The Effect of a Combination of Eucommia ulmoides and Achyranthes japonica on Alleviation of Testosterone Deficiency in Aged Rat Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Liquid Chromatography
2.3. Experimental Animals
2.4. Serum Testosterone Concentration Assay
2.5. SHBG Levels in Serum
2.6. Serum Estradiol Levels Measurement
2.7. DNA Synthesis and Quantitative Real-Time PCR
2.8. ALT, AST Analysis in the Serum
2.9. Biochemical Analysis
2.10. Sperm Count Measurement
2.11. Swimming Retention Time of Experimental Animals
2.12. PSA Levels in Serum
2.13. Statistical Analysis
3. Results
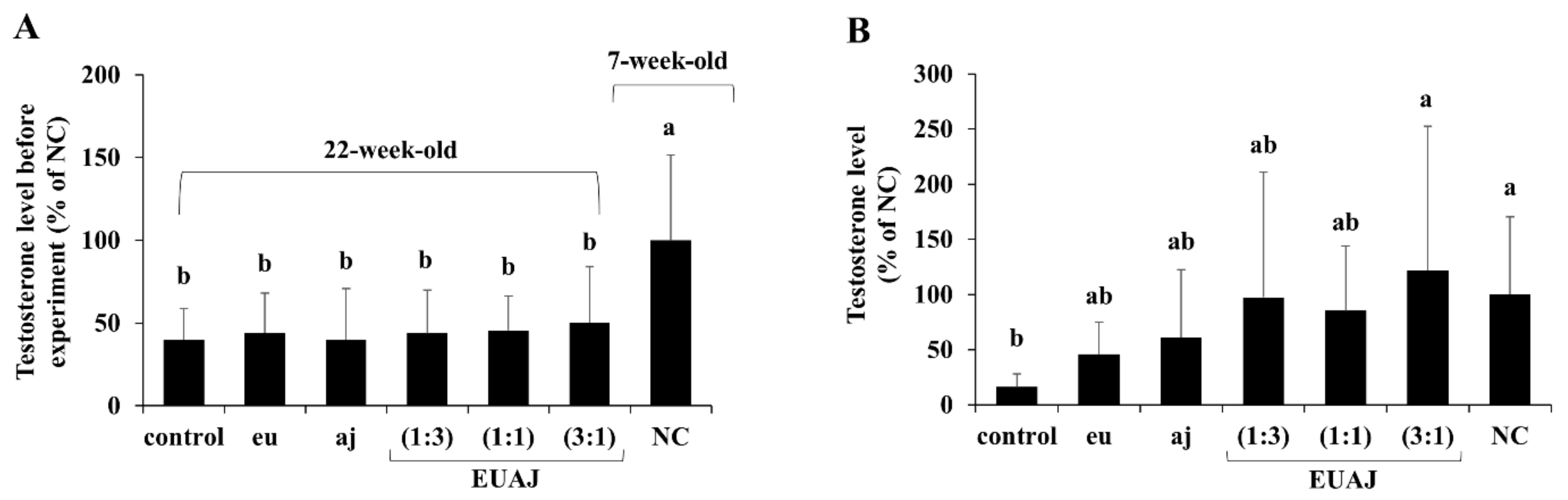
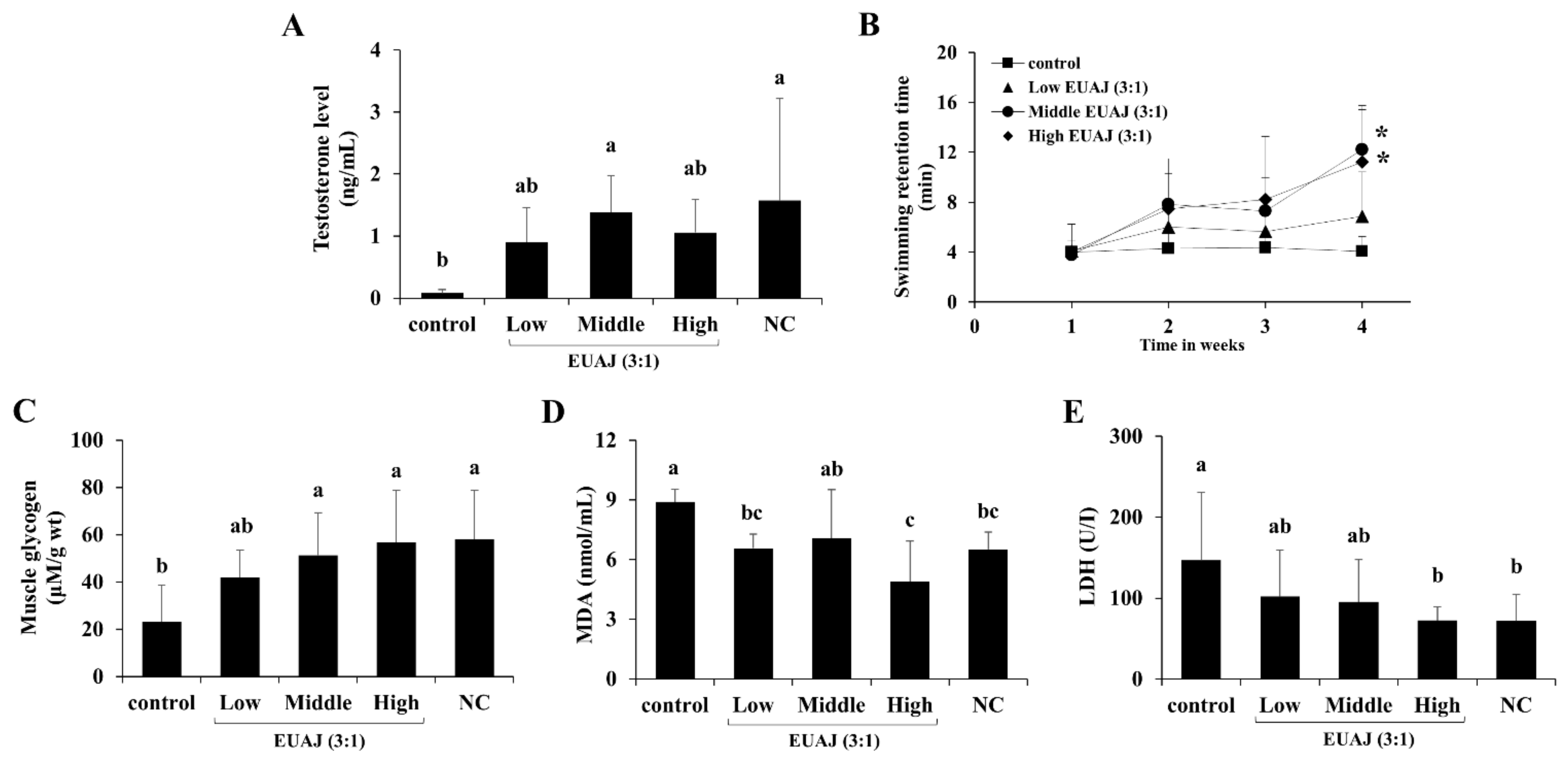
3.1. Effects of EUAJ (3:1) on Testosterone Concentration in Serum of Aged SD Rats
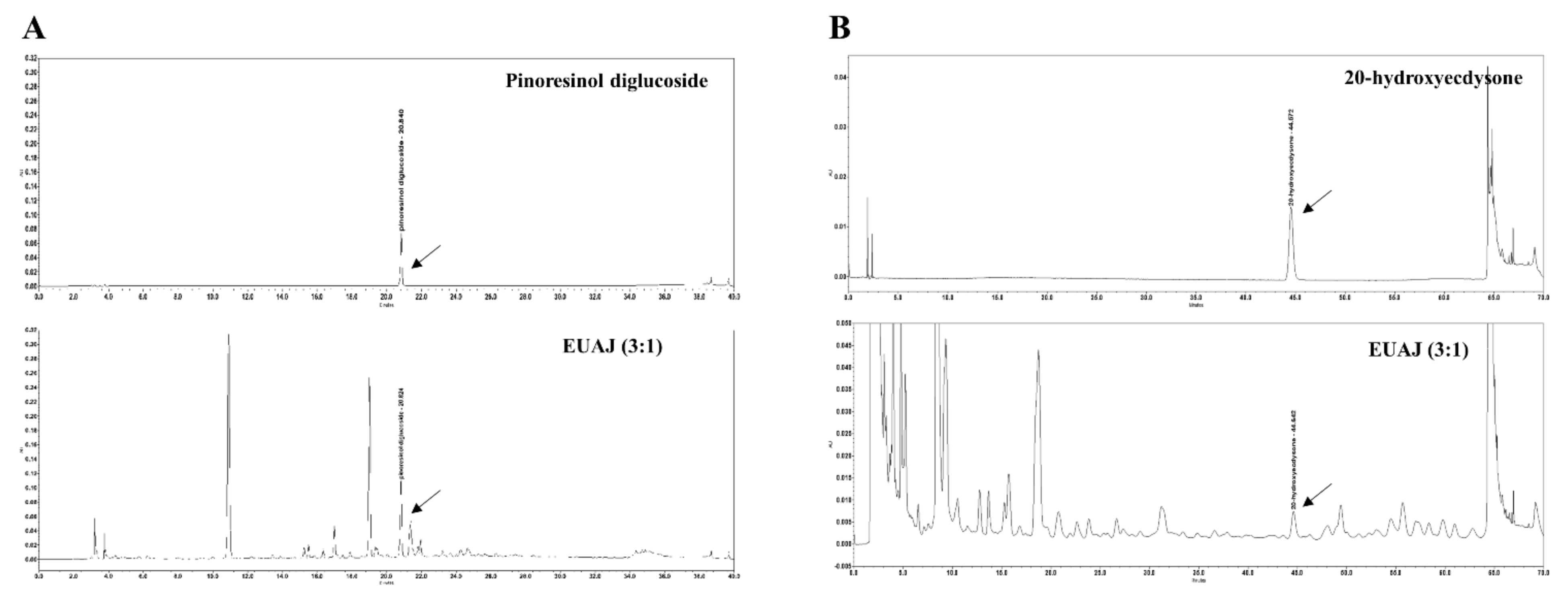
3.2. HPLC Analysis of Pinoresinol Diglucoside and 20-Hydroxyecdysone in EUAJ (3:1)
3.3. Effects of EUAJ (3:1) at the Various Concentrations of T, SHBG and Estradiol in Serum of Aged SD Rats
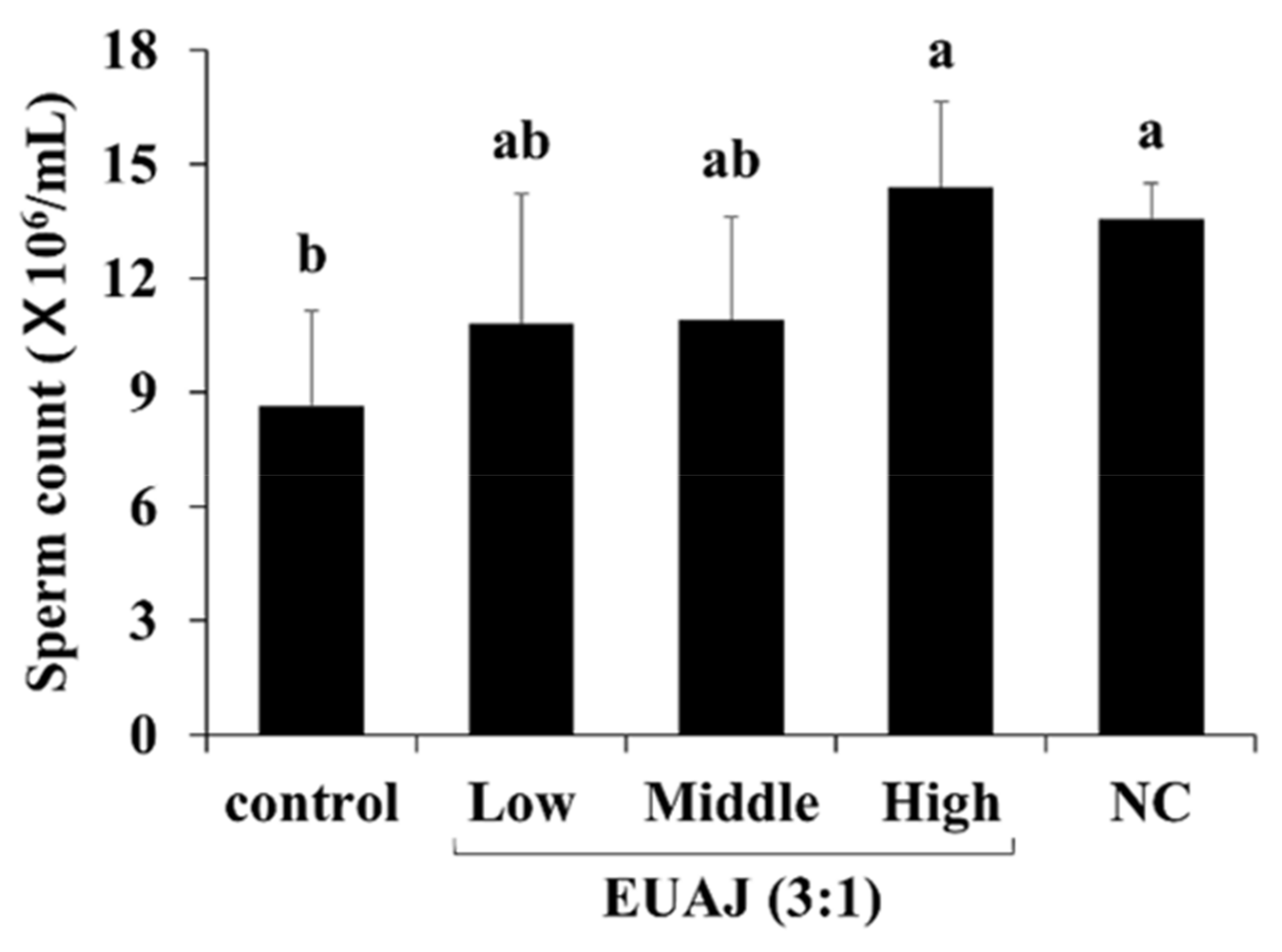
3.4. Effects of EUAJ (3:1) on fertility
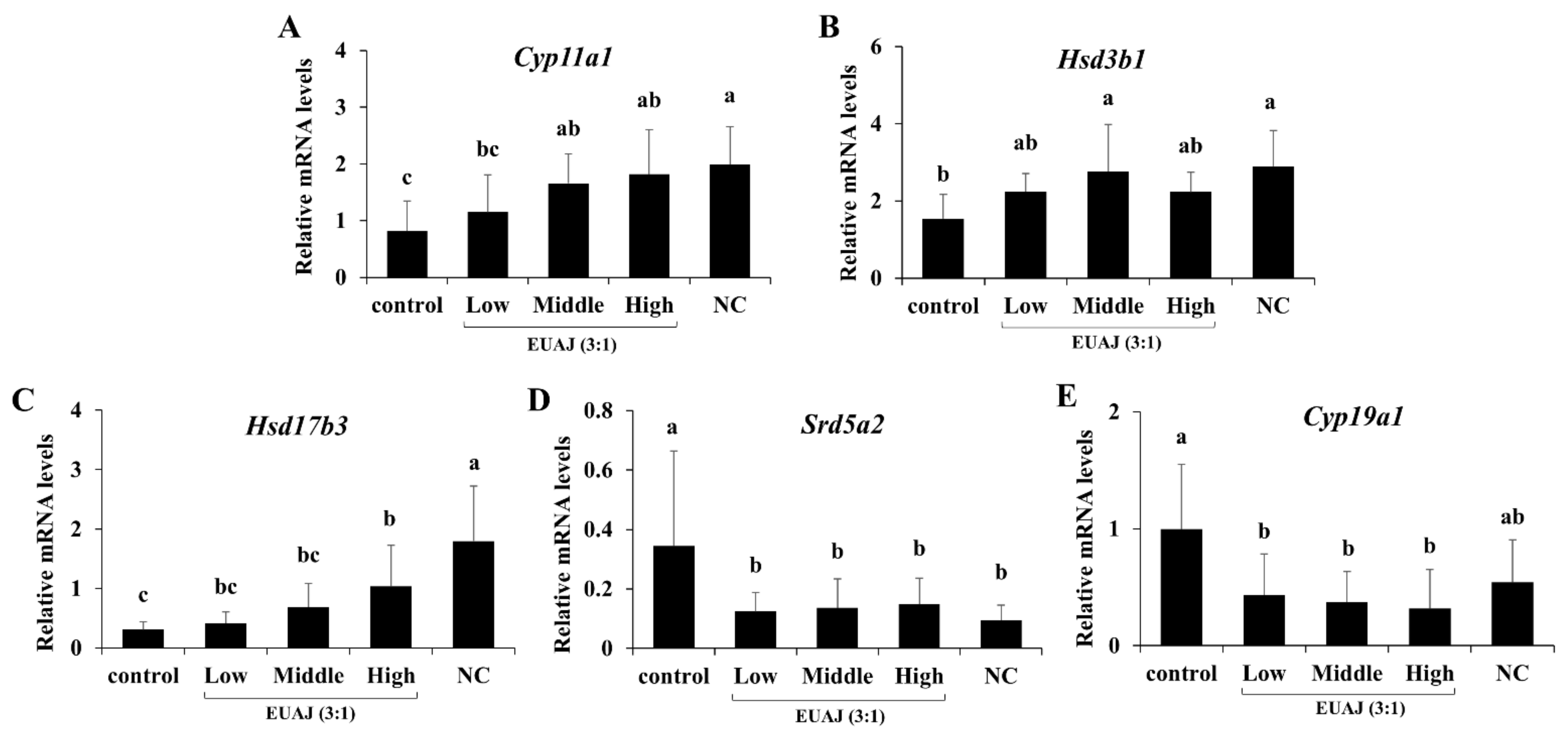
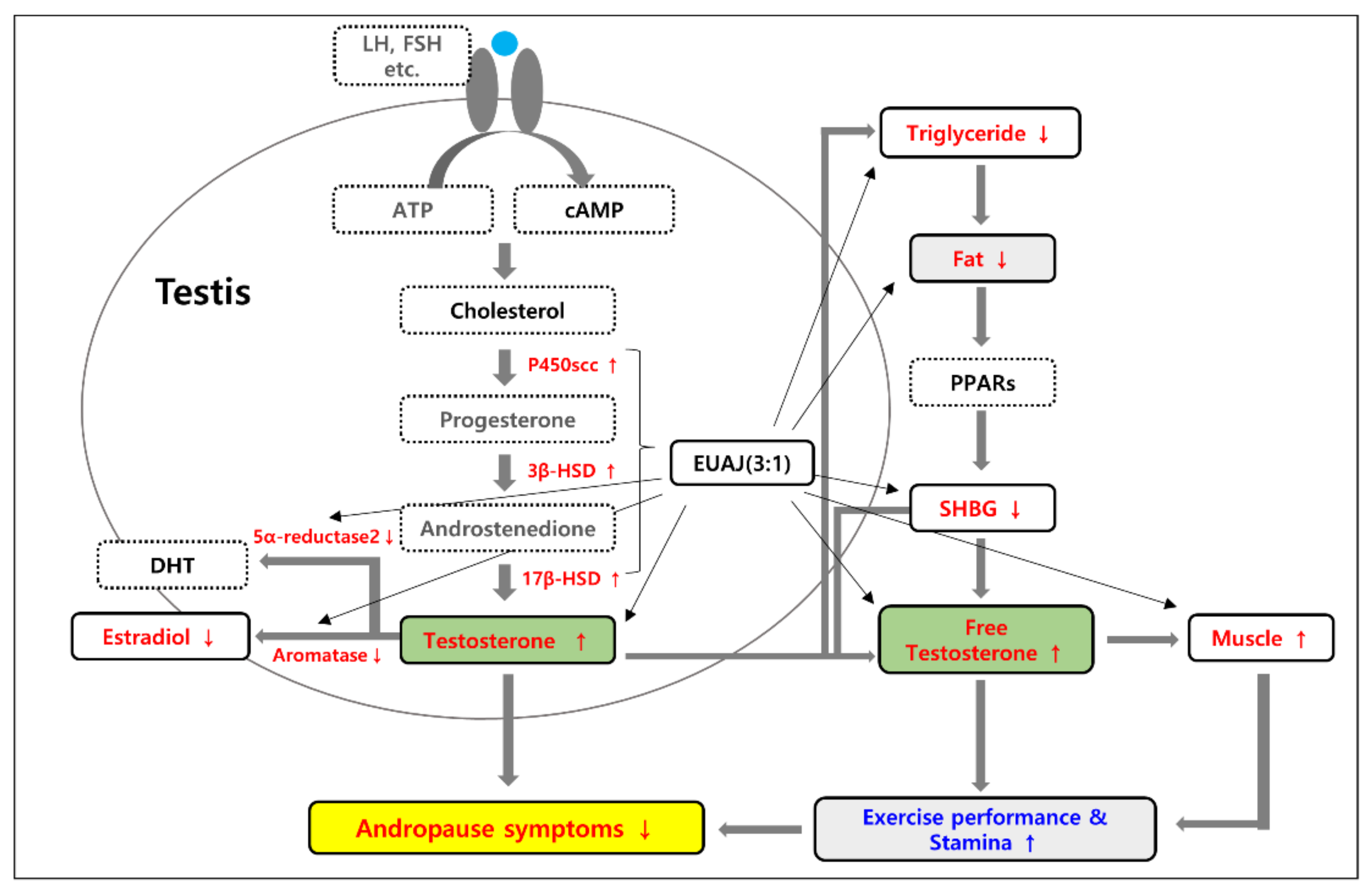
3.5. EUAJ (3:1) Stimulates Testosterone Synthesis Pathway by Upregulating Expression of Steroidogenic Genes
3.6. ALT and AST Levels
3.7. Effects of EUAJ (3:1) on Body Composition and Serum Lipid Profiles in Aged SD Rats
3.8. EUAJ (3:1) Enhances Physical Function and Stamina through Testosterone Elevation in ICR Mice
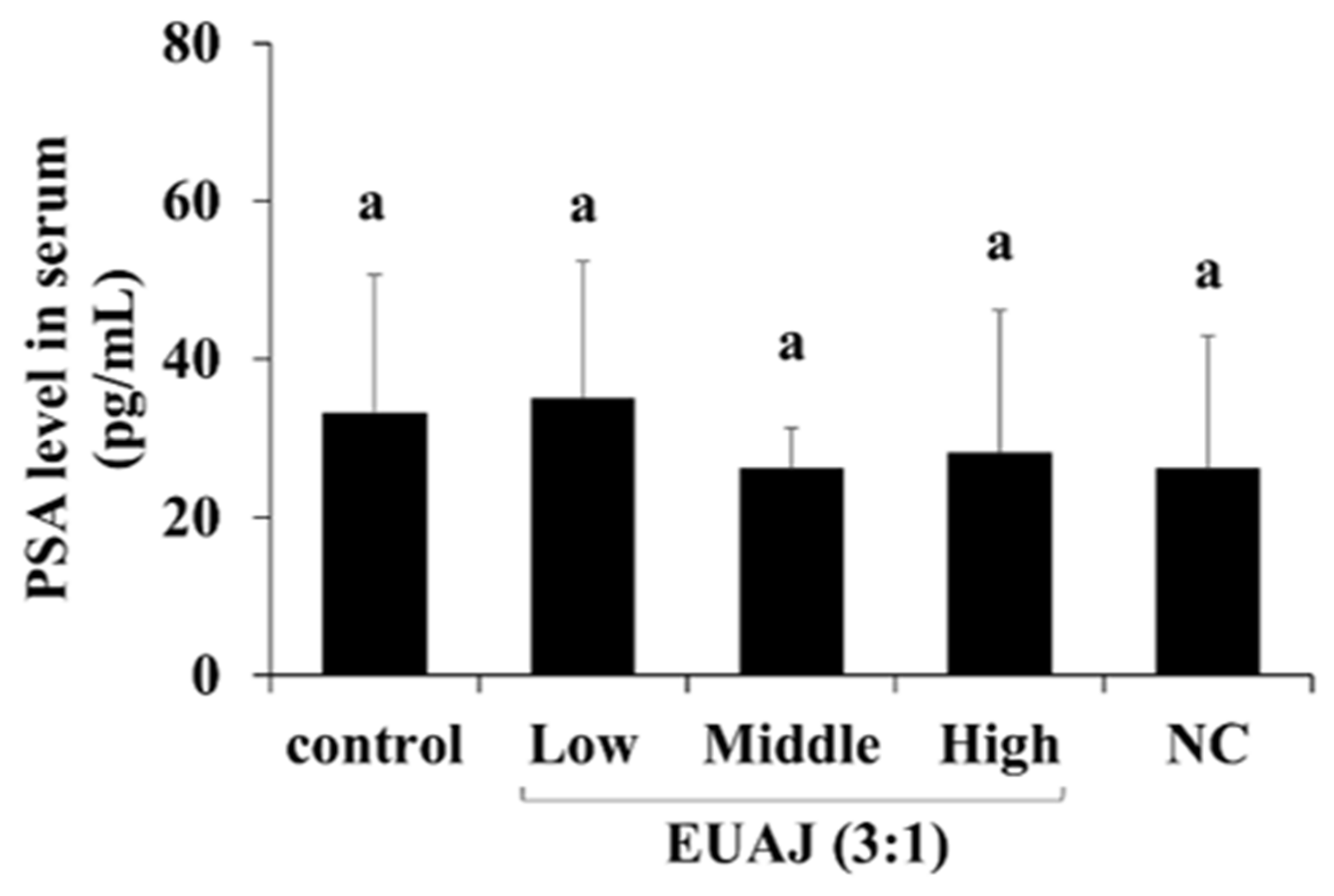
3.9. PSA Level According to the Administration of EUAJ (3:1) in SD Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, A.M. Andropause: Clinical Implications of the Decline in Serum Testosterone Levels with Aging in Men. J. Gerontol. A Biol. Sci. 2002, 57, M76–M99. [Google Scholar] [CrossRef] [PubMed]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. J. Clin. Endocr. 2001, 86, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J. Male Climacteric Syndrome: Late Onset Hypogonadism (LOH) in Males. Korean J. Med. 2016, 75, 262–266. [Google Scholar]
- Pye, S.R.; Huhtaniemi, I.T.; Finn, J.D.; Lee, D.M.; O’Neill, T.W.; Tajar, A.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; Forti, G.; et al. Late-Onset Hypogonadism and Mortality in Aging Men. J. Clin. Endocrinol. Metab. 2014, 99, 1357–1366. [Google Scholar] [CrossRef]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. J. Clin. Endocr. 1981, 53, 58–68. [Google Scholar] [CrossRef]
- Vermeulen, A.; Kaufman, J.M.; Giagulli, V.A. Influence of Some Biological Indexes on Sex Hormone-Binding Globulin and Androgen Levels in Aging or Obese Males. J. Clin. Endocrinol. Metab. 1996, 81, 1821–1826. [Google Scholar] [CrossRef]
- Gruenewald, D.A.; Matsumoto, A.M. Testosterone Supplementation Therapy for Older Men: Potential Benefits and Risks. J. Am. Geriatr. Soc. 2003, 51, 101–115. [Google Scholar] [CrossRef]
- Fu, H.; Bai, X.; Le, L.; Tian, D.; Gao, H.; Qi, L.X.; Hu, K.P. Eucommia Ulmoides Oliv. Leaf Extract Improves Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats by Protecting Endothelial Function and Ameliorating Hypothalamic-Pituitary-Gonadal Axis Function. Evid. Based Complement. Alternat. Med. 2019, 2019, 1782953. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Nie, Z.; Han, L.; Zhong, X.; Yan, X.; Gao, X. Eucommia Ulmoides Leaf Extract Alters Gut Microbiota Composition, Enhances Short-Chain Fatty Acids Production, and Ameliorates Osteoporosis in the Senescence-Accelerated Mouse P6 (SAMP6) Model. Food Sci. Nutr. 2020, 8, 4897–4906. [Google Scholar] [CrossRef]
- Woo, Y.M.; Kim, O.J.; Jo, E.S.; Kim, S.J.; Lee, Y.H.; Ahn, M.Y.; Lee, S.H.; Ha, J.M.; Kim, A. Anti-Inflammatory Effects of the Combined Extracts of Achyranthes Japonica Nakai and Aralia Continentalis Kitagawa In Vitro and In Vivo. Data Brief. 2019, 25, 104088. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Yeo, S.; Lim, E.; Choi, C.W.; Choi, S.; Li, W.Y.; Lee, J.W.; Park, J.H.; Huh, D.; et al. Anti-Osteoporotic Effects of Combined Extract of Lycii Radicis Cortex and Achyranthes Japonica in Osteoblast and Osteoclast Cells and Ovariectomized Mice. Nutrients 2019, 11, 2716. [Google Scholar] [CrossRef]
- Decaroli, M.C.; Rochira, V. Aging and Sex Hormones in Males. Virulence 2017, 8, 545–570. [Google Scholar] [CrossRef]
- Wu, D.; Yu, D.; Zhang, Y.; Dong, J.; Li, D.; Wang, D. Metabolite Profiles, Bioactivity, and HPLC Fingerprint of Different Varieties of Eucommia Ulmoides Oliv.: Towards the Utilization of Medicinal and Commercial Chinese Endemic Tree. Molecules 2018, 23, 1898. [Google Scholar] [CrossRef]
- Boo, K.H.; Lee, D.; Jeon, G.L.; Ko, S.H.; Cho, S.K.; Kim, J.H.; Park, S.P.; Hong, Q.; Lee, S.H.; Lee, D.S.; et al. Distribution and Biosynthesis of 20-Hydroxyecdysone in Plants of Achyranthes Japonica Nakai. Biosci. Biotechnol. Biochem. 2010, 74, 2226–2231. [Google Scholar] [CrossRef]
- Iwamoto, T.; Yanase, T.; Horie, H.; Namiki, M.; Okuyama, A. Late-Onset Hypogonadism (LOH) and Androgens: Validity of the Measurement of Free Testosterone Levels in the Diagnostic Criteria in Japan. Int. J. Urol. 2009, 16, 168–174. [Google Scholar] [CrossRef]
- Laurent, M.R.; Hammond, G.L.; Blokland, M.; Jardí, F.; Antonio, L.; Dubois, V.; Khalil, R.; Sterk, S.S.; Gielen, E.; Decallonne, B.; et al. Sex Hormone-Binding Globulin Regulation of Androgen Bioactivity in Vivo: Validation of the Free Hormone Hypothesis. Sci. Rep. 2016, 6, 35539. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig Cells: Formation, Function, and Regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Christenson, L.K.; Strauss, J.F. Steroidogenic Acute Regulatory Protein (StAR) and the Intramitochondrial Translocation of Cholesterol. Biochim. Biophys. Acta 2000, 1529, 175–187. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Scott, H.M.; Mason, J.I.; Sharpe, R.M. Steroidogenesis in the Fetal Testis and Its Susceptibility to Disruption by Exogenous Compounds. Endocr. Rev. 2009, 30, 883–925. [Google Scholar] [CrossRef]
- Sachdev, S.; Cucchiara, A.J.; Snyder, P.J. Prostate-Specific Antigen Concentrations in Response to Testosterone Treatment of Severely Hypogonadal Men. J. Endocr. Soc. 2020, 4, 141. [Google Scholar] [CrossRef]
- Luo, X.; Wu, J.; Li, Z.; Jin, W.; Zhang, F.; Sun, H.; Shi, Y. Safety Evaluation of Eucommia Ulmoides Extract. Regul. Toxicol. Pharmacol. 2020, 118, 104811. [Google Scholar] [CrossRef]
- Hyun, S.W.; Lee, T.G.; Song, S.J.; Kim, C.S. Evaluation of Oral Toxicity and Genotoxicity of Achyranthis Radix Extract. J. Ethnopharmacol. 2021, 274, 113944. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.K.; Choi, J.H.; Jung, J.Y.; Oh, W.Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.H.; et al. Hepato-protective Effect of Pinoresinol on Carbon Tetrachloride-Induced Hepatic Damage in Mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef]
- Gong, M.; Su, C.; Fan, M.; Wang, P.; Cui, B.; Guo, Z.; Liang, S.; Yang, L.; Liu, X.; Dai, L.; et al. Mechanism by Which Eu-commia Ulmoides Leaves Regulate Nonalcoholic Fatty Liver Disease Based on System Pharmacology. J. Ethnopharmacol. 2022, 282, 114603. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Yoon, Y.; Chae, H.J. Rhus Verniciflua and Eucommia Ulmoides Protects Against High-Fat Di-et-Induced Hepatic Steatosis by Enhancing Anti-Oxidation and AMPK Activation. Am. J. Chin. Med. 2019, 47, 1253–1270. [Google Scholar] [CrossRef]
- Jin, C.F.; Li, B.; Lin, S.M.; Yadav, R.K.; Kim, H.R.; Chae, H.J. Mechanism of the Inhibitory Effects of Eucommia Ul-moides Oliv. Cortex Extracts (EUCE) in the CCl 4 -Induced Acute Liver Lipid Accumulation in Rats. Int. J. Endocrinol. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Lutz, S.Z.; Wagner, R.; Fritsche, L.; Peter, A.; Rettig, I.; Willmann, C.; Fehlert, E.; Martus, P.; Todenhöfer, T.; Stefan, N.; et al. Sex-Specific Associations of Testosterone with Metabolic Traits. Front. Endocrinol. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Barrett-Connor, E.; Wedick, N.M.; Wingard, D.L. Endogenous Sex Hormones and the Development of Type 2 Diabetes in Older Men and Women: The Rancho Bernardo Study. Diabetes Care 2002, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Grosman, H.; Rosales, M.; Fabre, B.; Nolazco, C.; Mazza, O.; Berg, G.; Mesch, V. Association between Testosterone Levels and the Metabolic Syndrome in Adult Men. Aging Male 2014, 17, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Kwon, Y.J.; Lee, Y.J. High Triglyceride to HDL Cholesterol Ratio Is Associated with Low Testosterone and Sex Hormone-Binding Globulin Levels in Middle-Aged and Elderly Men. Aging Male 2020, 23, 93–97. [Google Scholar] [CrossRef]
- Sofikitis, N.; Giotitsas, N.; Tsounapi, P.; Baltogiannis, D.; Giannakis, D.; Pardalidis, N. Hormonal Regulation of Spermatogenesis and Spermiogenesis. J. Steroid. Biochem. Mol. Biol. 2008, 109, 323–330. [Google Scholar] [CrossRef]
- Neaves, W.B.; Johnson, L.; Porter, J.C.; Parker, C.R.; Petty, C.S. Leydig Cell Numbers, Daily Sperm Production, and Serum Gonadotropin Levels in Aging Men. J. Clin. Endocrinol. Metab. 1984, 59, 756–763. [Google Scholar] [CrossRef]
- Johnson, L.; Zane, R.S.; Petty, C.S.; Neaves, W.B. Quantification of the Human Sertoli Cell Population: Its Distribution, Relation to Germ Cell Numbers, and Age-Related Decline. Biol. Reprod. 1984, 31, 785–795. [Google Scholar] [CrossRef]
- Hou, Y.; Yuan, P.; Fu, Y.; Zhang, Q.; Wei, Y.; Gao, L.; Liu, L.; Wang, X.; Zheng, X.; Feng, W. Duzhong Butiansu Prescription Improves Heat Stress-Induced Spermatogenic Dysfunction by Regulating Sperm Formation and Heat Stress Pathway. Evid. Based Complement. Altern. Med. 2020, 2020, 6723204. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, J.; Zhu, Z.; Bao, X.; Zhang, M.; Ren, C.; Zhang, Q. Aucubin, a Natural Iridoid Glucoside, Attenuates Oxidative Stress-Induced Testis Injury by Inhibiting JNK and CHOP Activation via Nrf2 up-Regulation. Phytomedicine 2019, 64, 153057. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, D.H.; Kim, J.Y.; Park, J.; Kim, O.H.; Han, D.; Kim, W.Y.; Kim, S.S.; Lee, M.Y.; Heo, S.H.; et al. Improvement of Andropause Symptoms by Dandelion and Rooibos Extract Complex CRS-10 in Aging Male. Nutr. Res. Pract. 2012, 6, 505–512. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, E.K.; Kim, S.Y.; Kim, T.H.; Kim, H.P. Effect of a Mixed Extract of Fenugreek Seeds and Lespedeza Cuneata on Testosterone Deficiency Syndrome. Korean J. Food Sci. Technol. 2015, 47, 492–498. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Roth, S.M.; Lee, M.I.; Bhasin, S. Testosterone-Induced Muscle Hypertrophy Is Associated with an Increase in Satellite Cell Number in Healthy, Young Men. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E197–E205. [Google Scholar] [CrossRef]
- Dubois, V.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Androgens and Skeletal Muscle: Cellular and Molecular Action Mechanisms Underlying the Anabolic Actions. Cell Mol. Life Sci. 2012, 69, 1651–1667. [Google Scholar] [CrossRef]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone Administration to Elderly Men Increases Skeletal Muscle Strength and Protein Synthesis. Am. J. Physiol. 1995, 269, E820–E826. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.; Storer, T.W. Proof of the Effect of Testosterone on Skeletal Muscle. J. Endocrinol. 2001, 170, 27–38. [Google Scholar] [CrossRef]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of Testosterone on Muscle Mass and Muscle Protein Synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef]
- Jardí, F.; Laurent, M.R.; Kim, N.; Khalil, R.; de Bundel, D.; van Eeckhaut, A.; van Helleputte, L.; Deboel, L.; Dubois, V.; Schollaert, D.; et al. Testosterone Boosts Physical Activity in Male Mice via Dopaminergic Pathways. Sci. Rep. 2018, 8, 957. [Google Scholar] [CrossRef]
- Zhao, X.N.; Liang, J.L.; Chen, H.-B.; Liang, Y.E.; Guo, H.Z.; Su, Z.R.; Li, Y.C.; Zeng, H.F.; Zhang, X.J. Anti-Fatigue and Antioxidant Activity of the Polysaccharides Isolated from Millettiae Speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine Kinase Monitoring in Sport Medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Logan, A.C.; Wong, C. Chronic Fatigue Syndrome: Oxidative Stress and Dietary Modifications. Altern. Med. Rev. 2001, 6, 450–459. [Google Scholar]
- Kim, S.H.; Lee, J.Y.; Kim, S.H.; Kim, J.H.; Kwon, H.O.; Lee, J.S.; Jun, W.J.; Lee, Y.H. Effects of the Mixture of Eucommia ulmoides Oliver and Achyranthes japonica Nakai Extracts (KGC08EA) on Testosterone Synthesis in Late-Onset Hypogonadism (LOH) in vitro Models. J. Korean Soc. Food Sci. Nutr. 2020, 49, 1054–1060. [Google Scholar] [CrossRef]
- Muller, M.; den Tonkelaar, I.; Thijssen, J.H.H.; Grobbee, D.E.; van der Schouw, Y.T. Endogenous Sex Hormones in Men Aged 40–80 Years. Eur. J. Endocrinol. 2003, 149, 583–589. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Forti, G. Male Late-Onset Hypogonadism: Pathogenesis, Diagnosis and Treatment. Nat. Rev. Urol. 2011, 8, 335–344. [Google Scholar] [CrossRef]

| Group | Liver Weight Ratio 1 (%) | ALT (KU) | AST (KU) |
|---|---|---|---|
| control | 3.08 ± 0.24 a | 30.13 ± 3.44 a | 63.13 ± 14.84 a |
| Low | 2.99 ± 0.16 ab | 27.50 ± 6.68 ab | 67.88 ± 18.86 a |
| Middle | 2.86 ± 0.22 b | 27.00 ± 2.45 ab | 60.88 ± 10.95 a |
| High | 2.93 ± 0.20 ab | 24.63 ± 3.46 b | 56.38 ± 13.50 a |
| NC | 3.10 ± 0.13 a | 27.38 ± 2.26 ab | 68.13 ± 16.75 a |
| Group | Epididymal Fat Weight (g) | Epididymal Fat Weight Ratio 1 (%) | Muscle Weight Ratio 2 (%) |
|---|---|---|---|
| Control | 11.24 ± 0.99 a | 2.15 ± 0.20 a | 0.81 ± 0.02 c |
| Low | 10.10 ± 1.85 ab | 1.95 ± 0.33 ab | 0.86 ± 0.06b c |
| Middle | 8.68 ± 2.31 b | 1.65 ± 0.41 bc | 0.88 ± 0.03 ab |
| High | 9.88 ± 2.11 ab | 1.88 ± 0.40 ab | 0.89 ± 0.07 ab |
| NC | 5.83 ± 1.16 c | 1.47 ± 0.22 c | 0.93 ± 0.04 a |
| Group | TG (mg/dL) | Total-Cholesterol (mg/dL) | HDL-Cholesterol (mg/dL) | LDL-Cholesterol (mg/dL) |
|---|---|---|---|---|
| Control | 194.13 ± 63.34 a | 118.63 ± 14.70 ab | 38.88 ± 8.49 a | 40.98 ± 18.26 a |
| Low | 193.75 ± 54.99 a | 129.63 ± 18.49 a | 42.88 ± 10.26 a | 47.38 ± 15.14 a |
| Middle | 128.25 ± 42.47 b | 121.63 ± 26.01 ab | 38.38 ± 8.40 a | 56.88 ± 25.39 a |
| High | 127.63 ± 44.34 b | 115.50 ± 13.85 ab | 36.38 ± 9.71 a | 52.53 ± 18.80 a |
| NC | 112.88 ± 42.12 b | 107.50 ± 22.06 b | 35.63 ± 11.49 a | 47.90 ± 12.70 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, S.; Kwon, H.O.; Bae, B.S.; Shim, S.l.; Jun, W.; Lee, Y.-H. The Effect of a Combination of Eucommia ulmoides and Achyranthes japonica on Alleviation of Testosterone Deficiency in Aged Rat Models. Nutrients 2022, 14, 3341. https://doi.org/10.3390/nu14163341
Lee JY, Kim S, Kwon HO, Bae BS, Shim Sl, Jun W, Lee Y-H. The Effect of a Combination of Eucommia ulmoides and Achyranthes japonica on Alleviation of Testosterone Deficiency in Aged Rat Models. Nutrients. 2022; 14(16):3341. https://doi.org/10.3390/nu14163341
Chicago/Turabian StyleLee, Jeong Yoon, Seokho Kim, Han Ol Kwon, Bong Seok Bae, Sung lye Shim, Woojin Jun, and Yoo-Hyun Lee. 2022. "The Effect of a Combination of Eucommia ulmoides and Achyranthes japonica on Alleviation of Testosterone Deficiency in Aged Rat Models" Nutrients 14, no. 16: 3341. https://doi.org/10.3390/nu14163341
APA StyleLee, J. Y., Kim, S., Kwon, H. O., Bae, B. S., Shim, S. l., Jun, W., & Lee, Y.-H. (2022). The Effect of a Combination of Eucommia ulmoides and Achyranthes japonica on Alleviation of Testosterone Deficiency in Aged Rat Models. Nutrients, 14(16), 3341. https://doi.org/10.3390/nu14163341






