Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Measurement and Sample Collection
2.3. Liquid Chromatography–Mass Spectrometry (LC–MS)
2.4. Metabolites Analysis
2.5. Statistical Analysis
3. Results
3.1. Metabolic Features in All Participants
3.2. Correlation between Metabolites and Clinical Parameters
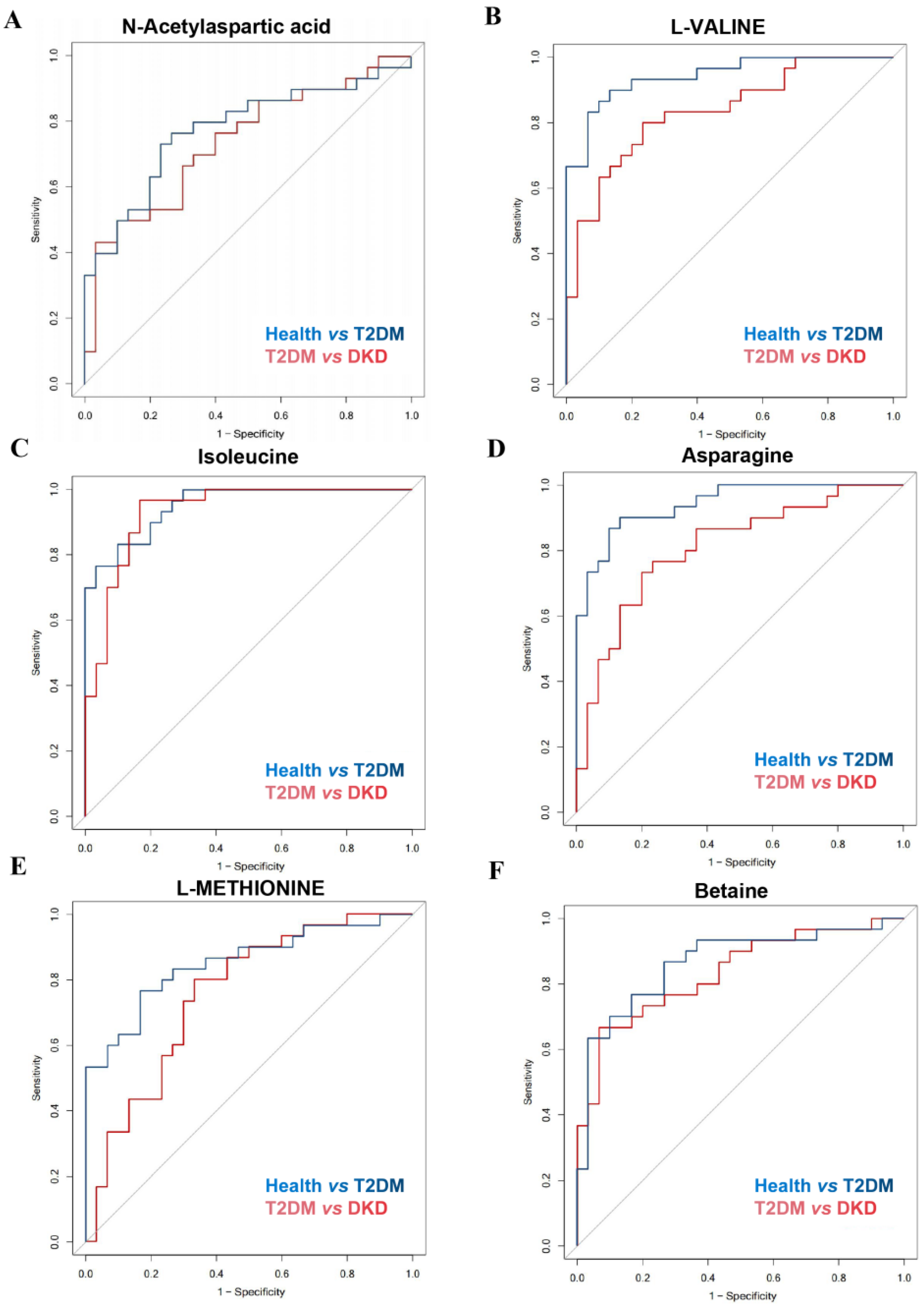
3.3. Validation of the Potential Biomarkers
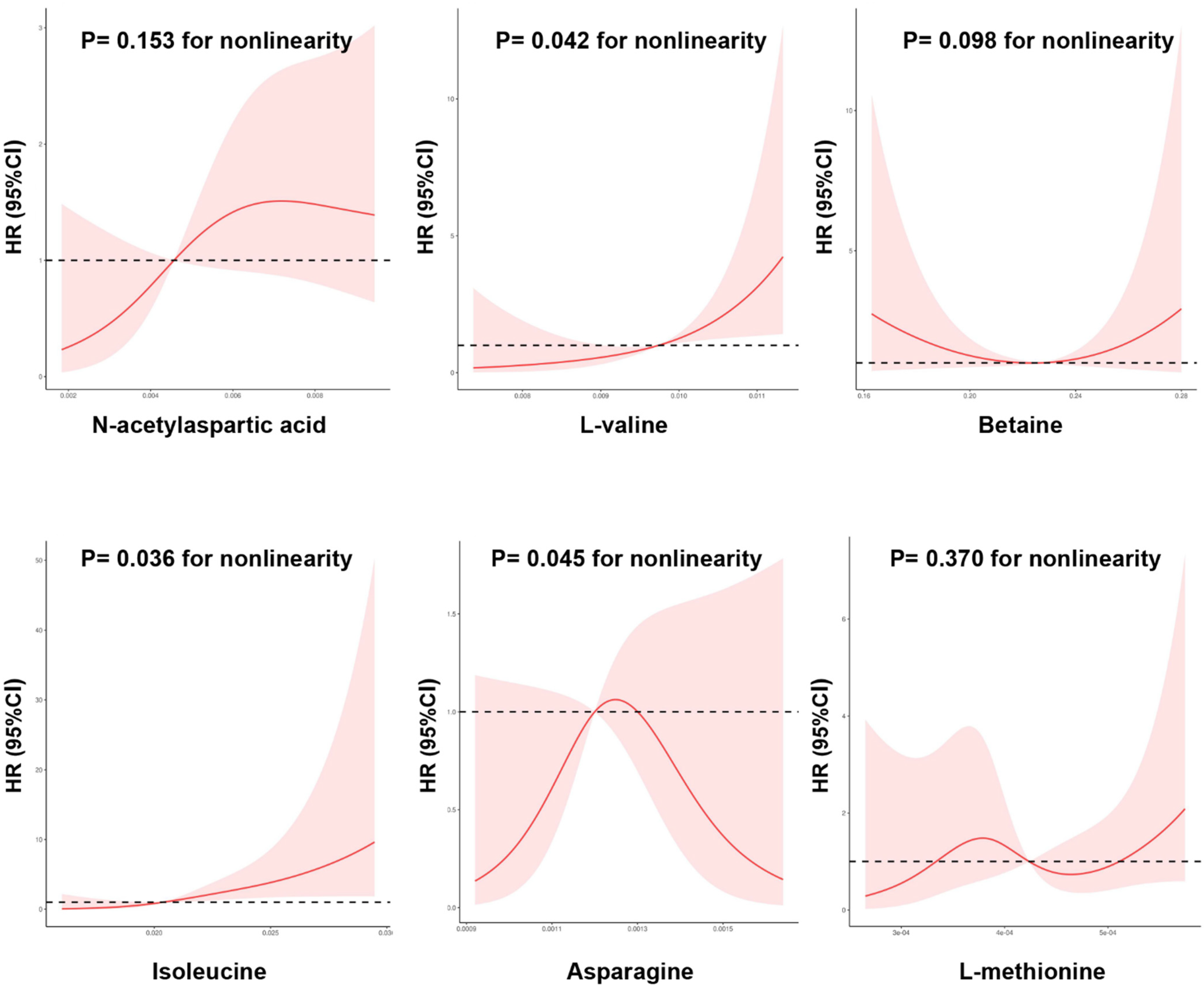
3.4. Correlation of Metabolites with Diabetes Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.-Z.; Gerszten, R.E. Metabolomics and Proteomics in Type 2 Diabetes. Circ. Res. 2020, 126, 1613–1627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, H.-Y.; Fan, Z.-Y.; He, Y.; Yan, Y.-X. Metabolomics Signatures in Type 2 Diabetes: A Systematic Review and Integrative Analysis. J. Clin. Endocrinol. Metab. 2020, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Peddinti, G.; Cobb, J.; Yengo, L.; Froguel, P.; Kravić, J.; Balkau, B.; Tuomi, T.; Aittokallio, T.; Groop, L. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia 2017, 60, 1740–1750. [Google Scholar] [CrossRef]
- Kwan, B.; Fuhrer, T.; Zhang, J.; Darshi, M.; Van Espen, B.; Montemayor, D.; de Boer, I.H.; Dobre, M.; Hsu, C.-Y.; Kelly, T.N.; et al. Metabolomic Markers of Kidney Function Decline in Patients with Diabetes: Evidence from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 76, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Lu, H.; Yang, M.; Liu, Y.; Yin, P.; Li, G.; Wang, Y.; Chen, L.; Chen, Q.; Zhao, C.; et al. Serum and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann. Transl. Med. 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-S.; Kim, J.H.; Jeong, H.; Yu, J.; Yeom, J.; Song, S.H.; Kim, S.S.; Kim, I.J.; Kim, K. Differential Urinary Proteome Analysis for Predicting Prognosis in Type 2 Diabetes Patients with and without Renal Dysfunction. Int. J. Mol. Sci. 2020, 21, 4236. [Google Scholar] [CrossRef]
- Hirayama, A.; Nakashima, E.; Sugimoto, M.; Akiyama, S.-I.; Sato, W.; Maruyama, S.; Matsuo, S.; Tomita, M.; Yuzawa, Y.; Soga, T. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal. Bioanal. Chem. 2012, 404, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Liao, W.-L.; Chang, C.-T.; Lin, Y.-N.; Tsai, F.-J. Identification of Urinary Metabolite Biomarkers of Type 2 Diabetes Nephropathy Using an Untargeted Metabolomic Approach. J. Proteome Res. 2018, 17, 3997–4007. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.J.G.; Sabrkhany, S.; Hundscheid, I.; Schellekens, D.; Lenaerts, K.; Olde Damink, S.W.; Blaak, E.E.; Dejong, C.H.C.; Rensen, S.S. Human splanchnic amino-acid metabolism. Amino Acids 2017, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Fairweather, S.J. Amino Acid Transport across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nakamura, K.; Wada, K.; Tsuji, M.; Tamai, Y.; Kawachi, T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: The Takayama study. Am. J. Epidemiol. 2013, 178, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.a.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.B.; et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-H.; Feng, X.-F.; Yang, X.-L.; Hou, R.-Q.; Fang, Z.-Z. Interactive effects of asparagine and aspartate homeostasis with sex and age for the risk of type 2 diabetes risk. Biol. Sex Differ. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Yu, B.; Zheng, Z.; Chang, P.; Tin, A.; Köttgen, A.; Wagenknecht, L.E.; Coresh, J.; Boerwinkle, E.; Selvin, E. Serum metabolomic profile of incident diabetes. Diabetologia 2018, 61, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Li, Y.; Zhang, L.; Dong, M. A novel hippocampus metabolite signature in diabetes mellitus rat model of diabetic encephalopathy. Metab. Brain Dis. 2020, 35, 895–904. [Google Scholar] [CrossRef]
- Zyśk, M.; Pikul, P.; Kowalski, R.; Lewandowski, K.; Sakowicz-Burkiewicz, M.; Pawełczyk, T. Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the -Acetylaspartate Network in Wistar Rat Brain Cells. Int. J. Mol. Sci. 2020, 21, 8541. [Google Scholar] [CrossRef] [PubMed]
- Mangia, S.; Kumar, A.F.; Moheet, A.A.; Roberts, R.J.; Eberly, L.E.; Seaquist, E.R.; Tkáč, I. Neurochemical profile of patients with type 1 diabetes measured by ¹H-MRS at 4 T. J. Cereb. Blood Flow Metab. 2013, 33, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Pinney, S.E. DNA methylation and its role in the pathogenesis of diabetes. Pediatr. Diabetes 2017, 18, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Kemppainen, E.; Trošt, K.; Siljander, H.; Hyöty, H.; Ilonen, J.; Toppari, J.; Veijola, R.; Hyötyläinen, T.; Knip, M.; et al. Circulating metabolites in progression to islet autoimmunity and type 1 diabetes. Diabetologia 2019, 62, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Chen, S.; Li, Y.; Han, H.; Gao, J.; Liu, G.; Wu, X.; Li, T.; Kim, S.W.; et al. Metabolic Regulation of Methionine Restriction in Diabetes. Mol. Nutr. Food Res. 2018, 62, e1700951. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Methionine abrogates the renoprotective effect of a low-protein diet against diabetic kidney disease in obese rats with type 2 diabetes. Aging 2020, 12, 4489–4505. [Google Scholar] [CrossRef]
- Cooke, D.; Ouattara, A.; Ables, G.P. Dietary methionine restriction modulates renal response and attenuates kidney injury in mice. FASEB J. 2018, 32, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.M.; Lee, B.; Lee, E.K.; Chung, K.W.; Moon, K.M.; An, H.J.; Kim, K.M.; Yu, B.P.; Chung, H.Y. Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J. Nutr. Biochem. 2017, 45, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-Y.; Won, S.-B.; Kim, J.; Jeon, S.; Han, A.; Kwon, Y.H. Betaine Alleviates Hypertriglycemia and Tau Hyperphosphorylation in db/db Mice. Toxicol. Res. 2013, 29, 7–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Chen, Y.-M.; Wang, L.-J.; Wei, J.; Tan, Y.-Z.; Zhou, J.-Y.; Yang, Y.; Chen, Y.-M.; Ling, W.-H.; Zhu, H.-L. Higher homocysteine and lower betaine increase the risk of microangiopathy in patients with diabetes mellitus carrying the GG genotype of PEMT G774C. Diabetes Metab. Res. Rev. 2013, 29, 607–617. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.; Bason, L.M.; Hayman, C.M.; Robson, R.A.; Chambers, S.T. Abnormal glycine betaine content of the blood and urine of diabetic and renal patients. Clin. Chim. Acta 1994, 230, 69–79. [Google Scholar] [CrossRef]
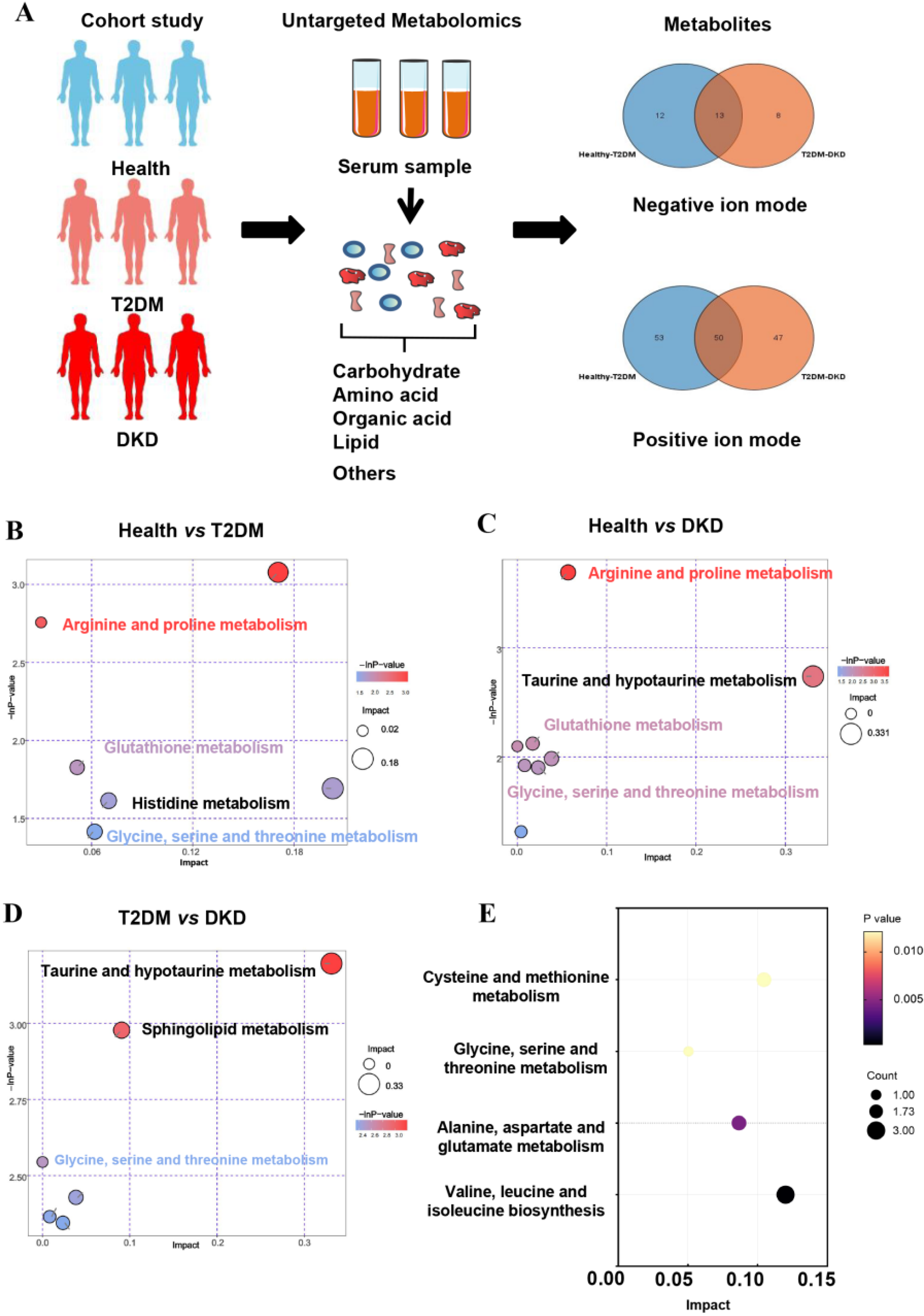


| Health (n = 30) | T2DM (n = 30) | DKD (n = 30) | p a | p b | |
|---|---|---|---|---|---|
| Age, years | 39.20 ± 10.68 | 53.30 ± 17.00 | 50.70 ± 10.36 | <0.001 | 0.478 |
| Male sex | 18 (60.00) | 15 (50.00) | 11 (36.70) | 0.436 | 0.297 |
| Duration of diabetes, years | / | 6.24 ± 5.70 | 7.08 ± 4.69 | / | 0.535 |
| Systolic blood pressure, mmHg | 119.40 ± 11.62 | 119.47 ± 21.38 | 142.87 ± 21.60 | 0.988 | <0.001 |
| Diastolic blood pressure, mmHg | 76.50 ± 6.12 | 75.53 ± 9.07 | 87.53 ± 11.82 | 0.630 | <0.001 |
| Total cholesterol, mmol/L | 4.32 (3.73, 4.96) | 3.83 (3.40, 4.55) | 5.06 (3.26, 6.41) | 0.017 | 0.012 |
| Triacylglycerol, mmol/L | 1.10 (0.81, 1.62) | 1.03 (0.72, 2.04) | 1.52 (1.06, 2.23) | 0.784 | 0.025 |
| HDL-cholesterol, mmol/L | 1.13 ± 0.36 | 0.93 (0.82, 1.31) | 1.24 ± 0.35 | 0.693 | 0.098 |
| LDL-cholesterol, mmol/L | 2.43 ± 0.60 | 1.95 (1.66, 2.68) | 2.90 ± 1.35 | 0.145 | 0.012 |
| Fasting glucose, mmol/L | 4.70 ± 0.37 | 8.29 ± 3.35 | 6.77 ± 3.15 | <0.001 | 0.081 |
| HbA1c, % | / | 10.81 ± 2.39 | 7.67 ± 2.35 | / | <0.001 |
| Creatinine, µmol/L | 66.00 (59.00, 80.25) | 59.00 (53.50, 83.00) | 107.00 (84.50, 146.75) | 0.325 | <0.001 |
| eGFR, ml/min/1.73 m2 | 104.01 ± 13.22 | 98.12 ± 21.77 | 63.23 ± 24.84 | 0.211 | <0.001 |
| Urea nitrogen, mmol/L | 4.72 (4.15, 6.20) | 5.20 (4.30, 6.40) | 7.25 (6.23, 10.08) | 0.520 | <0.001 |
| Uric acid, µmol/L | 327.83 ± 119.46 | 280.07 ± 82.59 | 390.23 ± 113.53 | 0.077 | <0.001 |
| Albumin, g/L | 47.95 (44.73, 50.15) | 42.10 (40.00, 44.60) | 30.75 (24.13, 40.03) | <0.001 | <0.001 |
| 24 h urine protein, g | / | 0.06 (0.04, 0.09) | 3.22 (1.11, 5.14) | / | <0.001 |
| eGFR | Serum Creatinine | Albuminuria | Serum Albumin | |||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value |
| N-acetylaspartic acid | −0.339 | 0.001 | 0.316 | 0.002 | 0.235 | 0.104 | −0.423 | <0.001 |
| L-valine | −0.537 | <0.001 | 0.419 | <0.001 | 0.593 | <0.001 | −0.617 | <0.001 |
| Betaine | −0.488 | <0.001 | 0.391 | <0.001 | 0.498 | <0.001 | −0.585 | <0.001 |
| Isoleucine | −0.584 | <0.001 | 0.482 | <0.001 | 0.698 | <0.001 | −0.727 | <0.001 |
| Asparagine | −0.423 | <0.001 | 0.383 | <0.001 | 0.389 | 0.006 | −0.599 | <0.001 |
| L-methionine | −0.427 | <0.001 | 0.348 | 0.001 | 0.422 | 0.003 | −0.497 | <0.001 |
| Metabolites | Pathway and Sub-Pathway | AUC (95% CI) | |
|---|---|---|---|
| Health vs. T2DM | T2DM vs. DKD | ||
| N-acetylaspartic acid | Alanine, aspartate and glutamate metabolism | 0.777 (0.655, 0.898) | 0.739 (0.612, 0.866) |
| L-valine | Valine, leucine and isoleucine degradation | 0.943 (0.889, 0.997) | 0.834 (0.733, 0.936) |
| Betaine | Glycine, serine and threonine metabolism | 0.863 (0.766, 0.960) | 0.834 (0.732, 0.937) |
| Isoleucine | Valine, leucine and isoleucine degradation | 0.951 (0.905, 0.997) | 0.932 (0.869, 0.995) |
| Asparagine | Alanine, aspartate and glutamate metabolism | 0.942 (0.889, 0.995) | 0.809 (0.698, 0.920) |
| L-methionine | Cysteine and methionine metabolism | 0.852 (0.754, 0.950) | 0.753 (0.628, 0.878) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Bai, M.; Xie, X.; Wang, J.; Weng, C.; Dai, H.; Chen, J.; Han, F.; Lin, W. Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3345. https://doi.org/10.3390/nu14163345
Zhu H, Bai M, Xie X, Wang J, Weng C, Dai H, Chen J, Han F, Lin W. Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients. 2022; 14(16):3345. https://doi.org/10.3390/nu14163345
Chicago/Turabian StyleZhu, Huanhuan, Mengqiu Bai, Xishao Xie, Junni Wang, Chunhua Weng, Huifen Dai, Jianghua Chen, Fei Han, and Weiqiang Lin. 2022. "Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus" Nutrients 14, no. 16: 3345. https://doi.org/10.3390/nu14163345
APA StyleZhu, H., Bai, M., Xie, X., Wang, J., Weng, C., Dai, H., Chen, J., Han, F., & Lin, W. (2022). Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients, 14(16), 3345. https://doi.org/10.3390/nu14163345






