Overgrowth of Squamocolumnar Junction and Dysregulation of Stem Cell Lineages in the Stomach of Vitamin A-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Tissue Processing for Histological, Immunohistochemical, and Lectin Binding Analyses
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
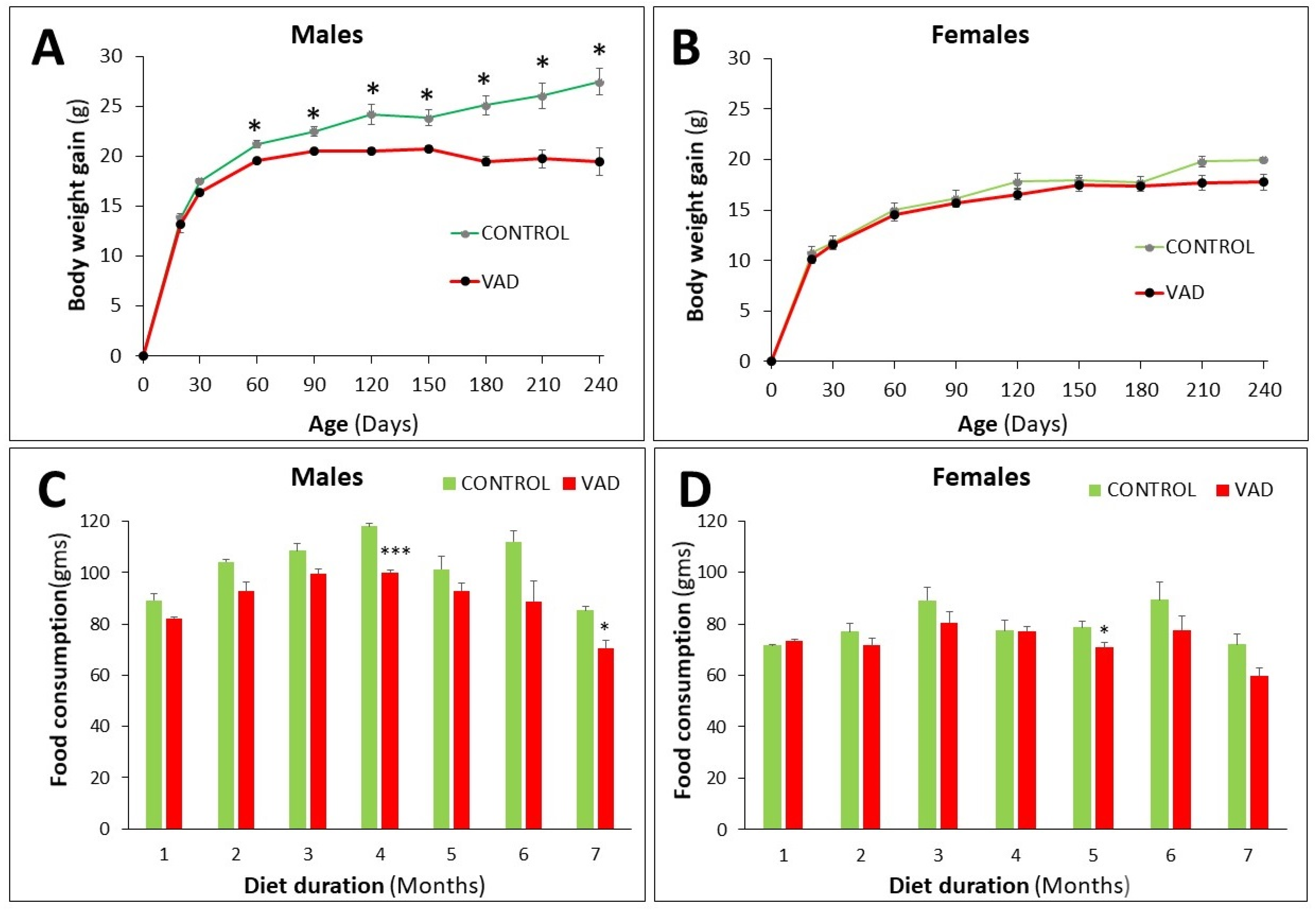
3.1. Differential Decrease in Body Weight and Food Consumption in VAD Mice
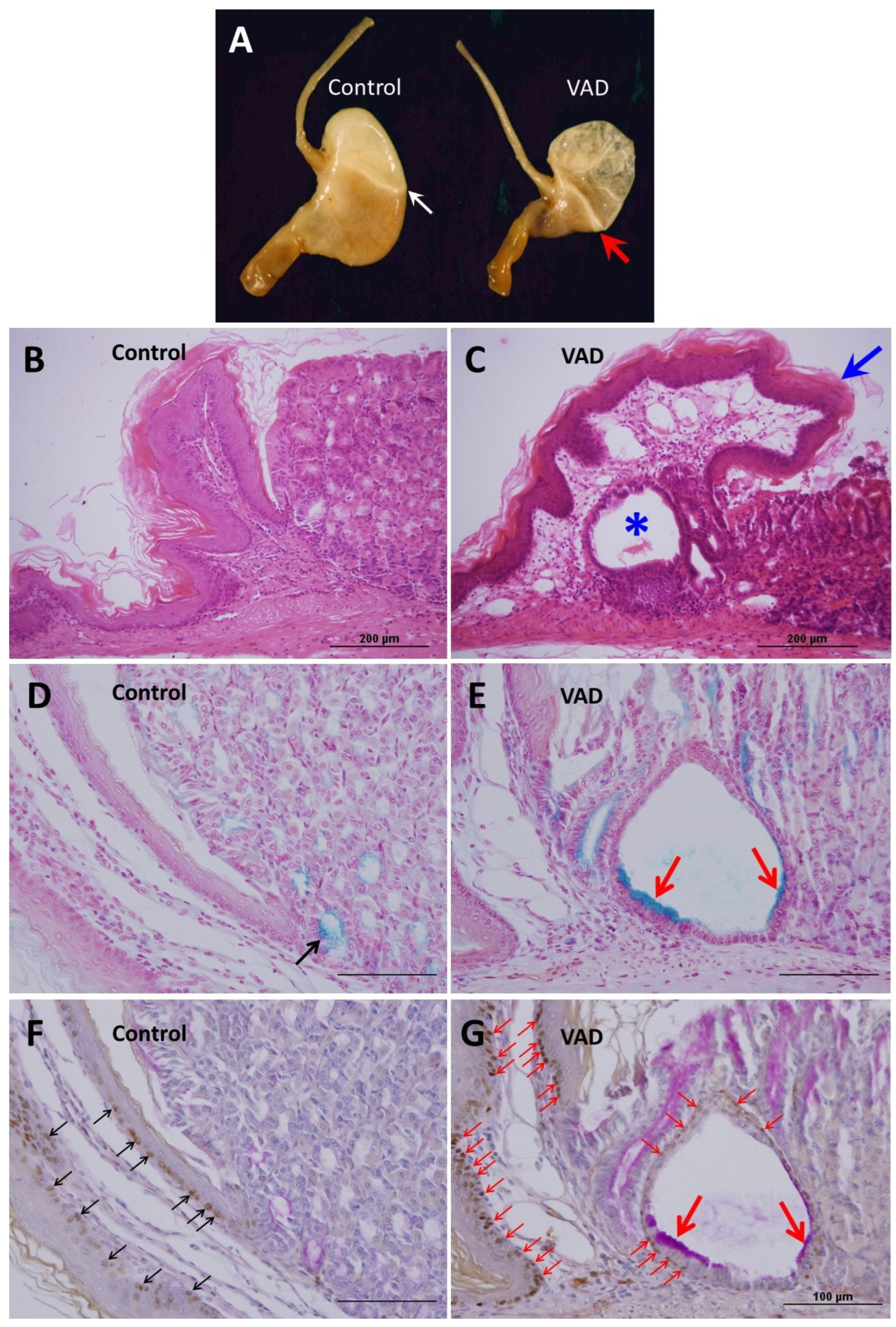
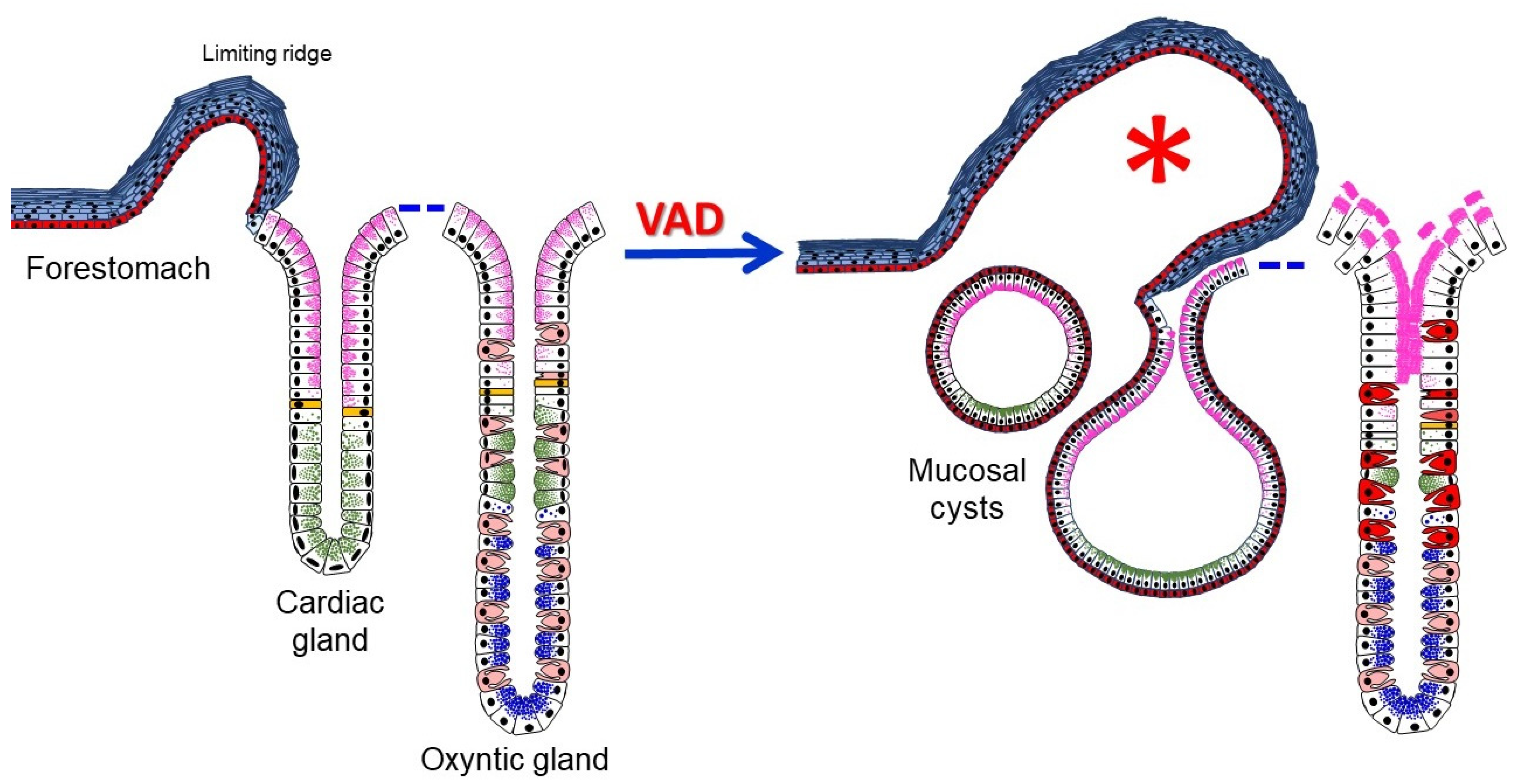
3.2. Anatomical and Histological Alterations of VAD Stomachs
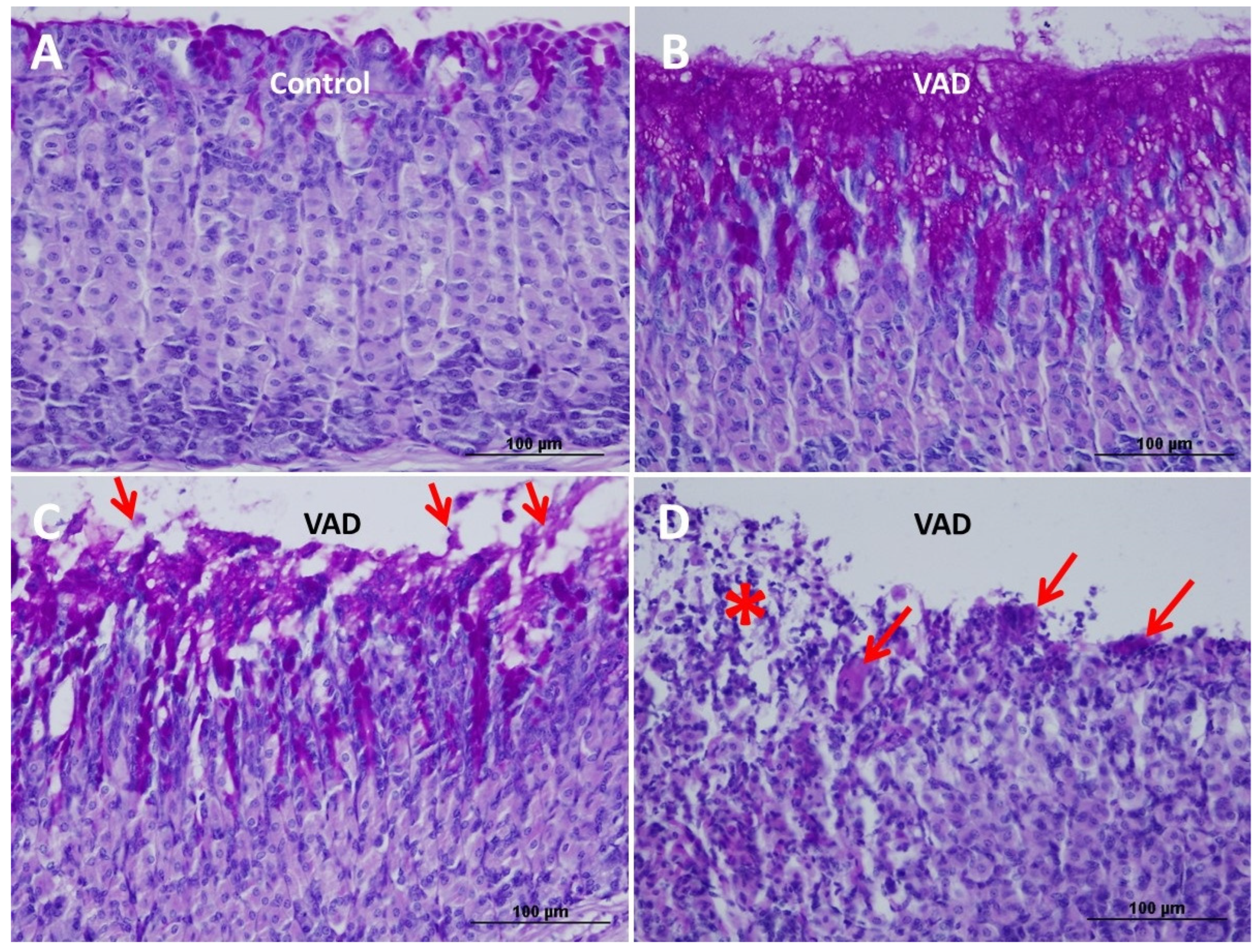
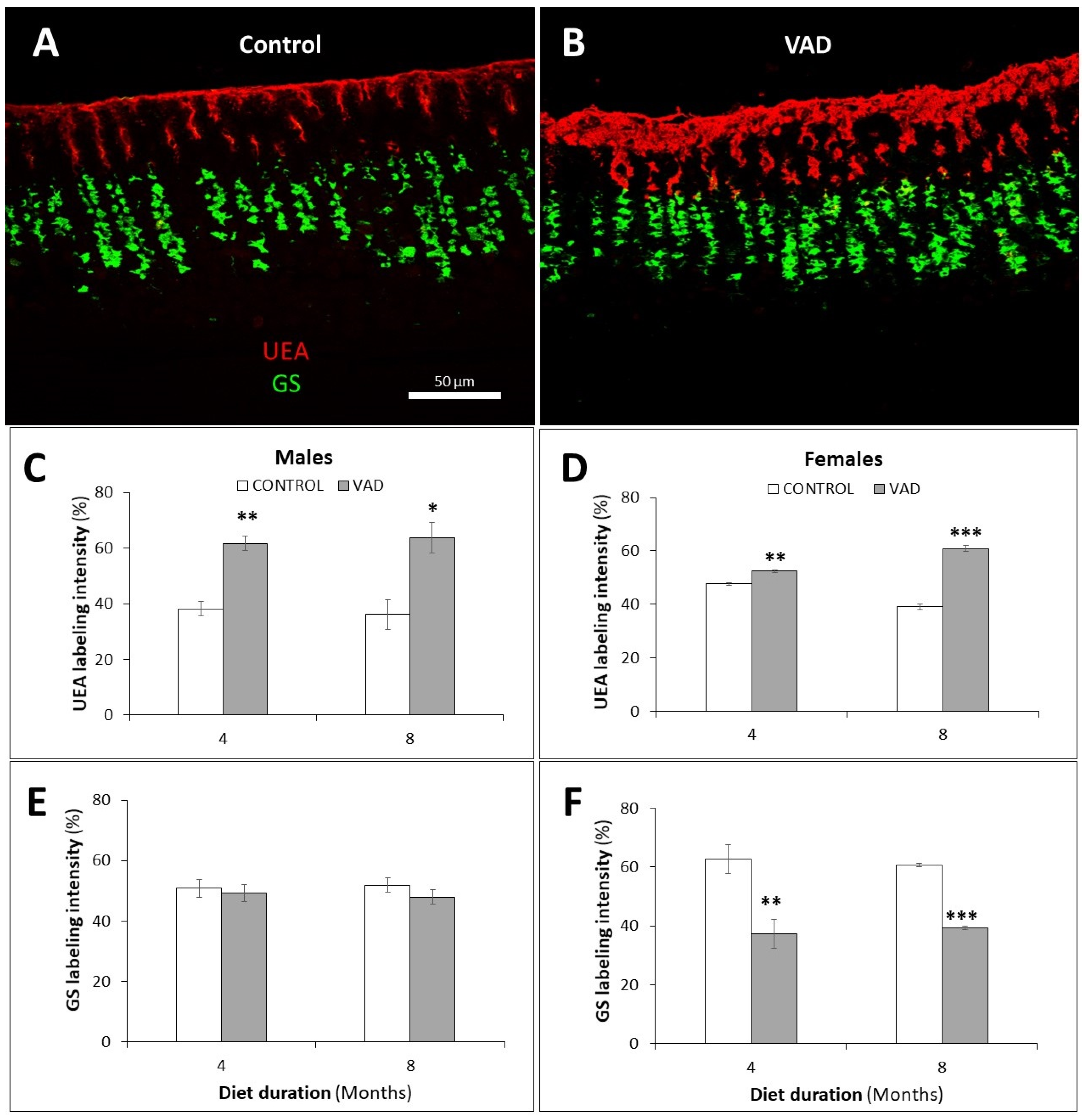
3.3. Loss of Mucosal Integrity of VAD Stomach
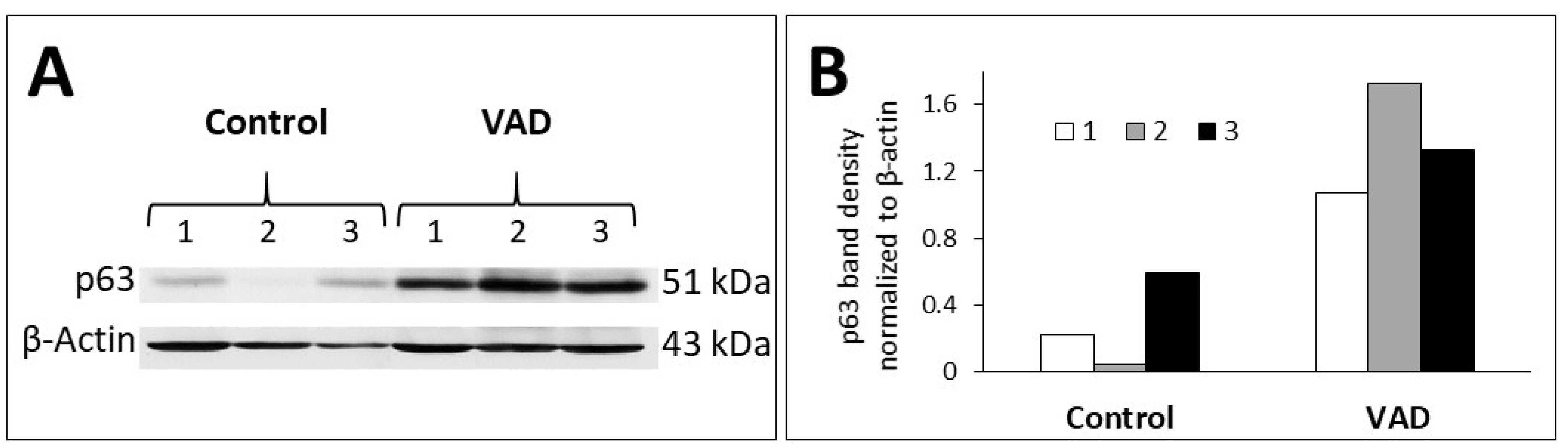
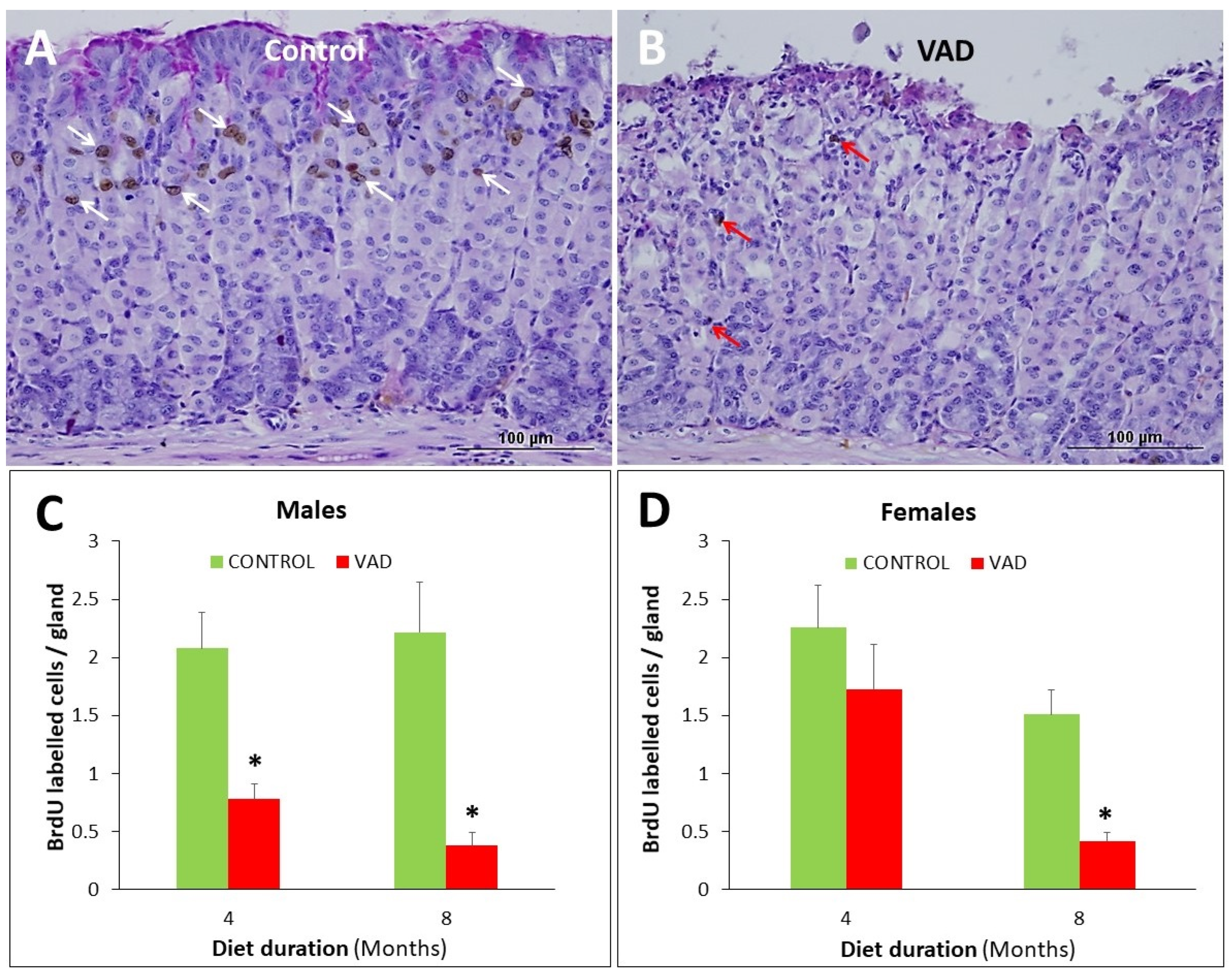
3.4. Inhibition of Cell Proliferation in VAD Gastric Epithelium
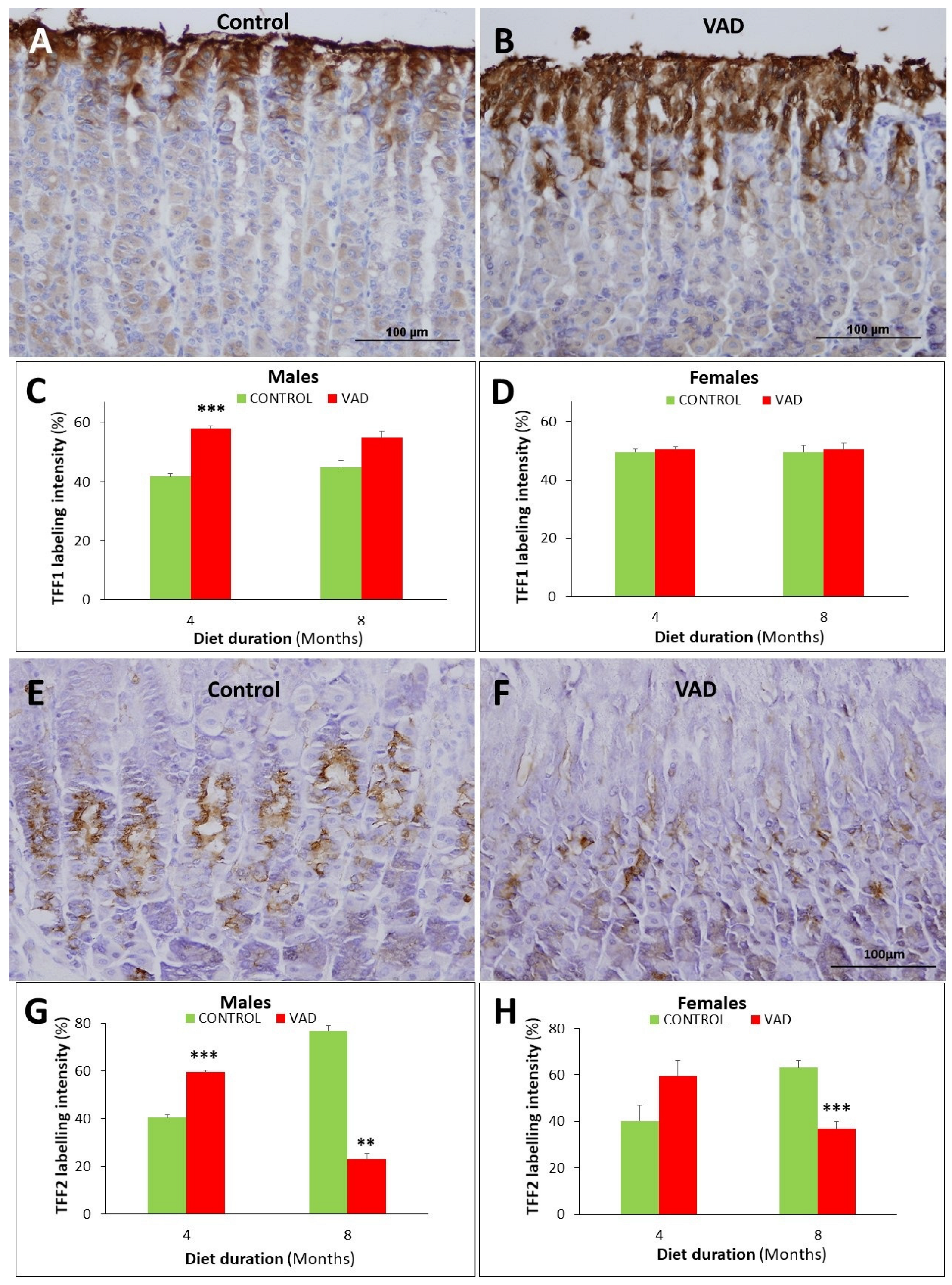
3.5. Dysregulation of Mucus- and TFF-Secreting Pit and Neck Cells in VAD Stomachs
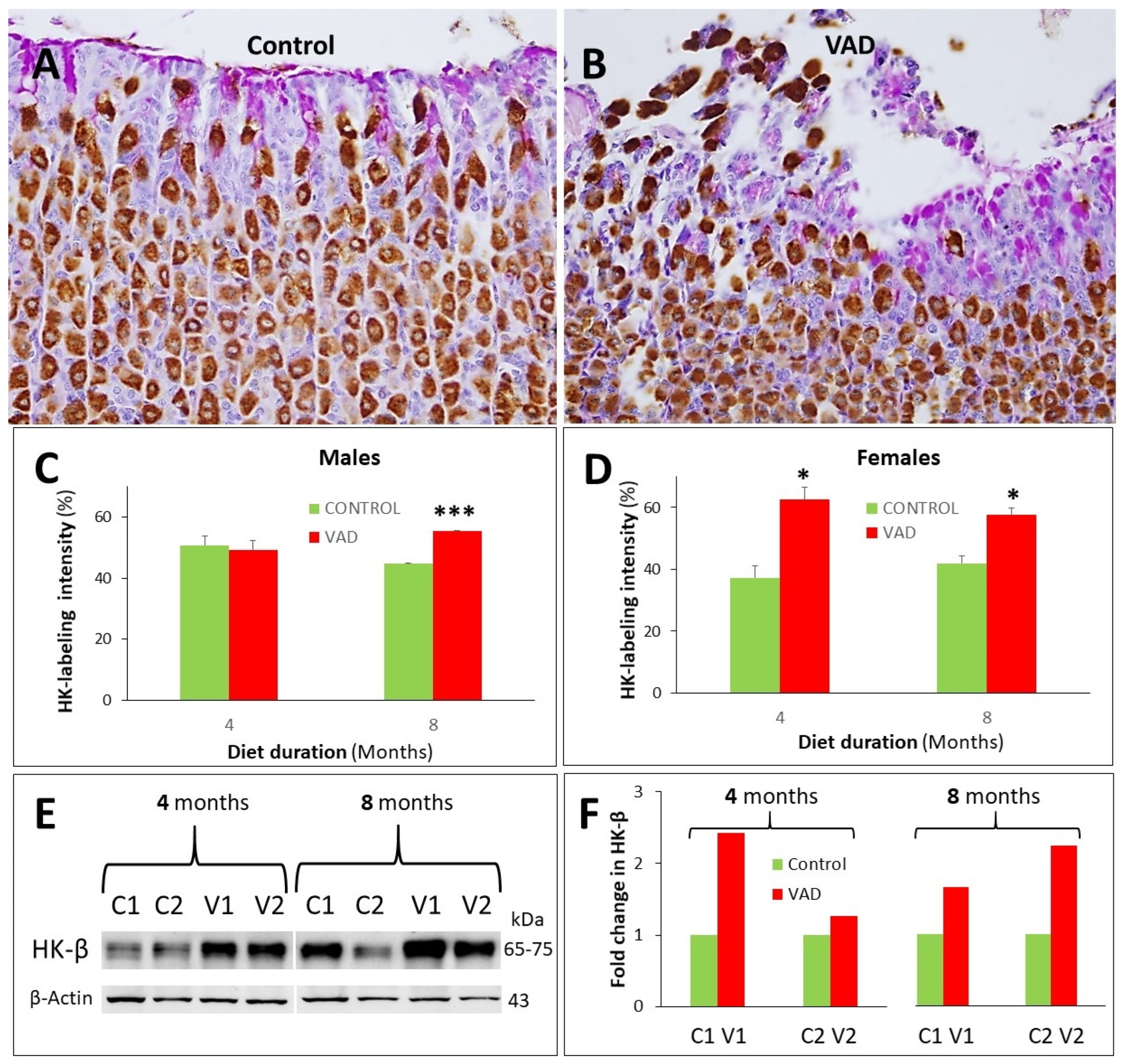
3.6. Increased Proton Pump in Parietal Cells of VAD Stomachs
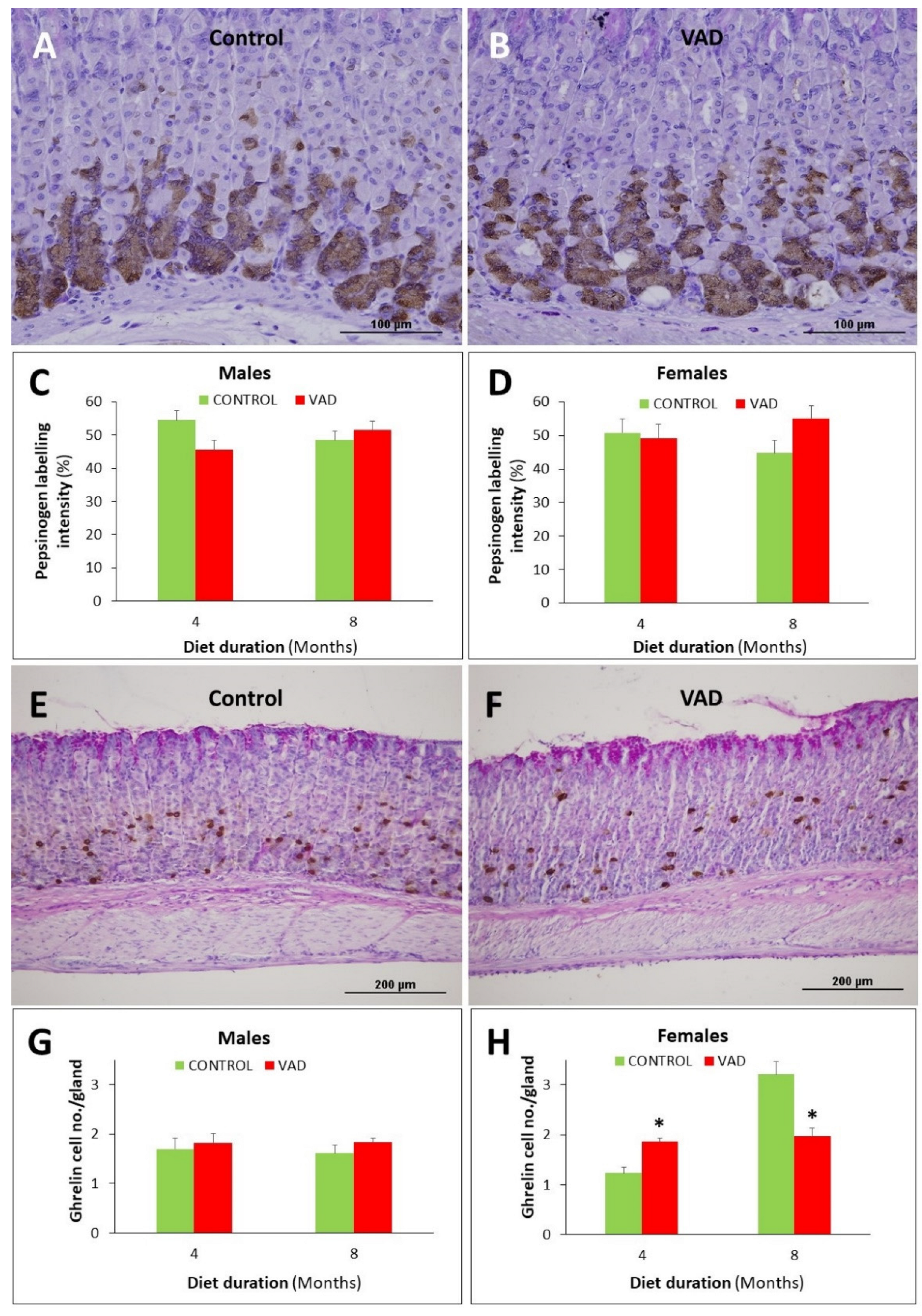
3.7. Ghrelin-Secreting Enteroendocrine Cells Are Reduced in Female VAD Gastric Glands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underwood, B.A. Vitamin A deficiency disorders: International efforts to control a preventable "pox". J. Nutr. 2004, 134, 231S–236S. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Griswold, M.D. The key role of vitamin A in spermatogenesis. J. Clin. Investig. 2010, 120, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A deficiency and the lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Cabezuelo, M.T.; Zaragozá, R.; Barber, T.; Viña, J.R. Role of Vitamin A in Mammary Gland Development and Lactation. Nutrients 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.; Taylor, C. Vitamin A Deficiency; StatPearls Publishing: Tampa, FL, USA, 2022; ISBN NBK567744. [Google Scholar]
- Lee, E.R.; Trasler, J.; Dwivedi, S.; Leblond, C.P. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am. J. Anat. 1982, 164, 187–207. [Google Scholar] [CrossRef]
- Karam, S.M. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 1999, 4, D286–D298. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat. Rec. 1993, 236, 259–279. [Google Scholar] [CrossRef]
- Al-Awadhi, H.; John, R.; Al-Marzooqi, F.; Vincze, A.; Branicki, F.; Karam, S.M. Sequential alterations in gastric biopsies and tumor tissues support the multistep process of carcinogenesis. Histol. Histopathol. 2011, 26, 1153–1164. [Google Scholar]
- Van den Brink, G.R.; Hardwick, J.C.; Tytgat, G.N.; Brink, M.A.; Ten Kate, F.J.; Van Deventer, S.J.; Tytgat, G.N.J.; Brink, M.A.; Ten Kate, F.J. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology 2001, 121, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Tomasetto, C.; Rio, M.C. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 2004, 53, 1408–1415. [Google Scholar] [CrossRef]
- Kim, T.H.; Shivdasani, R.A. Stomach development, stem cells and disease. Development 2016, 143, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.P.; Flaxman, B.A. The effect of vitamin A on growth and differentiation of human keratinocytes in vitro. J. Investig. Dermatol. 1975, 64, 19–22. [Google Scholar] [CrossRef]
- Chew, B.P.; Zamora, C.S.; Luedecke, L.O. Effect of vitamin A deficiency on mammary gland development and susceptibility to mastitis through intramammary infusion with Staphylococcus aureus in mice. Am. J. Vet. Res. 1985, 46, 287–293. [Google Scholar]
- An, M.; Yang, T.; Liu, H.; Xiao, L.; Li, C.; Zhu, J.; Chen, J.; Li, T. Maternal vitamin A deficiency impairs cholinergic and nitrergic neurons, leading to gastrointestinal dysfunction in rat offspring via RARβ. Life Sci. 2020, 264, 118688. [Google Scholar]
- McCullough, F.S.; Northrop-Clewes, C.A.; Thurnham, D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999, 58, 289–293. [Google Scholar] [CrossRef]
- Karam, S.M.; Hassan, W.M.; John, R. Expression of retinoid receptors in multiple cell lineages in the gastric mucosae of mice and humans. J. Gastroenterol. Hepatol. 2005, 20, 1892–1899. [Google Scholar] [CrossRef]
- Karam, S.M.; John, R.; Alpers, D.H.; Ponery, A.S. Retinoic acid stimulates the dynamics of mouse gastric epithelial progenitors. Stem Cells 2005, 23, 433–441. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, Y.; Shi, Y.; Li, P.; Xu, J.; Chen, K.; Xu, E.; Yang, J. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: A meta-analysis. Clin. Nutr. 2015, 34, 620–626. [Google Scholar] [CrossRef]
- Ghenimi, N.; Beauvieux, M.C.; Biran, M.; Pallet, V.; Higueret, P.; Gallis, J.L. Vitamin A deficiency in rats induces anatomic and metabolic changes comparable with those of neurodegenerative disorders. J. Nutr. 2009, 139, 696–702. [Google Scholar] [CrossRef]
- Zhang, M.; Ji, B.; Zou, H.; Shi, J.; Zhang, Z.; Li, X.; Zhu, H.; Feng, G.; Jin, M.; Yu, L.; et al. Vitamin A depletion alters sensitivity of motor behavior to MK-801 in C57BL/6J mice. Behav. Brain Funct. 2010, 6, 7. [Google Scholar] [CrossRef]
- Spechler, S.J.; Souza, R.F. Barrett’s esophagus. N. Engl. J. Med. 2014, 371, 836–845. [Google Scholar] [CrossRef]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; Doyle, L.A.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef]
- Jain, R.N.; Al-Menhali, A.A.; Keeley, T.M.; Ren, J.; El-Zaatari, M.; Chen, X.; Merchant, J.L.; Ross, T.S.; Chew, C.S.; Samuelson, L.C. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J. Clin. Investig. 2008, 118, 2459–2470. [Google Scholar] [CrossRef]
- Greaves, P.; Boiziau, J.L. Altered Patterns of Mucin Secretion in Gastric Hyperplasia in Mice. Vet. Pathol. 1984, 21, 224–228. [Google Scholar] [CrossRef]
- Glickman, J.N.; Yang, A.; Shahsafaei, A.; McKeon, F.; Odze, R.D. Expression of p53related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum. Pathol. 2001, 32, 1157–1165. [Google Scholar] [CrossRef]
- Mari, L.; Milano, F.; Parikh, K.; Straub, D.; Everts, V.; Hoeben, K.K.; Fockens, P.; Buttar, N.S.; Krishnadath, K.K. A pSMAD/CDX2 Complex Is Essential for the Intestinalization of Epithelial Metaplasia. Cell Rep. 2014, 7, 1197–1210. [Google Scholar] [CrossRef]
- Günther, J.; Seyfert, H.-M. The first line of defence: Insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin. Immunopathol. 2018, 40, 555–565. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, Y.; Tao, Y.; Wang, W. Effects of vitamin A deficiency on mucosal immunity and response to intestinal infection in rats. Nutrition 2011, 27, 227–232. [Google Scholar] [CrossRef]
- Harada, T.; Yamashiro, S.; Meade, P.D.; Basrur, P.K.; Maita, K.; Shirasu, Y. Stomach ulcers in vitamin A-deficient Syrian golden hamsters. Jpn. J. Vet. Sci. 1982, 44, 267–274. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, H.; Cho, Y.; Lee, J.; Nam, K.T.; Lee, H.W. Disruption of the Tff1 gene in mice using CRISPR/Cas9 promotes body weight reduction and gastric tumorigenesis. Lab. Anim. Res. 2018, 34, 257–263. [Google Scholar] [CrossRef][Green Version]
- Sturmer, R.; Muller, S.; Hanisch, F.G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef]
- Nitsche, H.; Ramamoorthy, S.; Sareban, M.; Pausawasdi, N.; Todisco, A. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G607–G614. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Reyher, K.K.; Wilkinson, L.B. Immunolocalization of oestrogen receptor alpha and beta in gastric epithelium and enteric neurons. J. Endocrinol. 2001, 171, 65–73. [Google Scholar] [CrossRef]
- Masui, F.; Matsuda, M.; Akazome, Y.; Imaoka, T.; Mori, T. Prevention of neonatal oestrogen imprinting by vitamin A as indicated by oestrogen receptor expression in the mouse vagina. Cell Tissue Res. 2001, 306, 441–447. [Google Scholar] [CrossRef]
- Clegg, D.J.; Brown, L.M.; Zigman, J.M.; Kemp, C.J.; Strader, A.D.; Benoit, S.C.; Woods, S.C.; Mangiaracina, M.; Geary, N. Estradiol-Dependent Decrease in the Orexigenic Potency of Ghrelin in Female Rats. Diabetes 2007, 56, 1051–1058. [Google Scholar] [CrossRef]
- Gualillo, O.; Caminos, J.; Kojima, M.; Kangawa, K.; Arvat, E.; Ghigo, E.; Casanueva, F.F.; Dieguez, C. Gender and gonadal influences on ghrelin mRNA levels in rat stomach. Eur. J. Endocrinol. 2001, 144, 687–690. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vins, N.; Sugathan, S.; Al Menhali, A.; Karam, S.M. Overgrowth of Squamocolumnar Junction and Dysregulation of Stem Cell Lineages in the Stomach of Vitamin A-Deficient Mice. Nutrients 2022, 14, 3334. https://doi.org/10.3390/nu14163334
Vins N, Sugathan S, Al Menhali A, Karam SM. Overgrowth of Squamocolumnar Junction and Dysregulation of Stem Cell Lineages in the Stomach of Vitamin A-Deficient Mice. Nutrients. 2022; 14(16):3334. https://doi.org/10.3390/nu14163334
Chicago/Turabian StyleVins, Neethu, Subi Sugathan, Asma Al Menhali, and Sherif M. Karam. 2022. "Overgrowth of Squamocolumnar Junction and Dysregulation of Stem Cell Lineages in the Stomach of Vitamin A-Deficient Mice" Nutrients 14, no. 16: 3334. https://doi.org/10.3390/nu14163334
APA StyleVins, N., Sugathan, S., Al Menhali, A., & Karam, S. M. (2022). Overgrowth of Squamocolumnar Junction and Dysregulation of Stem Cell Lineages in the Stomach of Vitamin A-Deficient Mice. Nutrients, 14(16), 3334. https://doi.org/10.3390/nu14163334






