Effect of Maternal Glucose and Triglyceride Levels during Early Pregnancy on Pregnancy Outcomes: A Retrospective Cohort Study
Abstract
:1. Introduction
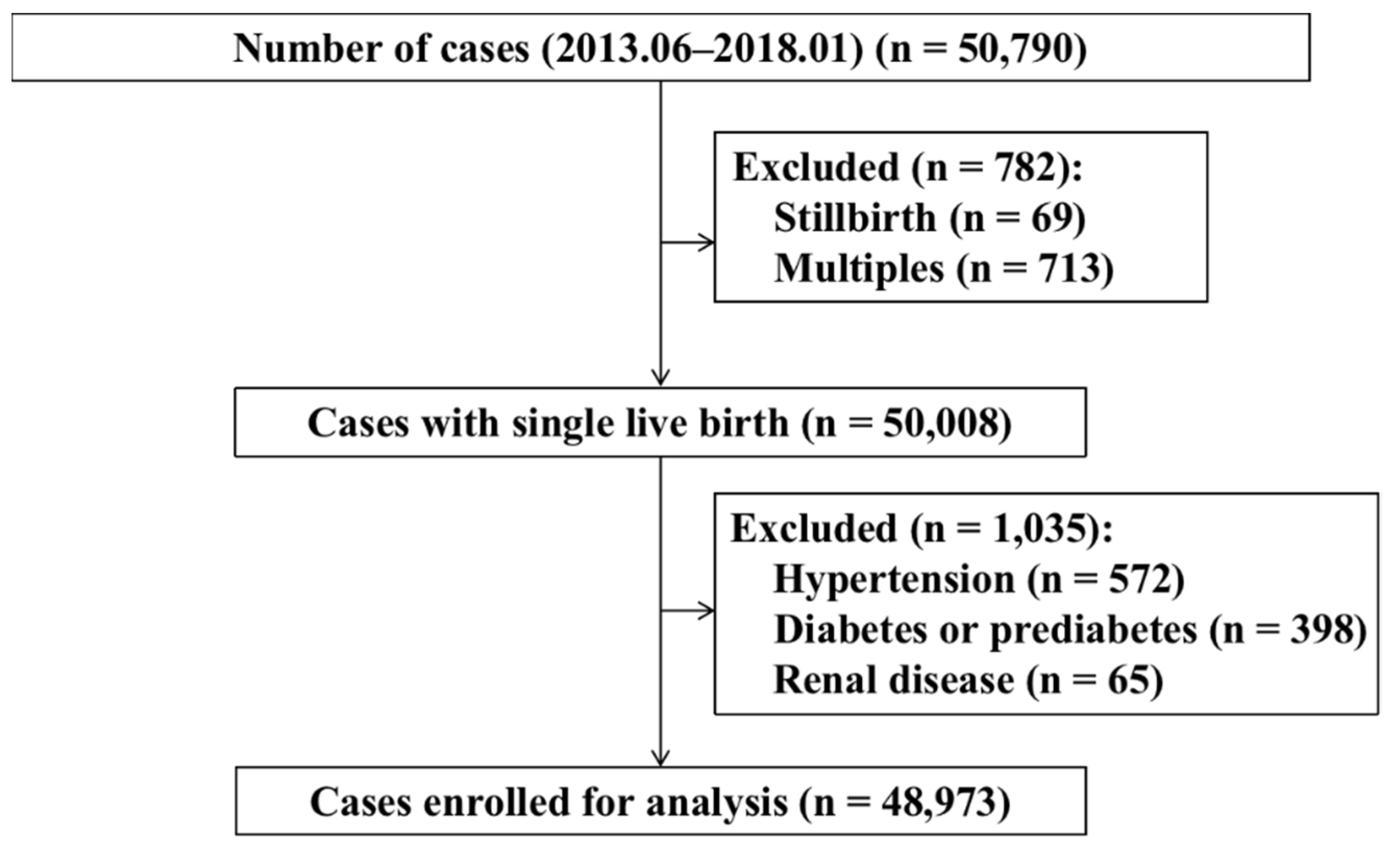
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. FPG Levels in Early Pregnancy and Risks of Pregnancy Outcomes
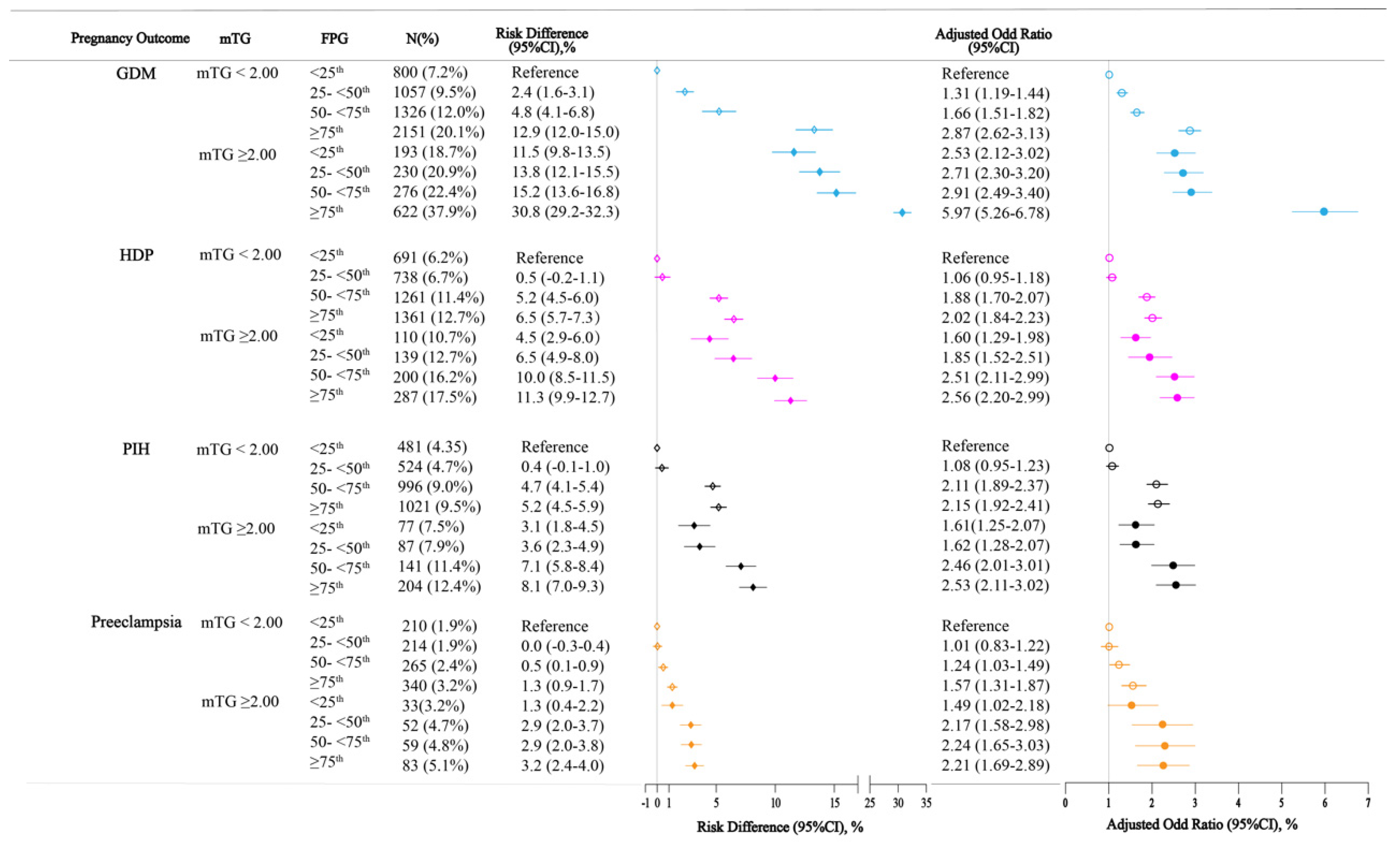
3.3. FPG Levels and TG Levels in Early Pregnancy and Risks of Pregnancy Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: A view from Denmark. Diabetologia 2016, 59, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. BMJ 2016, 354, i4694. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L., Jr.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Jovanovič, L.; Liang, Y.; Weng, W.; Hamilton, M.; Chen, L.; Wintfeld, N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes/Metab. Res. Rev. 2015, 31, 707–716. [Google Scholar] [CrossRef]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 130, e17–e37. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dainelli, L.; Yu, K.; Ma, L.; Silva Zolezzi, I.; Detzel, P.; Fang, H. The short-term health and economic burden of gestational diabetes mellitus in China: A modelling study. BMJ Open 2017, 7, e018893. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Wu, D.; Chen, Q.; Xiao, Z.; Wen, D.; Zhai, L.; Jia, L. Influence of Dietary Behaviors on Dyslipidemia in Pregnant Women and Its Effects on Physical Development of Fetuses and Infants: A Bidirectional Cohort Study. Nutrients 2021, 13, 3398. [Google Scholar] [CrossRef]
- Vrijkotte, T.G.; Krukziener, N.; Hutten, B.A.; Vollebregt, K.C.; van Eijsden, M.; Twickler, M.B. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J. Clin. Endocrinol. Metab. 2012, 97, 3917–3925. [Google Scholar] [CrossRef]
- Khaire, A.; Wadhwani, N.; Madiwale, S.; Joshi, S. Maternal fats and pregnancy complications: Implications for long-term health. Prostaglandins Leukot Essent Fat. Acids 2020, 157, 102098. [Google Scholar] [CrossRef]
- Gasevic, D.; Frohlich, J.; Mancini, G.B.; Lear, S.A. The association between triglyceride to high-density-lipoprotein cholesterol ratio and insulin resistance in a multiethnic primary prevention cohort. Metabolism 2012, 61, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Bridges, D.; Yang, Y.; Fisher, K.; Cheng, A.; Chang, L.; Meng, Z.X.; Lin, J.D.; Downes, M.; Yu, R.T.; et al. Metabolic crosstalk: Molecular links between glycogen and lipid metabolism in obesity. Diabetes 2014, 63, 2935–2948. [Google Scholar] [CrossRef]
- Persson, M.; Johansson, S.; Villamor, E.; Cnattingius, S. Maternal overweight and obesity and risks of severe birth-asphyxia-related complications in term infants: A population-based cohort study in Sweden. PLoS Med. 2014, 11, e1001648. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Schechtman, E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat—Which of these should we use? Value Health 2002, 5, 431–436. [Google Scholar] [CrossRef]
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014, 348, g2301. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Virally, M.; Laloi-Michelin, M. Methods for the screening and diagnosis of gestational diabetes mellitus between 24 and 28 weeks of pregnancy. Diabetes Metab. 2010, 36, 549–565. [Google Scholar] [CrossRef]
- Riskin-Mashiah, S.; Younes, G.; Damti, A.; Auslender, R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009, 32, 1639–1643. [Google Scholar] [CrossRef]
- Zhu, W.W.; Yang, H.X.; Wei, Y.M.; Yan, J.; Wang, Z.L.; Li, X.L.; Wu, H.R.; Li, N.; Zhang, M.H.; Liu, X.H.; et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Sacks, D.A.; Barbour, L.A.; Feig, D.S.; Catalano, P.M.; Damm, P.; McElduff, A. Issues with the Diagnosis and Classification of Hyperglycemia in Early Pregnancy. Diabetes Care 2016, 39, 53–54. [Google Scholar] [CrossRef]
- Jayasinghe, I.U.; Koralegedara, I.S.; Agampodi, S.B. Early pregnancy hyperglycaemia as a significant predictor of large for gestational age neonates. Acta Diabetol. 2022, 59, 535–543. [Google Scholar] [CrossRef]
- Sesmilo, G.; Meler, E.; Perea, V.; Rodríguez, I.; Rodríguez-Melcón, A.; Guerrero, M.; Serra, B. Maternal fasting glycemia and adverse pregnancy outcomes in a Mediterranean population. Acta Diabetol. 2017, 54, 293–299. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Jiang, F.; Chen, W.; Li, J.; Chen, X. Serum lipid levels in relation to clinical outcomes in pregnant women with gestational diabetes mellitus: An observational cohort study. Lipids Health Dis. 2021, 20, 125. [Google Scholar] [CrossRef]
- Lin, X.H.; Wu, D.D.; Li, C.; Xu, Y.J.; Gao, L.; Lass, G.; Zhang, J.; Tian, S.; Ivanova, D.; Tang, L.; et al. Maternal High Triglyceride Levels During Early Pregnancy and Risk of Preterm Delivery: A Retrospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 1249–1258. [Google Scholar] [CrossRef]
| Characteristics | n (%) | FPG Level (mM) | p Value | mTG Level (mM) | p Value |
|---|---|---|---|---|---|
| Total | 48,973 | 4.44 ± 0.36 | 1.34 ± 0.55 | ||
| Age, years | 0.000 | 0.000 | |||
| ≤24 | 1518 (3.1%) | 4.40 ± 0.35 | 1.20 ± 0.45 | ||
| 25–29 | 15,043 (30.7%) | 4.41 ± 0.36 | 1.27 ± 0.49 | ||
| 30–34 | 25,076 (51.2%) | 4.45 ± 0.36 | 1.35 ± 0.56 | ||
| ≥35 | 7336 (15.0%) | 4.51 ± 0.37 | 1.48 ± 0.61 | ||
| Pre-pregnancy BMI, kg/m2 | 0.000 | 0.000 | |||
| <18.5 | 5643 (11.5%) | 4.38 ± 0.36 | 1.15 ± 0.39 | ||
| 18.5–25 | 38,191 (78.0%) | 4.43 ± 0.36 | 1.33 ± 0.53 | ||
| ≥25 | 5139 (10.5%) | 4.56 ± 0.38 | 1.62 ± 0.69 | ||
| Parity, n | 0.000 | 0.000 | |||
| 0 | 39,520 (80.7%) | 4.43 ± 0.36 | 1.31 ± 0.53 | ||
| ≥1 | 9453 (19.3%) | 4.50 ± 0.37 | 1.46 ± 0.60 | ||
| Education, years | 0.000 | 0.000 | |||
| 9 | 843 (1.7%) | 4.51 ± 0.38 | 1.51 ± 0.68 | ||
| 10–12 | 2650 (5.5%) | 4.50 ± 0.36 | 1.41 ± 0.60 | ||
| 13–15 | 10,665 (22.0%) | 4.47 ± 0.36 | 1.36 ± 0.57 | ||
| ≥16 | 34,350 (70.8%) | 4.43 ± 0.36 | 1.32 ± 0.53 | ||
| Birthplace | 0.903 | 0.000 | |||
| Residents | 39,020 (79.7%) | 4.44 ± 0.36 | 1.33 ± 0.54 | ||
| Immigrants | 9953 (20.3%) | 4.44 ± 0.37 | 1.37 ± 0.57 | ||
| Category by FPG in early pregnancy | 0.000 | 0.000 | |||
| <25th percentile | 12,175 | 3.99 ± 0.19 | 1.30 ± 0.51 | ||
| 25– < 50th percentile | 12,181 | 4.33 ± 0.07 | 1.31 ± 0.53 | ||
| 50– < 75th percentile | 12,260 | 4.55 ± 0.07 | 1.34 ± 0.54 | ||
| ≥75th percentile | 12,357 | 4.90 ± 0.19 | 1.41 ± 0.61 | ||
| Category by mTG in early pregnancy | 0.000 | 0.000 | |||
| <90th percentile | 43,970 | 4.43 ± 0.36 | 1.20 ± 0.34 | ||
| ≥90th percentile | 5003 | 4.51 ± 0.39 | 2.52 ± 0.62 | ||
| GDM | 0.000 | 0.000 | |||
| No | 42,318 (86.4%) | 4.42 ± 0.35 | 1.30 ± 0.52 | ||
| Yes | 6655 (13.6%) | 4.60 ± 0.39 | 1.57 ± 0.67 | ||
| HDP | 0.000 | 0.000 | |||
| No | 44,186 (90.2) | 4.43 ± 0.36 | 1.33 ± 0.54 | ||
| Yes | 4787 (9.8%) | 4.55 ± 0.34 | 1.46 ± 0.64 | ||
| PIH | 3531 (7.2%) | 4.56 ± 0.33 | 1.44 ± 0.61 | ||
| Preeclampsia | 1256 (2.6%) | 4.53 ± 0.37 | 1.52 ± 0.70 | ||
| ICP | 0.039 | 0.606 | |||
| No | 48,518 (91.1%) | 4.45 ± 0.36 | 1.34 ± 0.55 | ||
| Yes | 455 (0.9%) | 4.41 ± 0.34 | 1.33 ± 0.50 | ||
| Postpartum hemorrhage | 0.011 | 0.000 | |||
| No | 48,237 (98.5%) | 4.44 ± 0.36 | 1.34 ± 0.55 | ||
| Yes | 736 (1.5%) | 4.48 ± 0.36 | 1.45 ± 0.63 |
| Variable | FPG in Early Pregnancy | p Value | |||
|---|---|---|---|---|---|
| <25th | 25– <50th | 50– <75th | ≥75th | ||
| Total | 12,175 | 12,181 | 12,260 | 12,357 | |
| GDM | 993 (8.2%) | 1287 (10.6%) | 1602 (13.1%) | 2773 (22.4%) | 0.000 |
| OR (95%CI) | reference | 1.33 (1.22–1.45) | 1.69 (1.56–1.84) | 3.26 (3.02–3.52) | |
| AOR (95%CI) | reference | 1.27 (1.16–1.38) | 1.56 (1.44–1.70) | 2.81 (2.60–3.05) | |
| HDP | 801 (6.6%) | 877 (7.2%) | 1461 (11.9%) | 1648 (13.3%) | 0.000 |
| OR (95%CI) | reference | 1.10 (0.99–1.22) | 1.92 (1.76–2.10) | 2.19 (2.00–2.39) | |
| AOR (95%CI) | reference | 1.08 (0.97–1.19) | 1.84 (1.68–2.02) | 1.98 (1.81–2.16) | |
| PIH | 558 (4.6%) | 611 (5.0%) | 1137 (9.3%) | 1225 (9.9%) | 0.000 |
| OR (95%CI) | reference | 1.10 (0.98–1.24) | 2.13 (1.92–2.36) | 2.29 (2.07–2.54) | |
| AOR (95%CI) | reference | 1.07 (0.95–1.21) | 2.03 (1.83–2.26) | 2.07 (1.87–2.30) | |
| Preeclampsia | 243 (2.0%) | 266 (2.2%) | 324 (2.6%) | 423 (3.4%) | 0.000 |
| OR (95%CI) | reference | 1.10 (0.92–1.31) | 1.33 (1.13–1.58) | 1.74 (1.48–2.04) | |
| AOR (95%CI) | reference | 1.07 (0.90–1.28) | 1.28 (1.08–1.52) | 1.57 (1.33–1.84) | |
| ICP | 126 (1.0%) | 117 (1.0%) | 113 (0.9%) | 99 (0.8%) | 0.282 |
| OR (95%CI) | reference | 0.93 (0.72–1.19) | 0.89 (0.70–1.15) | 0.77 (0.59–1.01) | |
| AOR (95%CI) | reference | 0.93 (0.72–1.20) | 0.88 (0.68–1.13) | 0.78 (0.60–1.02) | |
| Postpartum hemorrhage | 159 (1.3%) | 186 (1.5%) | 179 (1.5%) | 212 (1.7%) | 0.067 |
| OR (95%CI) | reference | 1.17 (0.95–1.45) | 1.12 (0.90–1.39) | 1.32 (1.07–1.62) | |
| AOR (95%CI) | reference | 1.14 (0.92–1.42) | 1.08 (0.87–1.34) | 1.22 (0.99–1.50) | |
| Preterm delivery | 468 (3.8%) | 518 (4.3%) | 438 (3.6%) | 511 (4.1%) | 0.029 |
| OR (95%CI) | reference | 1.11 (0.98–1.26) | 0.93 (0.81–1.06) | 1.08 (0.95–1.23) | |
| AOR (95%CI) | reference | 1.10 (0.97–1.26) | 0.90 (0.79–1.03) | 1.02 (1.05–1.39) | |
| SGA | 1254 (10.3%) | 1212 (9.9%) | 1182 (9.6%) | 1154 (9.3%) | 0.000 |
| OR (95%CI) | reference | 0.98 (0.90–1.06) | 0.95 (0.88–1.04) | 0.94 (0.86–1.02) | |
| AOR (95%CI) | reference | 0.99 (0.91–1.07) | 0.97 (0.89–1.05) | 0.96 (0.88–1.05) | |
| LGA | 1034 (8.5%) | 1192 (9.8%) | 1294 (10.6%) | 1477 (12.0%) | 0.000 |
| OR (95%CI) | reference | 1.17 (1.07–1.27) | 1.27 (1.16–1.38) | 1.45 (1.34–1.58) | |
| AOR (95%CI) | reference | 1.14 (1.04–1.25) | 1.21 (1.11–1.32) | 1.31 (1.20–1.42) | |
| Low birth weight (<2500 g) | 317 (2.6%) | 346 (2.8%) | 311 (2.5%) | 333 (2.7%) | 0.487 |
| OR (95%CI) | reference | 1.09 (0.94–1.28) | 0.97 (0.83–1.14) | 1.04 (0.89–1.21) | |
| AOR (95%CI) | reference | 1.09 (0.94–1.28) | 0.98 (0.83–1.14) | 1.02 (0.87–1.19) | |
| Macrosomia (≥4000 g) | 559 (4.6%) | 636 (5.2%) | 726 (5.9%) | 860 (7.0%) | 0.000 |
| OR (95%CI) | reference | 1.15 (1.02–1.29) | 1.31 (1.17–1.47) | 1.55 (1.39–1.73) | |
| AOR (95%CI) | reference | 1.10 (0.98–1.24) | 1.23 (1.10–1.38) | 1.35 (1.21–1.52) | |
| Apgar score ≤7 | 86 (0.7%) | 106 (0.9%) | 102 (0.8%) | 114 (0.9%) | 0.288 |
| OR (95%CI) | reference | 1.23 (0.93–1.64) | 1.18 (0.88–1.57) | 1.31 (0.99–1.73) | |
| AOR (95%CI) | reference | 1.22 (0.92–1.63) | 1.15 (0.86–1.53) | 1.23 (0.92–1.63) | |
| NICU | 1134 (9.3%) | 1280 (10.5%) | 1331 (10.9%) | 1360 (11.0%) | 0.000 |
| OR (95%CI) | reference | 1.14 (1.05–1.24) | 1.19 (1.09–1.29) | 1.20 (1.11–1.31) | |
| AOR (95%CI) | reference | 1.14 (1.05–1.24) | 1.19 (1.10–1.30) | 1.21 (1.11–1.32) | |
| TG Category Stratified by FPG Categories | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| FPG < 25th | 25th ≤ FPG < 50th | 50th ≤ FPG < 75th | FPG ≥ 75th | ||||||
| mTG < 2.00 | mTG ≥ 2.00 | mTG < 2.00 | mTG ≥ 2.00 | mTG < 2.00 | mTG ≥ 2.00 | mTG < 2.00 | mTG ≥ 2.00 | ||
| GDM | OR AOR | Reference | 2.97 (2.50–3.53) 2.53 (2.12–3.02) | Reference | 2.51 (2.15–2.95) 2.10 (1.78–2.48) | Reference | 2.11 (1.82–2.44) 1.77 (1.52–2.06) | Reference | 2.44 (2.18–2.72) 2.07 (1.85–2.32) |
| HDP | OR AOR | Reference | 1.81 (1.46–2.23) 1.57 (1.26–1.96) | Reference | 2.03 (1.68–2.46) 1.74 (1.43–2.13) | Reference | 1.50 (1.27–1.76) 1.34 (1.13–1.59) | Reference | 1.45 (1.27–1.68) 1.28 (1.10–1.48) |
| PIH | OR AOR | Reference | 1.79 (1.39–2.29) 1.51 (1.17–1.96) | Reference | 1.73 (1.37–2.20) 1.52 (1.19–1.93) | Reference | 1.30 (1.08–1.57) 1.16 (0.96–1.41) | Reference | 1.35 (1.15–1.59) 1.20 (1.02–1.42) |
| Preeclampsia | OR AOR | Reference | 1.72 (1.19–2.50) 1.60 (1.08–2.35) | Reference | 2.53 (1.85–3.44) 2.09 (1.52–2.89) | Reference | 2.04 (1.53–2.72) 1.85 (1.38–2.50) | Reference | 1.63 (1.27–2.08) 1.38 (1.07–1.78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Zhang, J.; Xiong, Y.; Wang, H.; Lu, D.; Guo, M.; Zhang, J.; Chen, L.; Fan, J.; Huang, H.; et al. Effect of Maternal Glucose and Triglyceride Levels during Early Pregnancy on Pregnancy Outcomes: A Retrospective Cohort Study. Nutrients 2022, 14, 3295. https://doi.org/10.3390/nu14163295
Wu D, Zhang J, Xiong Y, Wang H, Lu D, Guo M, Zhang J, Chen L, Fan J, Huang H, et al. Effect of Maternal Glucose and Triglyceride Levels during Early Pregnancy on Pregnancy Outcomes: A Retrospective Cohort Study. Nutrients. 2022; 14(16):3295. https://doi.org/10.3390/nu14163295
Chicago/Turabian StyleWu, Dandan, Jianlin Zhang, Yimeng Xiong, Hui Wang, Danyang Lu, Mengxi Guo, Jian Zhang, Lei Chen, Jianxia Fan, Hefeng Huang, and et al. 2022. "Effect of Maternal Glucose and Triglyceride Levels during Early Pregnancy on Pregnancy Outcomes: A Retrospective Cohort Study" Nutrients 14, no. 16: 3295. https://doi.org/10.3390/nu14163295
APA StyleWu, D., Zhang, J., Xiong, Y., Wang, H., Lu, D., Guo, M., Zhang, J., Chen, L., Fan, J., Huang, H., & Lin, X. (2022). Effect of Maternal Glucose and Triglyceride Levels during Early Pregnancy on Pregnancy Outcomes: A Retrospective Cohort Study. Nutrients, 14(16), 3295. https://doi.org/10.3390/nu14163295






