Diet and BMI Correlate with Metabolite Patterns Associated with Aggressive Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.3. Diet, BMI, and Covariate Data
2.4. Statistical Analysis
2.4.1. Participant Characteristics
2.4.2. Normalization of Metabolite Concentrations
2.4.3. Metabolite Patterns
2.4.4. Correlates of Metabolites
2.4.5. Individual Metabolite Analysis
3. Results
3.1. Participant Characteristics
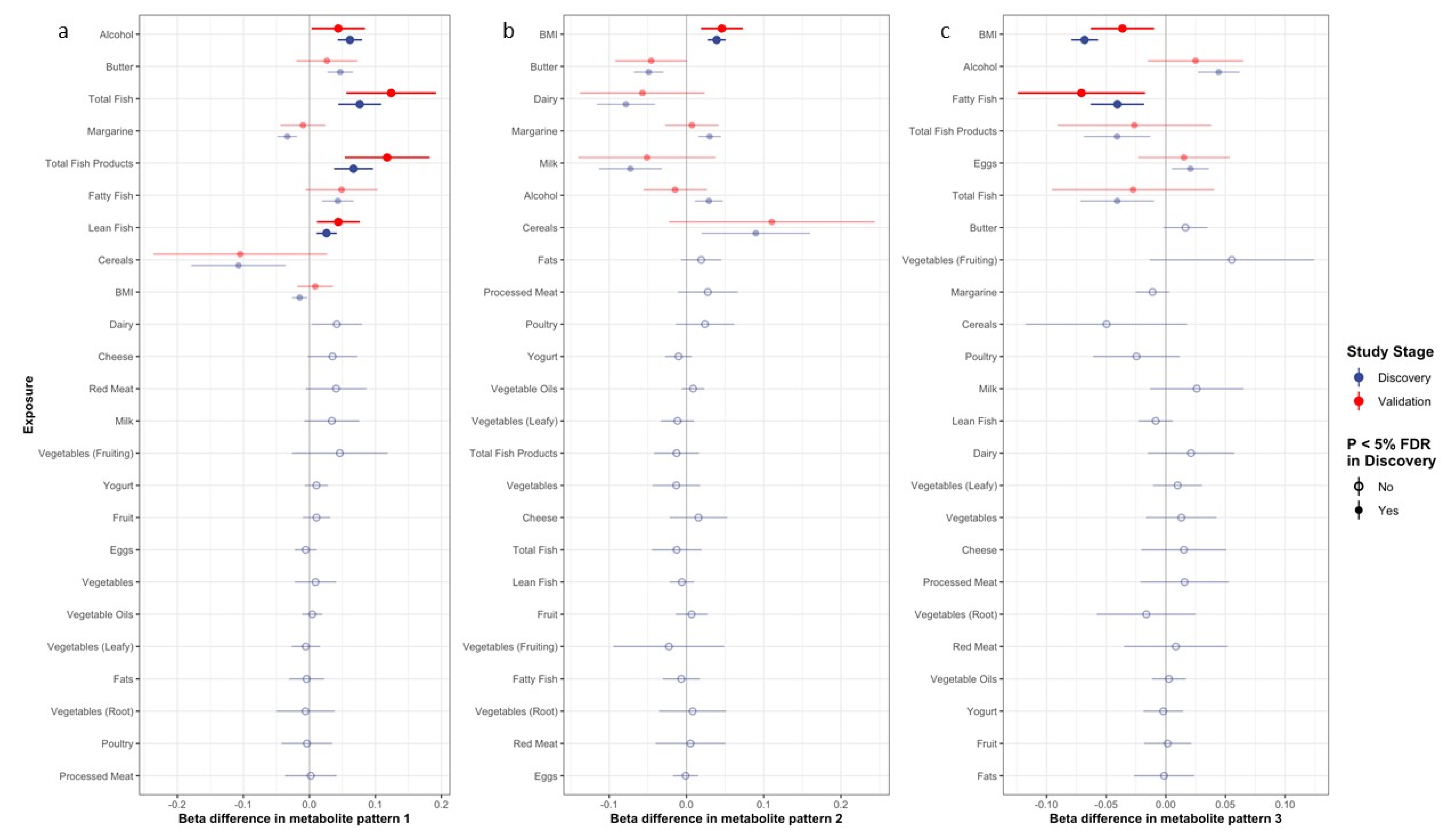
3.2. Correlates of Metabolite Patterns
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
IARC Disclaimer
References
- Schmidt, J.A.; Fensom, G.K.; Rinaldi, S.; Scalbert, A.; Appleby, P.N.; Achaintre, D.; Gicquiau, A.; Gunter, M.J.; Ferrari, P.; Kaaks, R.; et al. Patterns in Metabolite Profile Are Associated with Risk of More Aggressive Prostate Cancer: A Prospective Study of 3057 Matched Case–Control Sets from EPIC. Int. J. Cancer 2020, 146, 720–730. [Google Scholar] [CrossRef]
- Fages, A.; Duarte-Salles, T.; Stepien, M.; Ferrari, P.; Fedirko, V.; Pontoizeau, C.; Trichopoulou, A.; Aleksandrova, K.; Tjønneland, A.; Olsen, A.; et al. Metabolomic Profiles of Hepatocellular Carcinoma in a European Prospective Cohort. BMC Med. 2015, 13, 242. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Prospective Serum Metabolomic Profile of Prostate Cancer by Size and Extent of Primary Tumor. Oncotarget 2017, 8, 45190–45199. [Google Scholar] [CrossRef] [PubMed]
- Röhnisch, H.E.; Kyrø, C.; Olsen, A.; Thysell, E.; Hallmans, G.; Moazzami, A.A. Identification of Metabolites Associated with Prostate Cancer Risk: A Nested Case-Control Study with Long Follow-up in the Northern Sweden Health and Disease Study. BMC Med. 2020, 18, 187. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The Food Metabolome: A Window over Dietary Exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Fiehn, O.; Hankemeier, T.; Kristal, B.S.; van Ommen, B.; Pujos-Guillot, E.; Verheij, E.; Wishart, D.; Wopereis, S. Mass-Spectrometry-Based Metabolomics: Limitations and Recommendations for Future Progress with Particular Focus on Nutrition Research. Metabolomics 2009, 5, 435–458. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study Populations and Data Collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kliemann, N.; Viallon, V.; Murphy, N.; Beeken, R.J.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; van Roekel, E.H.; Schmidt, J.A.; Borch, K.B.; et al. Metabolic Signatures of Greater Body Size and Their Associations with Risk of Colorectal and Endometrial Cancers in the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2021, 19, 101. [Google Scholar] [CrossRef]
- Guida, F.; Tan, V.Y.; Corbin, L.J.; Smith-Byrne, K.; Alcala, K.; Langenberg, C.; Stewart, I.D.; Butterworth, A.S.; Surendran, P.; Achaintre, D.; et al. The Blood Metabolome of Incident Kidney Cancer: A Case-Control Study Nested within the MetKid Consortium. PLoS Med. 2021, 18, e1003786. [Google Scholar] [CrossRef]
- Stepien, M.; Duarte-Salles, T.; Fedirko, V.; Floegel, A.; Barupal, D.K.; Rinaldi, S.; Achaintre, D.; Assi, N.; Tjønneland, A.; Overvad, K.; et al. Alteration of Amino Acid and Biogenic Amine Metabolism in Hepatobiliary Cancers: Findings from a Prospective Cohort Study: Circulating Biomarkers of Hepatobiliary Cancer Risks. Int. J. Cancer 2016, 138, 348–360. [Google Scholar] [CrossRef]
- Guida, F.; Severi, G.; Giles, G.G.; Johansson, M. Metabolomics and Risk of Kidney Cancer. Rev. D’épidémiologie Et De St. Publique 2018, 66, S291. [Google Scholar] [CrossRef]
- Viallon, V.; His, M.; Rinaldi, S.; Breeur, M.; Gicquiau, A.; Hemon, B.; Overvad, K.; Tjønneland, A.; Rostgaard-Hansen, A.L.; Rothwell, J.A.; et al. A New Pipeline for the Normalization and Pooling of Metabolomics Data. Metabolites 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, H.; Perez-Cornago, A.; Casagrande, C.; Nicolas, G.; Versele, V.; Skeie, G.; Schulze, M.B.; Johansson, I.; María Huerta, J.; Oliverio, A.; et al. Comparing Calculated Nutrient Intakes Using Different Food Composition Databases: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Nutrients 2020, 12, 2906. [Google Scholar] [CrossRef] [PubMed]
- Great Britain Ministry of Agriculture, Fisheries, and Food. Food Portion Sizes, 2nd ed.; HMSO: London, UK, 1993. [Google Scholar]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Olsen, A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; et al. Consumption of Dairy Products and Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2013, 8, e72715. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Krogh, V.; Berrino, F.; Sieri, S.; Grioni, S.; Tjønneland, A.; Olsen, A.; Jakobsen, M.U.; Overvad, K.; Clavel-Chapelon, F.; et al. Meat, Eggs, Dairy Products, and Risk of Breast Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Am. J. Clin. Nutr. 2009, 90, 602–612. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Castañeda, J.; Rinaldi, S.; Cayssials, V.; Slimani, N.; Weiderpass, E.; Tsilidis, K.K.; Boutron-Ruault, M.-C.; Overvad, K.; Eriksen, A.K.; et al. Consumption of Fish Is Not Associated with Risk of Differentiated Thyroid Carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J. Nutr. 2017, 147, 1366–1373. [Google Scholar] [CrossRef]
- Linseisen, J.; Bergström, E.; Gafá, L.; González, C.; Thiébaut, A.; Trichopoulou, A.; Tumino, R.; Sánchez, C.N.; Garcia, C.M.; Mattisson, I.; et al. Consumption of Added Fats and Oils in the European Prospective Investigation into Cancer and Nutrition (EPIC) Centres across 10 European Countries as Assessed by 24-Hour Dietary Recalls. Public Health Nutr. 2002, 5, 1227–1242. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Béraud, V.; Franceschi, S.; Cayssials, V.; Tsilidis, K.K.; Boutron-Ruault, M.-C.; Weiderpass, E.; Overvad, K.; Tjønneland, A.; Eriksen, A.K.; et al. Consumption of Fruits, Vegetables and Fruit Juices and Differentiated Thyroid Carcinoma Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Fruits, Vegetables, and Fruit Juices and Thyroid Cancer in EPIC. Int. J. Cancer 2018, 142, 449–459. [Google Scholar] [CrossRef]
- Gorst-Rasmussen, A. Tt: Treelet Transform with Stata. Stata J. Promot. Commun. Stat. Stata 2012, 12, 130–146. [Google Scholar] [CrossRef]
- Gorst-Rasmussen, A.; Dahm, C.C.; Dethlefsen, C.; Scheike, T.; Overvad, K. Exploring Dietary Patterns by Using the Treelet Transform. Am. J. Epidemiol. 2011, 173, 1097–1104. [Google Scholar] [CrossRef]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Schuit, J.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and Repeatability of a Simple Index Derived from the Short Physical Activity Questionnaire Used in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Public Health Nutr. 2003, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pallister, T.; Jennings, A.; Mohney, R.P.; Yarand, D.; Mangino, M.; Cassidy, A.; MacGregor, A.; Spector, T.D.; Menni, C. Characterizing Blood Metabolomics Profiles Associated with Self-Reported Food Intakes in Female Twins. PLoS ONE 2016, 11, e0158568. [Google Scholar] [CrossRef] [PubMed]
- Jaremek, M.; Yu, Z.; Mangino, M.; Mittelstrass, K.; Prehn, C.; Singmann, P.; Xu, T.; Dahmen, N.; Weinberger, K.M.; Suhre, K.; et al. Alcohol-Induced Metabolomic Differences in Humans. Transl. Psychiatry 2013, 3, e276. [Google Scholar] [CrossRef]
- Lacruz, M.E.; Kluttig, A.; Tiller, D.; Medenwald, D.; Giegling, I.; Rujescu, D.; Prehn, C.; Adamski, J.; Frantz, S.; Greiser, K.H.; et al. Cardiovascular Risk Factors Associated with Blood Metabolite Concentrations and Their Alterations During a 4-Year Period in a Population-Based Cohort. Circ. Cardiovasc. Genet. 2016, 9, 487–494. [Google Scholar] [CrossRef]
- His, M.; Viallon, V.; Dossus, L.; Schmidt, J.A.; Travis, R.C.; Gunter, M.J.; Overvad, K.; Kyrø, C.; Tjønneland, A.; Lécuyer, L.; et al. Lifestyle Correlates of Eight Breast Cancer-Related Metabolites: A Cross-Sectional Study within the EPIC Cohort. BMC Med. 2021, 19, 312. [Google Scholar] [CrossRef]
- Hodson, L.; Crowe, F.L.; McLachlan, K.J.; Skeaff, C.M. Effect of Supplementation with Flaxseed Oil and Different Doses of Fish Oil for 2 Weeks on Plasma Phosphatidylcholine Fatty Acids in Young Women. Eur. J. Clin. Nutr. 2018, 72, 832–840. [Google Scholar] [CrossRef]
- Floegel, A.; von Ruesten, A.; Drogan, D.; Schulze, M.B.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Variation of Serum Metabolites Related to Habitual Diet: A Targeted Metabolomic Approach in EPIC-Potsdam. Eur. J. Clin. Nutr. 2013, 67, 1100–1108. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite Profiling Identifies Pathways Associated with Metabolic Risk in Humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Carayol, M.; Leitzmann, M.F.; Ferrari, P.; Zamora-Ros, R.; Achaintre, D.; Stepien, M.; Schmidt, J.A.; Travis, R.C.; Overvad, K.; Tjønneland, A.; et al. Blood Metabolic Signatures of Body Mass Index: A Targeted Metabolomics Study in the EPIC Cohort. J. Proteome Res. 2017, 16, 3137–3146. [Google Scholar] [CrossRef]
- Abdolahi, A.; Georas, S.N.; Thomas Brenna, J.; Cai, X.; Thevenet-Morrison, K.; Phipps, R.P.; Lawrence, P.; Mousa, S.A.; Block, R.C. The Effects of Aspirin and Fish Oil Consumption on Lysophosphatidylcholines and Lysophosphatidic Acids and Their Correlates with Platelet Aggregation in Adults with Diabetes Mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 61–68. [Google Scholar] [CrossRef]
- Terry, P.; Lichtenstein, P.; Feychting, M.; Ahlbom, A.; Wolk, A. Fatty Fish Consumption and Risk of Prostate Cancer. Lancet 2001, 357, 1764–1766. [Google Scholar] [CrossRef]
- Augustsson, K.; Michaud, D.S.; Rimm, E.B.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. A Prospective Study of Intake of Fish and Marine Fatty Acids and Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2003, 12, 64–67. [Google Scholar]
- Allen, N.E.; Sauvaget, C.; Roddam, A.W.; Appleby, P.; Nagano, J.; Suzuki, G.; Key, T.J.; Koyama, K. A Prospective Study of Diet and Prostate Cancer in Japanese Men. Cancer Causes Control 2004, 15, 911–920. [Google Scholar] [CrossRef]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort Study of Diet, Lifestyle, and Prostate Cancer in Adventist Men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A Prospective Study of Demographics, Diet, and Prostate Cancer among Men of Japanese Ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar]
- Hsing, A.W.; McLaughlin, J.K.; Schuman, L.M.; Bjelke, E.; Gridley, G.; Wacholder, S.; Chien, H.T.; Blot, W.J. Diet, Tobacco Use, and Fatal Prostate Cancer: Results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990, 50, 6836–6840. [Google Scholar]
- Le Marchand, L.; Kolonel, L.N.; Wilkens, L.R.; Myers, B.C.; Hirohata, T. Animal Fat Consumption and Prostate Cancer: A Prospective Study in Hawaii. Epidemiol. Camb. Mass 1994, 5, 276–282. [Google Scholar] [CrossRef]
- Grönberg, H.; Damber, L.; Damber, J.E. Total Food Consumption and Body Mass Index in Relation to Prostate Cancer Risk: A Case-Control Study in Sweden with Prospectively Collected Exposure Data. J. Urol. 1996, 155, 969–974. [Google Scholar] [CrossRef]
- Schuurman, A.G.; van den Brandt, P.A.; Dorant, E.; Goldbohm, R.A. Animal Products, Calcium and Protein and Prostate Cancer Risk in the Netherlands Cohort Study. Br. J. Cancer 1999, 80, 1107–1113. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity, and Prostate Cancer. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/prostate-cancer-report.pdf (accessed on 7 March 2022).
- Rohrmann, S.; Linseisen, J.; Key, T.J.; Jensen, M.K.; Overvad, K.; Johnsen, N.F.; Tjønneland, A.; Kaaks, R.; Bergmann, M.M.; Weikert, C.; et al. Alcohol Consumption and the Risk for Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.G.; Goldbohm, R.A.; van den Brandt, P.A. A Prospective Cohort Study on Consumption of Alcoholic Beverages in Relation to Prostate Cancer Incidence (The Netherlands). Cancer Causes Control 1999, 10, 597–605. [Google Scholar] [CrossRef]
- Breslow, R.A.; Wideroff, L.; Graubard, B.I.; Erwin, D.; Reichman, M.E.; Ziegler, R.G.; Ballard-Barbash, R. Alcohol and Prostate Cancer in the NHANES I Epidemiologic Follow-Up Study. Ann. Epidemiol. 1999, 9, 254–261. [Google Scholar] [CrossRef]
- Nilsen, T.I.L.; Vatten, L.J. Anthropometry and Prostate Cancer Risk: A Prospective Study of 22,248 Norwegian Men. Cancer Causes Control 1999, 10, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; Severi, G.; English, D.R.; Hopper, J.L.; Giles, G.G. Alcohol Consumption and Prostate Cancer Risk: Results from the Melbourne Collaborative Cohort Study. Int. J. Cancer 2006, 119, 1501–1504. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Stolzenberg-Solomon, R.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Dietary Factors of One-Carbon Metabolism and Prostate Cancer Risk. Am. J. Clin. Nutr. 2006, 84, 929–935. [Google Scholar] [CrossRef]
- Sesso, H.D.; Paffenbarger, R.S.; Lee, I.-M. Alcohol Consumption and Risk of Prostate Cancer: The Harvard Alumni Health Study. Int. J. Epidemiol. 2001, 30, 749–755. [Google Scholar] [CrossRef]
- Platz, E.A. Alcohol Intake, Drinking Patterns, and Risk of Prostate Cancer in a Large Prospective Cohort Study. Am. J. Epidemiol. 2004, 159, 444–453. [Google Scholar] [CrossRef]
- Velicer, C.M.; Kristal, A.; White, E. Alcohol Use and the Risk of Prostate Cancer: Results from the VITAL Cohort Study. Nutr. Cancer 2006, 56, 50–56. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef]
- Littman, A.J.; White, E.; Kristal, A.R. Anthropometrics and Prostate Cancer Risk. Am. J. Epidemiol. 2007, 165, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Freedland, S.J.; Deka, A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Thun, M.J.; Calle, E.E. Body Mass Index, Weight Change, and Risk of Prostate Cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2007, 16, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-Analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Möller, E.; Wilson, K.M.; Batista, J.L.; Mucci, L.A.; Bälter, K.; Giovannucci, E. Body Size across the Life Course and Prostate Cancer in the Health Professionals Follow-up Study: Body Size and Prostate Cancer. Int. J. Cancer 2016, 138, 853–865. [Google Scholar] [CrossRef]
- Siskos, A.P.; Jain, P.; Römisch-Margl, W.; Bennett, M.; Achaintre, D.; Asad, Y.; Marney, L.; Richardson, L.; Koulman, A.; Griffin, J.L.; et al. Interlaboratory Reproducibility of a Targeted Metabolomics Platform for Analysis of Human Serum and Plasma. Anal. Chem. 2017, 89, 656–665. [Google Scholar] [CrossRef]
- Margetts, B.; Pietinen, P. European Prospective Investigation into Cancer and Nutrition: Validity Studies on Dietary Assessment Methods. Int. J. Epidemiol. 1997, 26, S1. [Google Scholar] [CrossRef]
| Dietary Variable | Increment (Grams per Day) |
|---|---|
| Dairy | 200 g [14] |
| Milk | 200 g [14] |
| Cheese | 30 g [15] |
| Yogurt | 30 g [15] |
| Eggs | 7 g [16] |
| Total fish products | 30 g [17] |
| Total fish | 30 g [17] |
| Lean fish | 10 g [17] |
| Fatty fish | 10 g [17] |
| Red meat | 40 g [16] |
| Poultry | 20 g [16] |
| Processed meat | 40 g [16] |
| Fats and oils | 10 g [16] |
| Butter | 5 g [16] |
| Margarine | 5 g [16] |
| Vegetable oils | 5 g [18] |
| Fruits | 100 g [19] |
| Vegetables | 100 g [19] |
| Leafy vegetables | 25 g [19] |
| Root vegetables | 25 g [19] |
| Fruiting Vegetables | 100 g [19] |
| Cereals and cereal products | 200 g [14] |
| Alcohol | 10 g [14] |
| Metabolite Pattern | Contributing Metabolites All with Positive Loadings | Percent Explained Variance (%) |
|---|---|---|
| 1 | 64 diacyl and acyl-alkyl phosphatidylcholines; (SM (OH) C14:1, SM (OH) C16:1, and SM (OH) C22:2) | 21.5 |
| 2 | Acylcarnitines C18:1 and C18:2, glutamate, ornithine, and taurine | 5.2 |
| 3 | Lyso PC a C16:0, lyso PC a C16:1, lyso PC a C17:0, lyso PC a C18:0, lyso PC a C18:1, lyso PC a C18:2, lyso PC a C20:3, lyso PC a C20:4 | 4.7 |
| Participant Characteristics | Overall (n = 3198) | Discovery (n = 2640) | Validation (n = 558) |
|---|---|---|---|
| Age at blood collection (years) | 57.2 (7.2) | 57.5 (7.1) | 56.0 (7.8) |
| Fasting status at blood collection (time since last meal) (n (%)) | |||
| <3 h | 1402 (43.8) | 1225 (46.4) | 177 (31.7) |
| 3–6 h | 631 (19.7) | 526 (19.9) | 105 (18.8) |
| >6 h | 1100 (34.4) | 845 (32.0) | 255 (45.7) |
| Missing | 65 (2.0) | 44 (1.7) | 21 (3.8) |
| Socio-economic and lifestyle factors (n (%)) | |||
| Educational level | |||
| Primary/no schooling | 1216 (38.0) | 992 (37.6) | 224 (40.1) |
| Secondary | 347 (10.9) | 289 (11.0) | 58 (10.4) |
| Technical/professional | 744 (23.3) | 612 (23.2) | 132 (23.7) |
| University or higher | 761 (23.8) | 633 (24.0) | 128 (22.9) |
| Not specified | 99 (3.1) | 88 (3.3) | 11 (2.0) |
| Missing | 31 (0.9) | 26 (0.9) | 5 (0.9) |
| Physical activity (Cambridge Index) | |||
| Inactive | 722 (22.6) | 582 (22.1) | 140 (25.1) |
| Moderately inactive | 1048 (32.8) | 869 (32.9) | 179 (32.1) |
| Moderately active | 731 (22.9) | 609 (23.1) | 122 (21.9) |
| Active | 637 (19.9) | 523 (19.8) | 114 (20.4) |
| Missing | 60 (1.9) | 57 (2.2) | 3 (0.5) |
| Smoking status | |||
| Never | 1025 (32.1) | 843 (31.9) | 182 (32.6) |
| Former | 1374 (43.0) | 1129 (42.8) | 245 (43.9) |
| Current | 765 (23.9) | 640 (24.2) | 125 (22.4) |
| Missing | 34 (1.1) | 28 (1.1) | 6 (1.1) |
| Alcohol consumption at recruitment | |||
| Non-drinker (<0.1 g/day) | 286 (8.9) | 235 (8.9) | 51 (9.1) |
| >0.1–3 g/day | 432 (13.5) | 360 (13.6) | 72 (12.9) |
| >3–12 g/day | 730 (22.8) | 605 (22.9) | 125 (22.4) |
| >12–24 g/day | 644 (20.1) | 539 (20.4) | 105 (18.8) |
| >24 g/day | 1106 (34.6) | 901 (34.1) | 205 (36.7) |
| Anthropometric variables (mean (SD)) | |||
| Height (cm) | 172.7 (7.0) | 172.7 (7.1) | 173.0 (6.7) |
| BMI (kg/m2) | 26.9 (3.4) | 26.9 (3.4) | 26.9 (3.3) |
| Dietary variables (g/day) (mean (SD)) | |||
| Total energy (kcal/day) | 2390 (649) | 2375 (650) | 2440(641) |
| Dairy | 303 (229) | 302 (227) | 306 (237) |
| Milk | 198 (205) | 199 (204) | 195 (212) |
| Cheese | 34.5 (35.2) | 33.6 (34.1) | 38.7 (39.6) |
| Yogurt | 38.9 (70.4) | 37.5 (67.2) | 45.8 (83.5) |
| Egg | 18.6 (17.9) | 18.4 (18.1) | 19.5 (16.7) |
| Total fish products | 40.9 (41.8) | 40.9 (41.8) | 41.0 (41.6) |
| Total fish | 35.1 (38.3) | 35.2 (38.0) | 34.8 (39.4) |
| Lean fish | 24.9 (31.8) | 25.1 (31.6) | 24.2 (33.0) |
| Fatty fish | 12.8 (18.2) | 12.8 (18.4) | 13.0 (17.5) |
| Red meat | 49.6 (36.6) | 49.0 (36.2) | 52.5 (38.2) |
| Processed meat | 45.9 (42.7) | 45.9 (43.4) | 45.9 (38.8) |
| Poultry | 21.9 (21.2) | 21.9 (21.0) | 21.8 (22.4) |
| Fats and oils | 32.6 (17.4) | 32.3 (17.3) | 33.9 (17.6) |
| Butter | 5.24 (10.5) | 5.45(10.6) | 4.26 (9.68) |
| Margarine | 9.74 (14.7) | 9.69 (14.5) | 9.93 (15.8) |
| Vegetable oil | 16.5 (17.7) | 16.1 (17.5) | 18.4 (18.5) |
| Vegetables | 190 (129) | 191 (130) | 186 (128) |
| Leafy vegetables | 30.4 (49.0) | 30.0 (49.1) | 32.2 (48.6) |
| Fruiting vegetables | 67.6 (56.3) | 67.0 (56.1) | 70.2 (57.1) |
| Root vegetables | 19.6 (24.2) | 20.1 (24.6) | 17.5 (22.0) |
| Fruit | 236 (206) | 233 (204) | 251 (214) |
| Cereal | 257 (139) | 253 (134) | 273 (161) |
| Scores for metabolite patterns | |||
| Pattern 1 (geometric mean (SD)) | 10.2 (1.30) | 10.2 (1.30) | 10.2 (1.20) |
| Pattern 2 (geometric mean (SD)) | 1.98 (0.44) | 1.98 (0.44) | 1.98 (0.45) |
| Pattern 3 (geometric mean (SD)) | 6.13 (0.61) | 6.13 (0.61) | 6.13 (0.61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grenville, Z.S.; Noor, U.; His, M.; Viallon, V.; Rinaldi, S.; Aglago, E.K.; Amiano, P.; Brunkwall, L.; Chirlaque, M.D.; Drake, I.; et al. Diet and BMI Correlate with Metabolite Patterns Associated with Aggressive Prostate Cancer. Nutrients 2022, 14, 3306. https://doi.org/10.3390/nu14163306
Grenville ZS, Noor U, His M, Viallon V, Rinaldi S, Aglago EK, Amiano P, Brunkwall L, Chirlaque MD, Drake I, et al. Diet and BMI Correlate with Metabolite Patterns Associated with Aggressive Prostate Cancer. Nutrients. 2022; 14(16):3306. https://doi.org/10.3390/nu14163306
Chicago/Turabian StyleGrenville, Zoe S., Urwah Noor, Mathilde His, Vivian Viallon, Sabina Rinaldi, Elom K. Aglago, Pilar Amiano, Louise Brunkwall, María Dolores Chirlaque, Isabel Drake, and et al. 2022. "Diet and BMI Correlate with Metabolite Patterns Associated with Aggressive Prostate Cancer" Nutrients 14, no. 16: 3306. https://doi.org/10.3390/nu14163306
APA StyleGrenville, Z. S., Noor, U., His, M., Viallon, V., Rinaldi, S., Aglago, E. K., Amiano, P., Brunkwall, L., Chirlaque, M. D., Drake, I., Eichelmann, F., Freisling, H., Grioni, S., Heath, A. K., Kaaks, R., Katzke, V., Mayén-Chacon, A.-L., Milani, L., Moreno-Iribas, C., ... Schmidt, J. A. (2022). Diet and BMI Correlate with Metabolite Patterns Associated with Aggressive Prostate Cancer. Nutrients, 14(16), 3306. https://doi.org/10.3390/nu14163306








