The Relationship between Obesity and Clinical Outcomes in Young People with Duchenne Muscular Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Population
2.3. Procedures
2.4. Outcomes
2.4.1. Clinical Characteristics
2.4.2. Anthropometric Data
2.4.3. Clinical Milestones
2.5. Data Cleaning
2.6. Approvals and Patient Consents
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics
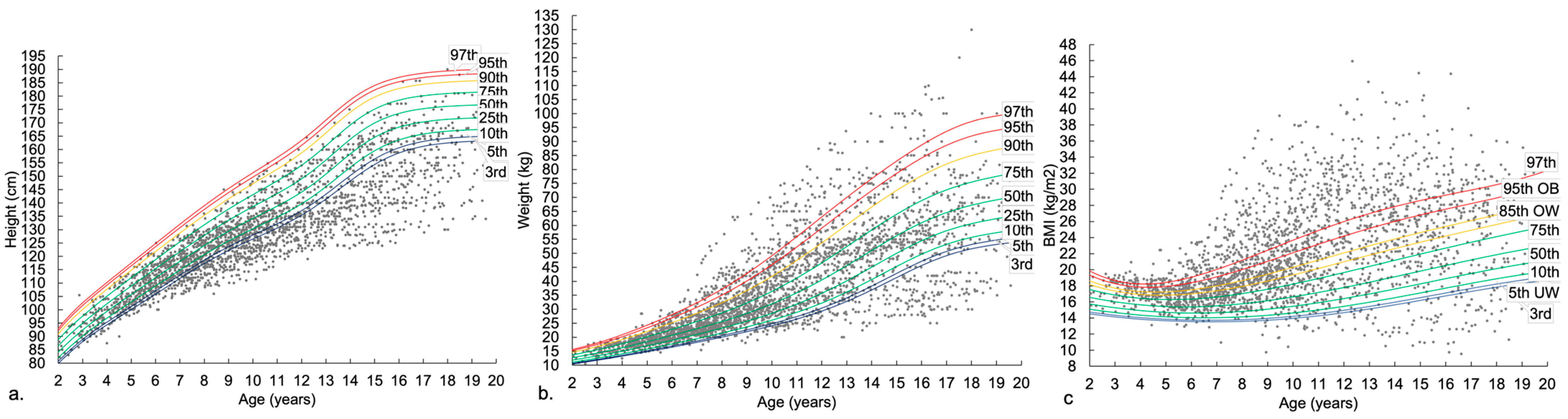
3.2. Anthropometry across Age Groups
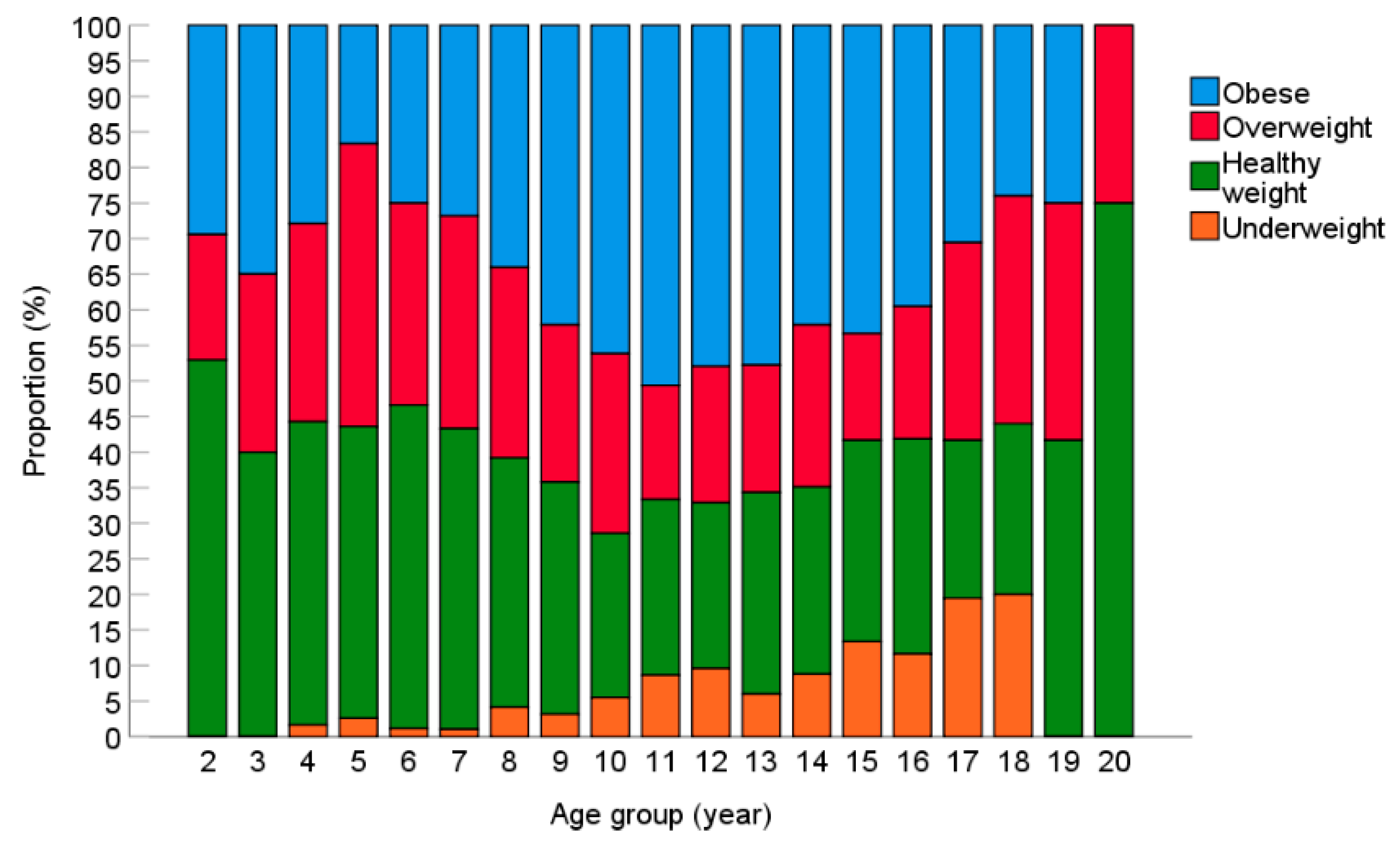
3.3. BMI Status
3.4. Impact of Obesity on Clinical Milestones
3.4.1. Obesity and Physical Function
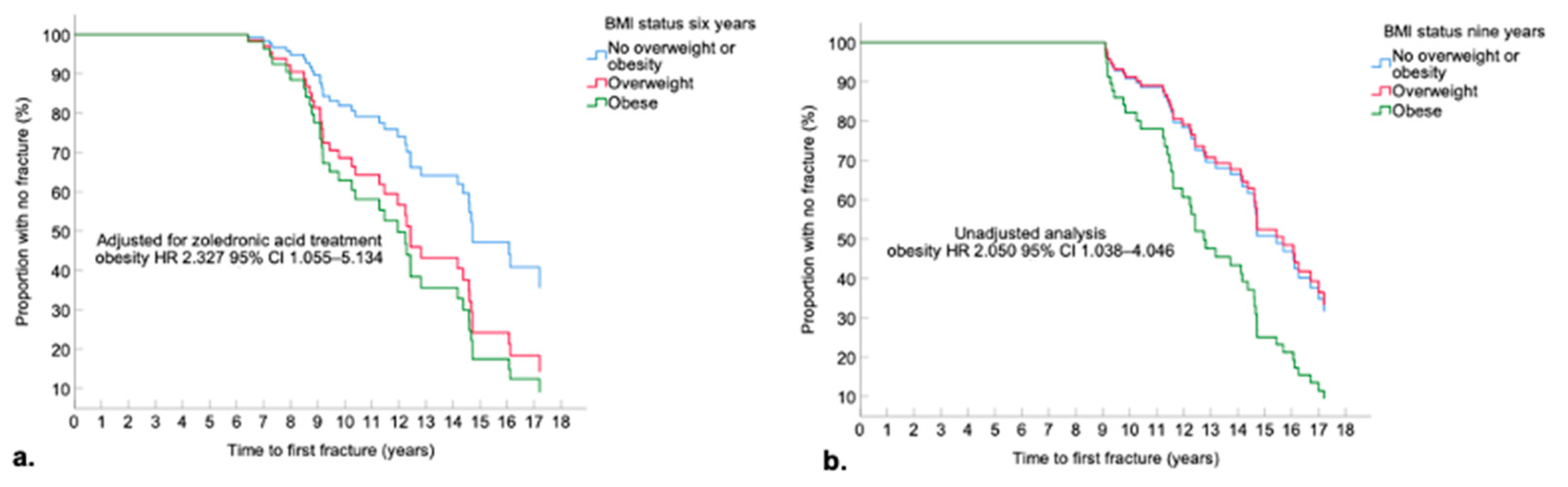
3.4.2. Obesity and First Fracture
3.4.3. Obesity and Respiratory Function
3.5. Factors Associated with Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mah, J.K.; Korngut, L.; Fiest, K.M.; Dykeman, J.; Day, L.J.; Pringsheim, T.; Jette, N. A Systematic Review and Meta-analysis on the Epidemiology of the Muscular Dystrophies. Can. J. Neurol. Sci. 2016, 43, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Shilling, C.; Leslie, N.D.; Flanigan, K.M.; al-Dahhak, R.; Gastier-Foster, J.; Kneile, K.; Dunn, D.M.; Duval, B.; Aoyagi, A.; et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol. 2012, 71, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Bradley, D.M.; Salmon, R.; Clarke, A.; Hartley, L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). Eur. J. Hum. Genet. 2013, 21, 1049–1053. [Google Scholar] [CrossRef]
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; de Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: An evidence review. Orphanet. J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef]
- Deconinck, N.; Dan, B. Pathophysiology of duchenne muscular dystrophy: Current hypotheses. Pediatr. Neurol. 2007, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shoji, E.; Sakurai, H.; Nishino, T.; Nakahata, T.; Heike, T.; Awaya, T.; Fujii, N.; Manabe, Y.; Matsuo, M.; Sehara-Fujisawa, A. Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci. Rep. 2015, 5, 12831. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 5, CD003725. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Duong, T.; Joyce, N.C.; Hu, F.; Clemens, P.R.; Hoffman, E.P.; Cnaan, A.; Gordish-Dressman, H.; et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study. Lancet 2018, 391, 451–461. [Google Scholar] [CrossRef]
- Lamb, M.M.; Cai, B.; Royer, J.; Pandya, S.; Soim, A.; Valdez, R.; DiGuiseppi, C.; James, K.; Whitehead, N.; Peay, H.; et al. The effect of steroid treatment on weight in nonambulatory males with Duchenne muscular dystrophy. Am. J. Med. Genet. A 2018, 176, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.M.; West, N.A.; Ouyang, L.; Yang, M.; Weitzenkamp, D.; James, K.; Ciafaloni, E.; Pandya, S.; DiGuiseppi, C.; Muscular Dystrophy Surveillance, R.; et al. Corticosteroid Treatment and Growth Patterns in Ambulatory Males with Duchenne Muscular Dystrophy. J. Pediatr. 2016, 173, 207–213.e203. [Google Scholar] [CrossRef] [PubMed]
- Davidson, Z.E.; Ryan, M.M.; Kornberg, A.J.; Sinclair, K.; Cairns, A.; Walker, K.Z.; Truby, H. Observations of body mass index in Duchenne muscular dystrophy: A longitudinal study. Eur. J. Clin. Nutr. 2014, 68, 892–897. [Google Scholar] [CrossRef]
- West, N.A.; Yang, M.L.; Weitzenkamp, D.A.; Andrews, J.; Meaney, F.J.; Oleszek, J.; Miller, L.A.; Matthews, D.; DiGuiseppi, C. Patterns of growth in ambulatory males with Duchenne muscular dystrophy. J. Pediatr. 2013, 163, 1759–1763.e1751. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC growth charts: United States. Adv. Data 2000, 8, 1–27. [Google Scholar]
- Martigne, L.; Salleron, J.; Mayer, M.; Cuisset, J.M.; Carpentier, A.; Neve, V.; Tiffreau, V.; Guimber, D.; Gottrand, F. Natural evolution of weight status in Duchenne muscular dystrophy: A retrospective audit. Br. J. Nutr. 2011, 105, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Abresch, R.T.; Carter, G.T.; Fowler, W.M., Jr.; Johnson, E.R.; Kilmer, D.D.; Sigford, B.J. Profiles of neuromus-cular diseases. Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 1995, 74, S70–S92. [Google Scholar] [CrossRef]
- Willig, T.N.; Carlier, L.; Legrand, M.; Riviere, H.; Navarro, J. Nutritional assessment in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 1993, 35, 1074–1082. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Okada, K.; Manabe, S.; Sakamoto, S.; Ohnaka, M.; Niiyama, Y. Protein and energy metabolism in patients with progressive muscular dystrophy. J. Nutr. Sci. Vitaminol. 1992, 38, 141–154. [Google Scholar] [CrossRef]
- Canapari, C.A.; Barrowman, N.; Hoey, L.; Walker, S.W.; Townsend, E.; Tseng, B.S.; Katz, S.L. Truncal fat distribution correlates with decreased vital capacity in Duchenne muscular dystrophy. Pediatr. Pulmonol. 2015, 50, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.; Carey, K.; Ho, G.; Mallitt, K.A.; Widger, J.; Farrar, M. The relationship of body habitus and respiratory function in Duchenne muscular dystrophy. Respir. Med. 2016, 119, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Goemans, N.; Vanden Hauwe, M.; Signorovitch, J.; Swallow, E.; Song, J.; Collaborative Trajectory Analysis, P. IndividualizedmPrediction of Changes in 6-Minute Walk Distance for Patients with Duchenne Muscular Dystrophy. PLoS ONE 2016, 11, e0164684. [Google Scholar] [CrossRef]
- Goemans, N.; Wong, B.; Van den Hauwe, M.; Signorovitch, J.; Sajeev, G.; Cox, D.; Landry, J.; Jenkins, M.; Dieye, I.; Yao, Z.; et al. Prognostic factors for changes in the timed 4-stair climb in patients with Duchenne muscular dystrophy, and implications for measuring drug efficacy: A multi-institutional collaboration. PLoS ONE 2020, 15, e0232870. [Google Scholar] [CrossRef]
- Rodriguez-Cruz, M.; Cruz-Guzman, O.R.; Escobar, R.E.; Lopez-Alarcon, M. Leptin and metabolic syndrome in patients with Duchenne/Becker muscular dystrophy. Acta Neurol. Scand. 2016, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cruz, M.; Sanchez, R.; Escobar, R.E.; Cruz-Guzman Odel, R.; Lopez-Alarcon, M.; Bernabe Garcia, M.; Coral-Vazquez, R.; Matute, G.; Velazquez Wong, A.C. Evidence of Insulin Resistance and Other Metabolic Alterations in Boys with Duchenne or Becker Muscular Dystrophy. Int. J. Endocrinol. 2015, 2015, 867273. [Google Scholar] [CrossRef] [PubMed]
- Saure, C.; Caminiti, C.; Weglinski, J.; de Castro Perez, F.; Monges, S. Energy expenditure, body composition, and prevalence of metabolic disorders in patients with Duchenne muscular dystrophy. Diabetes Metab. Syndr. 2018, 12, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Sawnani, H.; Thampratankul, L.; Szczesniak, R.D.; Fenchel, M.C.; Simakajornboon, N. Sleep disordered breathing in young boys with Duchenne muscular dystrophy. J. Pediatr. 2015, 166, 640–645.e641. [Google Scholar] [CrossRef]
- Van de Velde, N.M.; Roest, A.A.W.; van Zwet, E.W.; Niks, E.H. Increased Blood Pressure and Body Mass Index as Potential Modifiable Factors in The Progression of Myocardial Dysfunction in Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2019, 6, 65–73. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef]
- Cole, T.J. The LMS method for constructing normalized growth standards. Eur. J. Clin. Nutr. 1990, 44, 45–60. [Google Scholar] [PubMed]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Florence, J.M.; Eagle, M.; Gappmaier, E.; Glanzman, A.M.; Group, P.G.-D.S.; Spiegel, R.; Barth, J.; et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 2013, 48, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, V.; Ridout, D.A.; Pane, M.; Main, M.; Mayhew, A.; Mercuri, E.; Manzur, A.Y.; Muntoni, F.; Network, U.K.N.C. The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: Considerations for the design of clinical trials. J. Neurol. Neurosurg. Psychiatry 2016, 87, 149–155. [Google Scholar] [CrossRef]
- McDonald, C.M.; Gordish-Dressman, H.; Henricson, E.K.; Duong, T.; Joyce, N.C.; Jhawar, S.; Leinonen, M.; Hsu, F.; Connolly, A.M.; Cnaan, A.; et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: Long-term natural history with and without glucocorticoids. Neuromuscul. Disord. 2018, 28, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Elm, E.v.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of obser-vational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control and Prevention US. Percentile Data Files with LMS Values; National Center for Health Statistics: Atlanta, GA, USA, 2000.
- Zacharin, M.; Lim, A.; Gryllakis, J.; Siafarikas, A.; Jefferies, C.; Briody, J.; Heather, N.; Pitkin, J.; Emmanuel, J.; Lee, K.J.; et al. Randomized Controlled Trial Evaluating the Use of Zoledronic Acid in Duchenne Muscular Dystrophy. J. Clin. Endocrinol. Metab. 2021, 106, 2328–2342. [Google Scholar] [CrossRef]
- Australian Institute of Health Welfare. Overweight and Obesity among Australian Children and Adolescents; Australian Institute of Health Welfare: Canberra, Australia, 2020; p. 76.
- Kessler, J.; Koebnick, C.; Smith, N.; Adams, A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. Orthop. Relat. Res. 2013, 471, 1199–1207. [Google Scholar] [CrossRef]
- King, W.M.; Ruttencutter, R.; Nagaraja, H.N.; Matkovic, V.; Landoll, J.; Hoyle, C.; Mendell, J.R.; Kissel, J.T. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology 2007, 68, 1607–1613. [Google Scholar] [CrossRef]
- Guglieri, M.; Bushby, K.; McDermott, M.P.; Hart, K.A.; Tawil, R.; Martens, W.B.; Herr, B.E.; McColl, E.; Speed, C.; Wilkinson, J.; et al. Effect of Different Corticosteroid Dosing Regimens on Clinical Outcomes in Boys with Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA 2022, 327, 1456–1468. [Google Scholar] [CrossRef]
- Yildiz, S.; Glanzman, A.M.; Estilow, T.; Flickinger, J.; Brandsema, J.F.; Tennekoon, G.; Banwell, B.L.; Yum, S. Retrospective Analysis of Fractures and Factors Causing Ambulation Loss After Lower Limb Fractures in Duchenne Muscular Dystrophy. Am. J. Phys. Med. Rehabil. 2020, 99, 789–794. [Google Scholar] [CrossRef] [PubMed]
- El-Aloul, B.; Speechley, K.N.; Wei, Y.; Wilk, P.; Campbell, C. Fatigue in young people with Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2020, 62, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.G.; Terhorst, L.; Little, N.; Bendixen, R.M. Uncovering sleep in young males with Duchenne muscular dystrophy. Eur. J. Paediatr. Neurol. 2020, 26, 20–28. [Google Scholar] [CrossRef]
- Suresh, S.; Wales, P.; Dakin, C.; Harris, M.A.; Cooper, D.G. Sleep-related breathing disorder in Duchenne muscular dystrophy: Disease spectrum in the paediatric population. J. Paediatr. Child Health 2005, 41, 500–503. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Greenberg-Dotan, S.; Simon-Tuval, T.; Freidman, B.; Goldbart, A.D.; Tal, A.; Reuveni, H. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am. J. Respir Crit. Care Med. 2007, 175, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Teoh, L.J.; Geelhoed, E.A.; Bayley, K.; Leonard, H.; Laing, N.G. Health care utilization and costs for children and adults with duchenne muscular dystrophy. Muscle Nerve 2016, 53, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Finder, J.D.; Birnkrant, D.; Carl, J.; Farber, H.J.; Gozal, D.; Iannaccone, S.T.; Kovesi, T.; Kravitz, R.M.; Panitch, H.; Schramm, C.; et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med. 2004, 170, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Vuillerot, C.; Braillon, P.; Fontaine-Carbonnel, S.; Rippert, P.; Andre, E.; Iwaz, J.; Poirot, I.; Berard, C. Influence of a two-year steroid treatment on body composition as measured by dual X-ray absorptiometry in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2014, 24, 467–473. [Google Scholar] [CrossRef]
- Sussman, M.D.; Sienko, S.E.; Buckon, C.E.; Hilton, C.; De Mattos, C.B.; d’Amato, C. Efficacy of corticosteroid in decreasing scoliosis and extending time to loss of ambulation in a single clinic: An effectiveness trial. J. Child. Orthop. 2020, 14, 421–432. [Google Scholar] [CrossRef]
- Cheuk, D.K.; Wong, V.; Wraige, E.; Baxter, P.; Cole, A. Surgery for scoliosis in Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2015, 1, CD005375. [Google Scholar] [CrossRef]
- Malik, A.T.; Tamer, R.; Yu, E.; Kim, J.; Khan, S.N. The Impact of Body Mass Index (BMI) on 30-day Outcomes FollowingPosterior Spinal Fusion in Neuromuscular Scoliosis. Spine 2019, 44, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.P.; Zuckerman, K.E.; Fombonne, E. Obesity and Autism. Pediatrics 2015, 136, 1051–1061. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Wittenbrook, W. Position of the Academy of Nutrition and Dietetics: Nutrition services for individuals with intellectual and developmental disabilities and special health care needs. J. Acad. Nutr. Diet 2015, 115, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Darmahkasih, A.J.; Rybalsky, I.; Tian, C.; Shellenbarger, K.C.; Horn, P.S.; Lambert, J.T.; Wong, B.L. Neurodevelopmental, behavioral, and emotional symptoms common in Duchenne muscular dystrophy. Muscle Nerve 2020, 61, 466–474. [Google Scholar] [CrossRef] [PubMed]

| Outcome Measure (Observations n) | Time to Clinical Milestone |
|---|---|
| Loss of ambulation (77) | First documentation of loss of ambulation |
| Timed 10 m walk/run (528) | First >10 s time for a 10 m walk/run [32,33], first 7–10 s time for a 10 m walk/run |
| Timed supine-to-stand (420) | First >30 s time to stand from supine [32,33], First >7 s time to stand from supine |
| Timed stair climb (470) | First >8 s time for a 4 stair climb [32,33] |
| NSAA (468) | First NSAA score 9 [34] |
| 6MWD (83) | First 6MWD < 325 m [32,33] |
| FVC (1002) | First FVC < 1 L [35] |
| OSA diagnosis (71) | Polysomnography-confirmed OSA diagnosis |
| Nocturnal hypoventilation diagnosis (25) | Polysomnography-confirmed nocturnal hypoventilation diagnosis |
| CPAP commencement (26) | CPAP initiation |
| Bi-level commencement (22) | Bi-level initiation |
| Scoliosis diagnosis (48) | First documentation of xray-confirmed scoliosis |
| Scoliosis surgery (15) | Scoliosis corrective surgery |
| First fracture (crush or other) (71) | First fracture |
| Outcome Category | Outcome Measure 1 | |
|---|---|---|
| Age and length of follow-up | Age at diagnosis (years) (n = 157), mean ± SD | 4.2 ± 2.1 |
| Age at first neurologist appointment (years), mean ± SD | 4.5 ± 2.5 2 | |
| Length of total follow-up (years), mean ± SD | 8.7 ± 4.7 3 | |
| DMD mutation type, n (N%) | Deletion | 93 (58.9) |
| Duplication or duplication/triplication | 18 (11.4) | |
| Point mutation | 29 (18.4) | |
| Genetic testing conducted but mutation not identified | 5 (3.2) | |
| Genetic report not found (diagnosed with muscle biopsy) | 13 (8.2) | |
| Dystrophin isoforms maintained, n (N%) | Dp260, Dp140, Dp116 and Dp71 | 36 (22.8) |
| Dp140, Dp116 and Dp71 | 21 (13.3) | |
| Dp116 and Dp71 | 70 (44.3) | |
| Dp71 | 5 (3.2) | |
| Nothing maintained | 8 (5.1) | |
| Exons affected not available | 18 (12.7) | |
| Steroid treatment | Steroid-treated, n (N%) | 142 (89.9) 4 |
| Age at steroid commencement (years, n = 139) | 6.6 ± 2.3 | |
| Prednisolone only, n (N%) | 81 (51.3) | |
| Prednisolone then deflazacort, n (N%) | 58 (36.7) | |
| Other corticosteroid, n (N%) | 3 (1.9) 5 | |
| Steroid-naive, n (N%) | 16 (10.1) | |
| Anthropometric measures (n = 156) | Height z-score (observations n = 2480), mean ± SD [range] | −1.47 ± 1.40 [−6.06–2.97] |
| Weight z-score (observations n = 2902), mean ± SD [range] | 0.10 ± 1.56 [−8.51–3.24] | |
| BMI observations per individual over total follow-up | 16 ± 10 | |
| BMI observations per individual per year of follow-up | 2 ± 1 | |
| BMI z-score (observations n = 2456), mean ± SD [range] | 1.00 ± 1.60 [−14.23–3.36] | |
| Change in BMI z-score across total follow-up (n = 150), mean ± SD [range] | 0.23 ± 1.58 [−7.36–4.59] | |
| Co-morbidities, n (N%) | Any neurodevelopmental disability diagnosis | 39 (24.7) 6 |
| Any mental health diagnosis | 22 (13.9) | |
| Deaths | Age at death (n = 10), mean ± SD | 15.7 ± 3.0 |
| Milestone Type | Clinical Milestone | Event Occurred during Follow-Up, n (%) | Time to Event (Age in Years) Median (IQR) |
|---|---|---|---|
| Physical function | Non-ambulant | 82 (51.9) | 11.0 (9.3, 12.4), n = 77 |
| 10 m walk/run in 7–10 s | 56 (35.4) | 9.7 (8.2, 11.0) | |
| 10 m walk/run in >10 s | 35 (22.2) | 10.8 (9.5, 12.0) | |
| Supine-to-stand in >7 s | 58 (36.7) | 9.5 (8.3, 10.6) | |
| Four stair climb in >8 s | 45 (28.5) | 10.2 (8.4, 11.6) | |
| NSAA total score 9 | 31 (19.6) | 11.6 (9.4, 13.1) | |
| 6MWD < 325 m | 17 (10.8) | 11.6 (10.5, 13.6) | |
| Respiratory function | OSA diagnosed | 72 (45.6) | 13.1 (9.7, 15.0), n = 71 |
| Nocturnal hypoventilation diagnosed | 27 (17.1) | 15.3 (13.3, 16.5), n = 25 | |
| CPAP initiated | 24 (15.2) | 13.7 (12.5, 16.0) | |
| Bi-level initiated | 20 (12.7) | 15.5 (14.0, 16.5) | |
| Both CPAP and Bi-level initiated | 2 (1.3) | - | |
| FVC < 1 L on lung function test | 27 (17.1) | 13.8 (11.2, 16.4) | |
| Fractures | ≥1 fracture | 71 (44.9) | 11.4 (8.8, 14.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billich, N.; Adams, J.; Carroll, K.; Truby, H.; Evans, M.; Ryan, M.M.; Davidson, Z.E. The Relationship between Obesity and Clinical Outcomes in Young People with Duchenne Muscular Dystrophy. Nutrients 2022, 14, 3304. https://doi.org/10.3390/nu14163304
Billich N, Adams J, Carroll K, Truby H, Evans M, Ryan MM, Davidson ZE. The Relationship between Obesity and Clinical Outcomes in Young People with Duchenne Muscular Dystrophy. Nutrients. 2022; 14(16):3304. https://doi.org/10.3390/nu14163304
Chicago/Turabian StyleBillich, Natassja, Justine Adams, Kate Carroll, Helen Truby, Maureen Evans, Monique M. Ryan, and Zoe E. Davidson. 2022. "The Relationship between Obesity and Clinical Outcomes in Young People with Duchenne Muscular Dystrophy" Nutrients 14, no. 16: 3304. https://doi.org/10.3390/nu14163304
APA StyleBillich, N., Adams, J., Carroll, K., Truby, H., Evans, M., Ryan, M. M., & Davidson, Z. E. (2022). The Relationship between Obesity and Clinical Outcomes in Young People with Duchenne Muscular Dystrophy. Nutrients, 14(16), 3304. https://doi.org/10.3390/nu14163304






