The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study
Highlights
- Whole grain intake is associated with a marginally increased risk of total mortality among breast cancer survivors.
- Among breast cancer survivors with high intakes of refined grains, higher intakes of whole grains were linked to an increased risk of mortality compared to lower intakes of whole grains.
- Whole grains are widely recognized as healthy carbohydrates in dietary guidelines. However, the optimal number of whole grains for consumption may vary depending on individual characteristics, a consideration that current dietary guidelines might not fully address.
- High intakes of whole grains could be challenging for people with weakened digestive systems, such as breast cancer survivors, potentially leading to discomfort or other adverse effects. More mechanistic studies are needed to confirm these findings.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Total Dietary Intakes, Whole Grain, and Refined Grain Intakes
2.3. Assessment of Mortality
2.4. Assessment of Demographic Factors, Lifestyle Habits, and Treatment Regimens
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
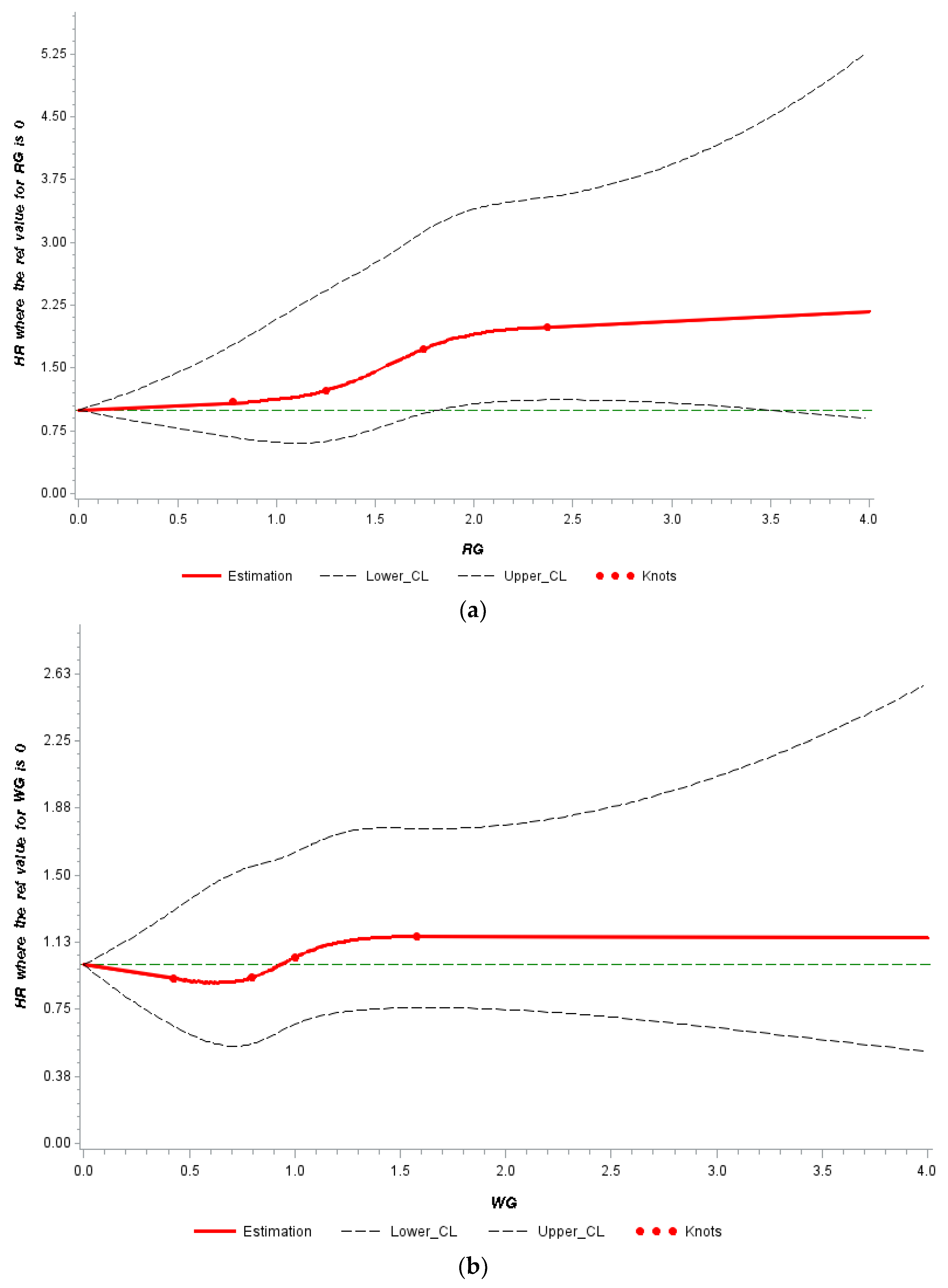
3.2. Independent Associations of Whole Grain and Refined Grain with Mortality
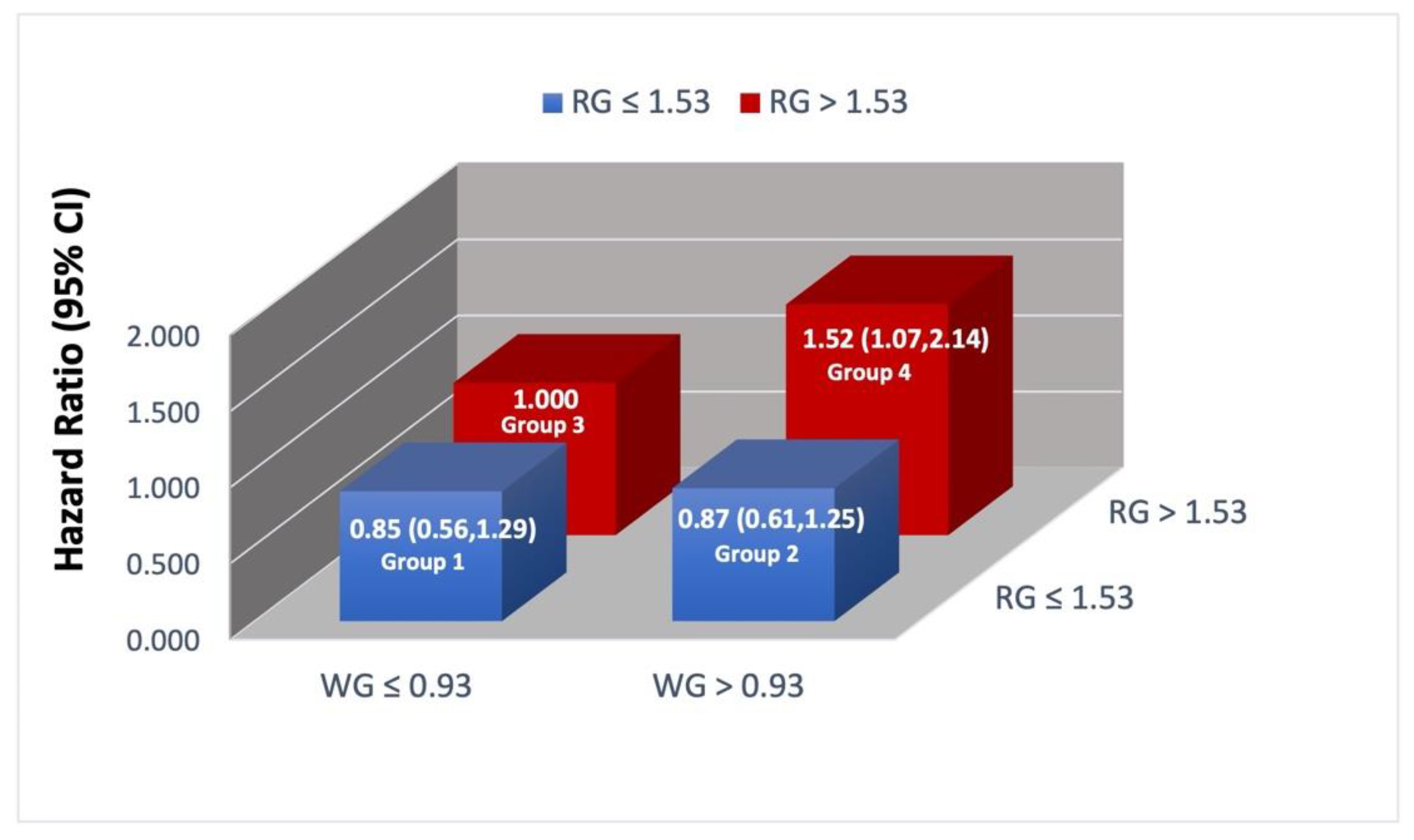
3.3. Joint Associations of Whole Grain and Refined Grain with Total Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Health NIo. Cancer Statistics. 2020. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 15 October 2021).
- American Cancer Society. Survival Rates for Breast Cancer. 2021. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html (accessed on 15 October 2021).
- American Cancer Society. About Breast Cancer: American Cancer Society. 2021. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 15 October 2021).
- American Cancer Society. Breast Cancer Facts & Figures: American Cancer Society. 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html# (accessed on 12 April 2022).
- O’Reilly, M.; Mellotte, G.; Ryan, B.; O’Connor, A. Gastrointestinal side effects of cancer treatments. Ther. Adv. Chronic. Dis. 2020, 11, 2040622320970354. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary Guidelines for Breast Cancer Patients: A Critical Review. Adv. Nutr. 2017, 8, 613–623. [Google Scholar] [CrossRef]
- Clinic, C. The Best Foods to Eat When You Have Breast Cancer Cleveland Clinic—Cancer Care: Cleveland Clinic. 2019. Available online: https://health.clevelandclinic.org/the-best-foods-to-eat-when-you-have-breast-cancer/ (accessed on 21 November 2021).
- Xiao, Y.; Ke, Y.; Wu, S.; Huang, S.; Li, S.; Lv, Z.; Yeoh, E.-K.; Lao, X.; Wong, S.; Kim, J.H. Association between whole grain intake and breast cancer risk: A systematic review and meta-analysis of observational studies. Nutr. J. 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- NIH. 2020–2030 Strategic Plan for NIH Nutrition Research. 2020. Available online: https://dpcpsi.nih.gov/sites/default/files/2020NutritionStrategicPlan_508.pdf (accessed on 4 August 2022).
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Rock, C.L.; Newman, V.; Flatt, S.W.; Kealey, S.; Jones, V.E.; Caan, B.J.; Gold, E.B.; et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women’s Healthy Eating and Living (WHEL) Study. Control Clin. Trials 2002, 23, 728–756. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.; Ottesen, R. Survival 101—Just Learning to Survive. PharmaSUG 2017—Paper HT03. 2017. Available online: https://www.pharmasug.org/proceedings/2017/HT/PharmaSUG-2017-HT03.pdf (accessed on 15 March 2022).
- Introduction to Survival Analysis in SAS UCLA Advanced Research Computing, Statistical Methods and Data Analytics University of California Los Angeles. Available online: https://stats.oarc.ucla.edu/sas/seminars/sas-survival/ (accessed on 15 March 2022).
- Powell, T.; Bagnell, M. Your “Survival” Guide to Using Time-Dependent Covariates; Deployment Health Research Department: San Diego, CA, USA, 2016. [Google Scholar]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Custódio, I.D.; Marinho, E.a.C.; Gontijo, C.A.; Pereira, T.S.; Paiva, C.E.; Maia, Y.C. Impact of Chemotherapy on Diet and Nutritional Status of Women with Breast Cancer: A Prospective Study. PLoS ONE 2016, 11, e0157113. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef]
- Wu, H.; Flint, A.J.; Qi, Q.; van Dam, R.M.; Sampson, L.A.; Rimm, E.B.; Holmes, M.D.; Willett, W.C.; Hu, F.B.; Sun, Q. Association between dietary whole grain intake and risk of mortality: Two large prospective studies in US men and women. JAMA Intern. Med. 2015, 175, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Dilsiz, S.A.; Başaran, N.; Başaran, A.A. Effects of phytochemicals against diabetes. Adv. Food Nutr. Res. 2019, 89, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Kritchevsky, D. Phytochemicals and cardiovascular disease. A statement for healthcare professionals from the American Heart Association. Circulation 1997, 95, 2591–2593. [Google Scholar] [CrossRef] [PubMed]
- Benisi-Kohansal, S.; Saneei, P.; Salehi-Marzijarani, M.; Larijani, B.; Esmaillzadeh, A. Whole-Grain Intake and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2016, 7, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.I.; Riethoven, J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr. J. 2018, 17, 72. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, Q.; Feng, J.; Du, L.; Li, K.; Zhou, Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine 2018, 97, e12995. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.W.; Baydoun, M.; Piedalue, K.A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef]
- Ruo, S.W.; Alkayyali, T.; Win, M.; Tara, A.; Joseph, C.; Kannan, A.; Srivastava, K.; Ochuba, O.; Sandhu, J.K.; Went, T.R.; et al. Role of Gut Microbiota Dysbiosis in Breast Cancer and Novel Approaches in Prevention, Diagnosis, and Treatment. Cureus 2021, 13, e17472. [Google Scholar] [CrossRef]
- Hidalgo, D.; Nukavarapu, M.; Smith, A.; Chaudhari, D.; Young, M. Breast Cancer as a Cause of Intestinal Obstruction. Am. J. Gastroenterol. 2018, 113, S959. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e5. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020.e4. [Google Scholar] [CrossRef] [PubMed]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J.D. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef] [PubMed]
- Tabariès, S.; McNulty, A.; Ouellet, V.; Annis, M.G.; Dessureault, M.; Vinette, M.; Hachem, Y.; Lavoie, B.; Omeroglu, A.; Simon, H.G.; et al. Afadin cooperates with Claudin-2 to promote breast cancer metastasis. Genes Dev. 2019, 33, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y. Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma. Hum. Pathol. 2004, 35, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Andersen, L.F.; Blomhoff, R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2007, 85, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef]
- Swaminathan, S.; Dehghan, M.; Raj, J.M.; Thomas, T.; Rangarajan, S.; Jenkins, D.; Mony, P.; Mohan, V.; Lear, S.A.; Avezum, A.; et al. Associations of cereal grains intake with cardiovascular disease and mortality across 21 countries in Prospective Urban and Rural Epidemiology study: Prospective cohort study. BMJ 2021, 372, m4948. [Google Scholar] [CrossRef]
- Gaesser, G. Perspective: Refined Grains and Health: Genuine Risk, or Guilt by Association? Adv. Nutr. 2019, 10, 361–371. [Google Scholar] [CrossRef]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control 2016, 27, 1303–1314. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality: A prospective study of over half a million people. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef]
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef]
- Sergi, C.; Villanacci, V.; Carroccio, A. Non-celiac wheat sensitivity: Rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: A review. BMC Gastroenterol. 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
| Overall | Mortality Status | p-Value | ||
|---|---|---|---|---|
| No (n = 2767) | Yes (n = 314) | |||
| Age at diagnosis (years) | 50.7 ± 8.85 | 50.66 ± 8.7 | 51.45 ± 10.08 | 0.13 |
| Age at randomization (years) | 52.7 ± 8.97 | 52.63 ± 8.81 | 53.20 ± 10.29 | 0.30 |
| BMI (kg/m2) | 27.25 ± 6.07 | 27.16 ± 6.01 | 28.06 ± 6.52 | 0.01 |
| Physical activity (Mets) | 839.04 ± 878.83 | 857.30 ± 891.21 | 678.16 ± 743.15 | 0.0007 |
| Total calorie intake (kcal) | 1717.64 ± 407.59 | 1719.39 ± 403.81 | 1702.22 ± 439.87 | 0.48 |
| Randomization group (N, %) | 0.59 | |||
| Intervention | 1534 (49.79) | 1379 (49.84) | 155 (49.36) | |
| Comparison | 1547 (50.21) | 1388 (50.16) | 159 (50.64) | |
| Chemotherapy (N, %) | 0.02 | |||
| Yes | 2154 (69.91) | 1909 (68.99) | 245 (78.03) | |
| No | 925 (30.02) | 856 (30.94) | 69 (21.97) | |
| Radiation therapy (N, %) | 0.72 | |||
| Yes | 1894 (61.47) | 1701 (61.47) | 193 (61.46) | |
| No | 1183 (38.40) | 1062 (38.38) | 121 (38.54) | |
| Hormone status (N, %) | 0.0005 | |||
| ER+/PR+ | 1905 (61.83) | 1742 (62.96) | 163 (51.91) | |
| ER+/PR- | 493 (16.00) | 432 (15.61) | 61 (19.43) | |
| ER-/PR+ | 618 (20.06) | 532 (19.23) | 86 (27.39) | |
| ER-/PR- | 65 (2.11) | 61 (2.20) | 4 (1.27) | |
| Ethnicity (N, %) | 0.32 | |||
| White | 2627 (85.3) | 2368 (85.58) | 259 (82.48) | |
| Non-white | 454 (14.7) | 399 (14.42) | 55 (17.52) | |
| Education (N, %) | 0.0197 | |||
| High school or less | 377 (12.24) | 329 (11.89) | 48 (15.29) | |
| Post high school to college | 1912 (62.06) | 1715 (61.98) | 197 (62.74) | |
| Post college | 792 (25.71) | 723 (26.13) | 69 (21.97) | |
| Employment status (N, %) | 0.20 | |||
| Employed | 2210 (71.73) | 2003 (72.39) | 105 (33.44) | |
| Unemployed | 853 (27.69) | 748 (27.03) | 207 (65.92) | |
| Marital status (N, %) | 0.0417 | |||
| Single | 338 (10.97) | 299 (10.81) | 39 (12.42) | |
| Married | 2154 (69.91) | 1945 (70.29) | 209 (66.56) | |
| Other | 589 (19.12) | 523 (18.90) | 66 (21.02) | |
| Whole Grains (Servings/Day) | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Value | |
| Refined Grains (servings/day) | 2.13 ± 1.08 | 2.05 ± 1.04 | 1.80 ± 1.19 | 1.7 ± 1.02 | <0.0001 |
| Age at diagnosis (years) | 50 ± 8.59 | 50 ± 8.87 | 51 ± 9.05 | 50 ± 8.89 | 0.31 |
| BMI | 26.62 ± 6.45 | 25.95 ± 6.06 | 26.07 ± 5.69 | 24.97 ± 6.01 | 0.0002 |
| Physical activity (METs) | 450 ± 876.08 | 555 ± 880.49 | 600 ± 803.98 | 750 ± 940.95 | <0.0001 |
| Total calorie intake (kcal) | 1597 ± 424.69 | 1631 ± 380.67 | 1687 ± 382.54 | 1816 ± 403.72 | <0.0001 |
| Smoking status (N, %) | 0.0013 | ||||
| Never | 421 (25.66) | 409 (24.92) | 427 (26.02) | 384 (23.40) | |
| Former | 329 (25.84) | 299 (23.49) | 315 (24.74) | 330 (25.92) | |
| Current | 52 (37.96) | 41 (29.93) | 27 (19.71) | 17 (12.41) | |
| Chemotherapy (N, %) | 0.14 | ||||
| No | 223 (24.11) | 233 (25.19) | 236 (25.51) | 233(25.19) | |
| Yes | 586 (27.21) | 524 (24.33) | 544 (25.26) | 500 (23.21) | |
| Cancer stage (N, %) | 0.46 | ||||
| Stage I | 309 (26.01) | 299 (25.17) | 294 (24.75) | 286 (24.07) | |
| Stage II | 467 (26.85) | 423 (24.32) | 449 (25.82) | 400 (23.00) | |
| Stage III | 33 (21.43) | 37 (24.03) | 37 (24.03) | 47 (30.52) | |
| Hormone status (N, %) | 0.78 | ||||
| ER+/PR+ | 497 (26.09) | 472 (24.78) | 472 (24.78) | 464 (24.36) | |
| ER+/PR− | 132 (26.77) | 111 (22.52) | 125 (25.35) | 125 (25.35) | |
| ER−/PR+ | 163 (26.38) | 158 (25.57) | 168 (27.18) | 129 (20.87) | |
| ER−/PR− | 17 (26.15) | 18 (27.69) | 15 (23.08) | 15 (23.08) | |
| Total Mortality | Breast-Cancer-Specific Mortality | ||||
|---|---|---|---|---|---|
| Event | HR (95% CI) | Event | HR (95% CI) | ||
| Whole Grains (servings/day) | Range | ||||
| Quartile 1 | ≤0.5 | 142 | Ref | 102 | Ref |
| Quartile 2 | 0.6–0.98 | 140 | 1.06 (0.74, 1.58) | 110 | 0.99 (0.93, 1.06) |
| Quartile 3 | 0.99–1.56 | 146 | 1.30 (0.91, 1.86) | 119 | 1.03 (0.96, 1.10) |
| Quartile 4 | ≥1.57 | 130 | 1.36 (0.94, 1.97) | 105 | 1.02 (0.95, 1.09) |
| P for trend | 0.07 | 0.55 | |||
| Refined Grains (servings/day) | Range | ||||
| Quartile 1 | ≤0.91 | 98 | Ref | 70 | Ref |
| Quartile 2 | 0.92–1.53 | 141 | 1.18 (0.81, 1.71) | 113 | 1.07 (1.00, 1.15) |
| Quartile 3 | 1.54–2.31 | 142 | 1.40 (0.95, 2.06) | 107 | 1.16 (1.08, 1.25) |
| Quartile 4 | ≥2.32 | 177 | 1.74 (1.17, 2.59) | 146 | 1.16 (1.08, 1.26) |
| P for trend | 0.0047 | <0.0001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernest, D.K.; Lemus, H.; Hsu, F.-C.; Pierce, J.P.; Wu, T. The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients 2022, 14, 3333. https://doi.org/10.3390/nu14163333
Ernest DK, Lemus H, Hsu F-C, Pierce JP, Wu T. The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients. 2022; 14(16):3333. https://doi.org/10.3390/nu14163333
Chicago/Turabian StyleErnest, Deepali Karina, Hector Lemus, Fang-Chi Hsu, John P. Pierce, and Tianying Wu. 2022. "The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study" Nutrients 14, no. 16: 3333. https://doi.org/10.3390/nu14163333
APA StyleErnest, D. K., Lemus, H., Hsu, F.-C., Pierce, J. P., & Wu, T. (2022). The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients, 14(16), 3333. https://doi.org/10.3390/nu14163333







