Chicago/Turabian StyleHu, Qin, Aneesha Acharya, Wai Keung Leung, and George Pelekos. 2022. "Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study" Nutrients 14, no. 16: 3409. https://doi.org/10.3390/nu14163409
APA StyleHu, Q., Acharya, A., Leung, W. K., & Pelekos, G. (2022). Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study. Nutrients, 14(16), 3409. https://doi.org/10.3390/nu14163409







