The Pathogenetic Role of Melatonin in Migraine and Its Theoretic Implications for Pharmacotherapy: A Brief Overview of the Research
Abstract
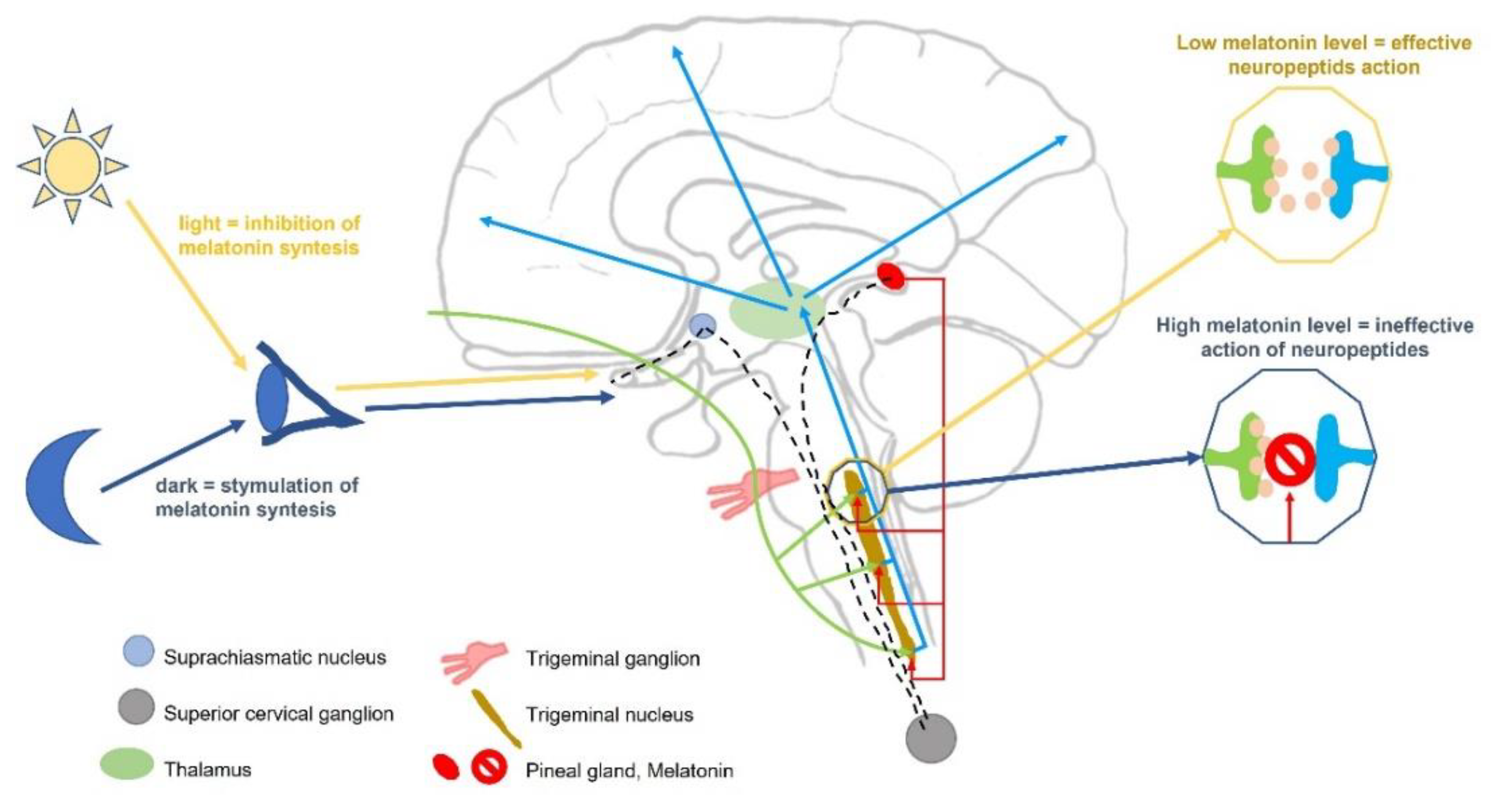
1. Introduction
2. Migraine and Melatonin—Nutrition and Health Interactions
3. Determinations of Melatonin Level in Episodic Migraine
4. Determinations of Melatonin Level in Chronic Migraine
5. Melatonin in the Treatment of Migraine
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and systems of care. Lancet 2021, 17, 1485–1495. [Google Scholar] [CrossRef]
- de Tommaso, M.; Ambrosini, A.; Brighina, F.; Coppola, G.; Perrotta, A.; Pierelli, F.; Sandrini, G.; Valeriani, M.; Marinazzo, D.; Stramaglia, S.; et al. Altered processing of sensory stimuli in patients with migraine. Nat. Rev. Neurol. 2014, 10, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R. An update: Pathophysiology of migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef]
- Karsan, N.; Goadsby, P.J. Biological insights from the premonitory symptoms of migraine. Nat. Rev. Neurol. 2018, 14, 699–710. [Google Scholar] [CrossRef]
- Peng, K.P.; May, A. Redefining migraine phases—a suggestion based on clinical, physiological, and functional imaging evidence. Cephalalgia 2020, 40, 866–870. [Google Scholar] [CrossRef]
- Skorobogatykh, K.; van Hoogstraten, W.S.; Degan, D.; Prischepa, A.; Savitskaya, A.; Ileen, B.M.; Bentivegna, E.; Skiba, I.; D’Acunto, L.; Ferri, L.; et al. Functional connectivity studies in migraine: What have we learned? J. Headache Pain 2019, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.P.; Westerfield, M.; Lopes, M.; Maruta, C.; Gil-da-Costa, R. Brain state monitoring for the future prediction of migraine attacks. Cephalalgia 2020, 40, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rahmani, B.; Gandhi, J.; Seyam, O.; Joshi, G.; Reid, I.; Smith, N.L.; Waltzer, W.C.; Khan, S.A. Revisiting the pineal gland: A review of calcification, masses, precocious puberty, and melatonin functions. Int. J. Neurosci. 2020, 130, 464–475. [Google Scholar] [CrossRef]
- Long, R.; Zhu, Y.; Zhou, S. Therapeutic role of melatonin in migraine prophylaxis: A systematic review. Medicine 2019, 98, e14099. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.P. Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia 2005, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kurganova, Y.M.; Danilov, A.B. Melatonin in Chronic Pain Syndromes. Neurosci. Behav. Physiol. 2017, 47, 806–812. [Google Scholar] [CrossRef]
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev. Neurol. (Paris) 2020, 176, 148–165. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s impact on antioxidative and anti-Inflammatory reprogramming in homeostasis and disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Raghavendra, V.; Singh, V.; Kulkarni, S.K.; Agrewala, J.N. Melatonin enhances Th2 cell mediated immune responses: Lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol. Cell. Biochem. 2001, 221, 57–62. [Google Scholar] [CrossRef]
- Prusiński, A.; Rożniecki, J. Triptans in migraine-here and now (15 years from the introduction to therapy). Neurol. Neurochir. Polska 2005, 39, 68–77. [Google Scholar]
- Noghani, M.T.; Namdar, H. Migraine associated with gastrointestinal disorders: A pathophysiological explanation. Med. Hypotheses 2019, 1, 90–93. [Google Scholar] [CrossRef]
- Vogler, B.; Rapoport, A.M.; Tepper, S.J.; Sheftell, F.; Bigal, M.E. Role of melatonin in the pathophysiology of migraine. Implications and treatment. CNS Drugs 2006, 20, 343–350. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Tan, D.-X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 13, 15. [Google Scholar] [CrossRef]
- Dodick, D.W.; Eross, E.J.; Parish, J.M. Clinical, anatomical, and physiological relationship between sleep and headache. Headache 2003, 43, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.P.; Masruha, M.R.; Zukerman, E.; Moreira-Filho, C.A.; Cavalheiro, E.A. Potential therapeutic use of melatonin in migraine and other headache disorders. Expert. Opin. Investig. Drugs 2006, 15, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Chuchuen, U.; Ebadi, M.; Govitrapong, P. The stimulatory effect of mu- and delta-opioid receptors on bovine pinealocyte melatonin synthesis. J. Pineal Res. 2004, 37, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, M.; Amirain, I.; Reiter, R.J.; Rosenberg, J.; Gogennur, I. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 2011, 51, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.D. Retiono-hypothalamic-pineal hypothesis in the pathophysiology of primary headaches. Med. Hypotheses 2006, 66, 1146–1151. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in Humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain 2013, 14, 62. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and headache: Part 2. Headache 2016, 56, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Migraine and diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef] [PubMed]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The role of diet and nutrition in migraine triggers and treatment: A systematic literature review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef] [PubMed]
- Boutrid, N.; Rahmoune, H. “3M”: Migraine, Microbiota and Melatonin. Med. Hypotheses 2019, 127, 90. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, S.; Breedveld, A.C.; Rovers, J.M.; Vermeiden, J.P.; Witteman, B.J.; Smits, M.G.; de Roos, N.M. Migraine associated with gastrointestinal disorders: Review of the literature and clinical implications. Front. Neurol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Hindiyeh, N.; Aurora, S.K. What the gut can teach us about migraine. Curr. Pain Headache Rep. 2015, 19, 33. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Yang, C.-P.; Su, K.-P.; Chen, T.-Y.; Wu, Y.-C.; Tu, Y.-K.; Lin, P.-Y.; Stubbs, B.; Carvalho, A.F.; Matsuoka, Y.J.; et al. The association between melatonin and episodic migraine: A pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J. Pineal Res. 2020, 69, e12663. [Google Scholar] [CrossRef]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Lipton, R.B.; Silberstein, S.D. Episodic and chronic migraine headache: Breaking down barriers to optimal treatment and prevention. Headache 2015, 55, 103–122. [Google Scholar] [CrossRef]
- Claustrat, B.; Loisy, C.; Brun, J.; Beorchia, S.; Arnaud, J.L.; Chazot, G. Nocturnal plasma melatonin levels in migraine: A preliminary report. Headache 1989, 29, 242–245. [Google Scholar] [CrossRef]
- Masruha, M.R.; De Souza Vieira, D.S.; Minett, T.S.C.; Cipolla-Neto, J.; Zukerman, E.; Vilanova, L.C.P.; Peres, M.F.P. Low urinary 6- sulphatoxymelatonin concentrations in acute migraine. J. Headache Pain 2008, 9, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, A.; Cegielska, J.; Zduński, S.; Białobrzewski, M.; Kochanowski, J. Variability in melatonin concentration in blood serum of patients with episodic migraine: A pilot study. Neurol. Neurochir. Polska 2021, 55, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kozak, H.H.; Boysan, M.; Uca, A.U.; Aydın, A.; Kılınç, I.; Genç, E.; Altaş, M.; Güngör, D.C.; Turgut, K.; Özer, N. Sleep quality, morningness-eveningness preference, mood profile, and levels of serum melatonin in migraine patients: A case-control study. Acta Neurol. Belg. 2017, 117, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Claustrat, B.; Saddier, P.; Chazot, G. Nocturnal melatonin excretion is decreased in patients with migraine without aura attacks associated with menses. Cephalalgia 1995, 15, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Murialdo, G.; Fonzi, S.; Costelli, P.; Solinas, G.P.; Parodi, C.; Marabini, S.; Fanciullacci, M.; Polleri, A. Urinary melatonin excretion throughout the ovarian cycle in menstrually related migraine. Cephalalgia 1994, 14, 205–209. [Google Scholar] [CrossRef]
- Peres, M.F.P.; Sanchez del Rio, M.; Seabra, M.L.; Tufik, S.; Abucham, J.; Cipolla-Neto, J.; Silberstein, S.D.; Zukerman, E. Hypothalamic involvement in chronic migraine. J. Neurol. Neurosurg. Psychiatry 2001, 71, 747–751. [Google Scholar] [CrossRef]
- Bruera, O.; Sances, G.; Leston, J.; Levin, G.; Cristina, S.; Medina, C.; Barontini, M.; Nappi, G.; Figuerola, M.A.A. Plasma melatonin pattern in chronic and episodic headaches. Evaluation during sleep and waking. Funct. Neurol. 2008, 23, 77–81. [Google Scholar]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Ebrahimi-Monfared, M.; Sharafkhah, M.; Abdolrazaghnejad, A.; Mohammadbeigi, A.; Faraji, F. Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor. Neurol. Neurosci. 2017, 35, 385–393. [Google Scholar] [CrossRef]
- Ali, A.A.; Al-Tamimi, J.J. Clinical efficacy of melatonin for prevention of common migraine headache in comparison with propranolol. Karbala J. Pharm. Sci. 2014, 7, 131–139. [Google Scholar]
- Gonçalves, A.L.; Martini Ferreira, A.; Ribeiro, R.T.; Zukerman, E.; Cipolla-Neto, J.; Peres, M.F. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Spantideas, N.; Lyras, V.; Avramidis, T.; Thomaidis, T. Melatonin 4 mg as prophylactic therapy for primary headaches: A pilot study. Funct. Neurol. 2016, 31, 33–37. [Google Scholar] [CrossRef]
- Alstadhaug, K.B.; Odeh, F.; Salvesen, R.; Bekkelund, S.I. Prophylaxis of migraine with melatonin. A randomized controlled trial. Neurology 2010, 75, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.P.; Zukerman, E.; Da Cunha Tanuri, F.; Moreira, F.R.; Cipolla-Neto, J. Melatonin, 3 mg, is effective for migraine prevention. Neurology 2004, 63, 757. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.L.; Latorraca, C.O.C.; Costa, A.A.L.F.; Martimbianco, A.L.C.; Pachito, D.V.; Riera, R. Melatonin for preventing primary headache: A systematic review. Int. J. Clin. Pract. 2018, 72, e13203. [Google Scholar] [CrossRef]
- Guglielmo, R.; Martinotti, G.; Di Giannantonio, M.; Janiri, L. A possible new option for migraine management: Agomelatine. Clin. Neuropharmacol. 2013, 36, 65–67. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lai, C.-H. The Relief Effects of Ramelteon on Refractory Chronic Migraine: A Case Report. Clin. Psychopharmacol. Neurosci. 2016, 14, 405–406. [Google Scholar] [CrossRef][Green Version]
- Lavedan, C.; Forsberg, M.; Gentile, A.J. Tasimelteon: A selective and unique receptor binding profile. Neuropharmacology 2015, 91, 142–147. [Google Scholar] [CrossRef]
- Alstadhaug, K.B. Migraine and hypothalamus. Cephalalgia 2009, 29, 809–817. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and patophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef]
- Liampas, I.; Siokas, V.; Brotis, A.; Vikelis, M. Endogenous melatonin levels and therapeutic use of exogenous melatonin in migraine: Systematic review and meta-analysis. J. Headache 2020, 60, 1273–1299. [Google Scholar] [CrossRef] [PubMed]
| Reference | Method of Study | Patients No | Diagnosis | Melatonin Test Method | Outcome |
|---|---|---|---|---|---|
| Claustrat et al., 1989 [40] | case control | 93 + 46 | migraine + control | serum melatonin RIA | Lowering the plasma melatonin level in the entire migraine population compared to the control group |
| Masruha et al., 2008 [41] | case control | 146 + 74 | migraine + control | urine aMT6s ELISA | Urinary aMT6s concentration in patients with migraine attack during sample collection was significantly lower than in migraine patients without headache and in the control group. There was no significant difference in the urinary aMT6s concentration in migraine patients without pain on the day of their urine samples collection and in controls |
| Zduńska et al., 2021 [42] | case control | 29 + 29 | migraine + control | serum melatonin RIA | No statistically significant differences between melatonin concentrations in patients with episodic migraine in the interictal period and control group |
| Kozak et al., 2016 [43] | case control | 55 + 57 | migraine + control | serum melatonin ELISA | The level of melatonin was significantly lower in the migraine patients than in control |
| Brun et al., 1995 [44] | case control | 10 + 9 | menstrual related migraine + control | urine melatonin RIA | The mean nocturnal melatonin excretion throughout the cycle was significantly lower in women with migraine than in the control group. In the control group, the excretion of melatonin increased significantly from the follicular to luteal phase, while in migraine patients, this phenomenon was not observed |
| Murialdo et al., 1994 [45] | case control | 12 + 8 | menstrual related migraine + control | urine melatonin RIA | Nocturnal urinary melatonin excretion was significantly lower in migraine patients than in the control group. The increase in urinary melatonin excretion during the luteal phase was less marked in migraine patients. The decrease in melatonin excretion increased even more during the headache |
| Peres et al., 2001 [46] | case control | 17 + 9 | chronic migraine + control | serum melatonin RIA | Delayed nocturnal peak of melatonin level in chronic migraine patients as well lower melatonin levels in chronic migraine patients with insomnia |
| Bruera et al., 2008 [47] | case control | 30 + 10 | various idiopathic headaches + control; during NREM and REM sleep as well waking up | serum melatonin RIA | Significantly lower levels of melatonin after waking up and no characteristic increase of REM sleep, compared to the control group, were found only in patients with chronic migraine and chronic tension headache |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zduńska, A.; Cegielska, J.; Domitrz, I. The Pathogenetic Role of Melatonin in Migraine and Its Theoretic Implications for Pharmacotherapy: A Brief Overview of the Research. Nutrients 2022, 14, 3335. https://doi.org/10.3390/nu14163335
Zduńska A, Cegielska J, Domitrz I. The Pathogenetic Role of Melatonin in Migraine and Its Theoretic Implications for Pharmacotherapy: A Brief Overview of the Research. Nutrients. 2022; 14(16):3335. https://doi.org/10.3390/nu14163335
Chicago/Turabian StyleZduńska, Anna, Joanna Cegielska, and Izabela Domitrz. 2022. "The Pathogenetic Role of Melatonin in Migraine and Its Theoretic Implications for Pharmacotherapy: A Brief Overview of the Research" Nutrients 14, no. 16: 3335. https://doi.org/10.3390/nu14163335
APA StyleZduńska, A., Cegielska, J., & Domitrz, I. (2022). The Pathogenetic Role of Melatonin in Migraine and Its Theoretic Implications for Pharmacotherapy: A Brief Overview of the Research. Nutrients, 14(16), 3335. https://doi.org/10.3390/nu14163335






