Short-Chain Fatty Acid Reference Ranges in Pregnant Women from a Mediterranean Region of Northern Spain: ECLIPSES Study
Abstract
:1. Introduction
2. Materials and Methods
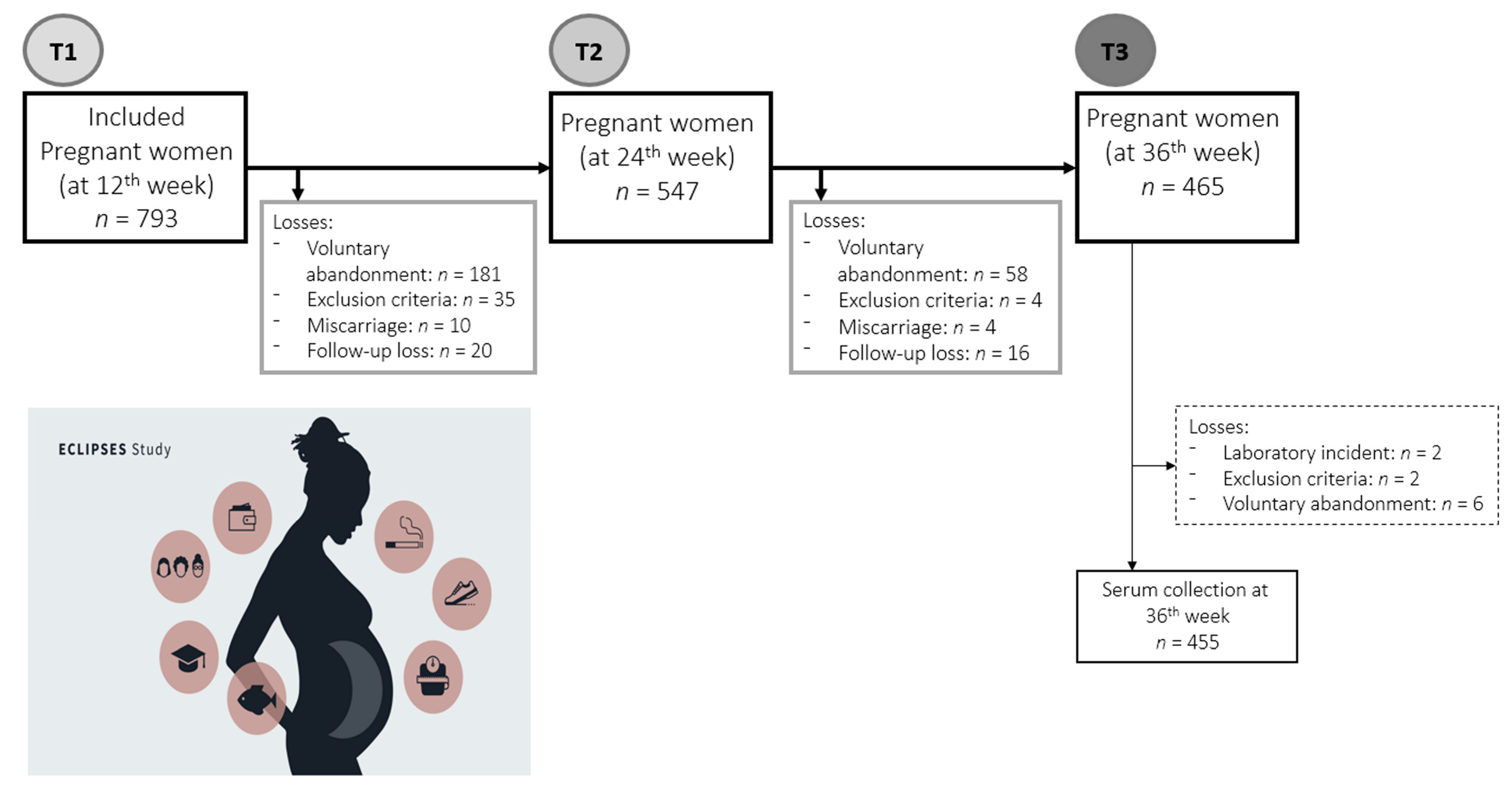
2.1. Study Design and Participants
2.2. Data Collection
2.3. Short-Chain Fatty Acids Measuress
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Short Chain Fatty Acids in Serum of Pregnant Women
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R.; Nguyen-Ngo, C.; Lappas, M. Short-chain fatty acids as novel therapeutics for gestational diabetes. J. Mol. Endocrinol. 2020, 65, 21–34. [Google Scholar] [CrossRef]
- Voltolini, C.; Battersby, S.; Etherington, S.L.; Petraglia, F.; Norman, J.E.; Jabbour, H.N. A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 2012, 153, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Al Mahri, S.; Malik, S.S.; Al Ibrahim, M.; Haji, E.; Dairi, G.; Mohammad, S. Free Fatty Acid Receptors (FFARs) in Adipose: Physiological Role and Therapeutic Outlook. Cells 2022, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Cai, S.; Wang, S.; Zeng, X.; Ye, C.; Chen, M.; Zeng, X.; Qiao, S. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J. Nutr. Biochem. 2019, 69, 98–107. [Google Scholar] [CrossRef]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, aaw8429. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Blaser, M.J.; Dominguez-Bello, M.G. The Human Microbiome before Birth. Cell Host Microbe 2016, 20, 558–560. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.; Feng, Q.Q.; Ojo, O.O.; Wang, X.H. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Thomas, A.; Alexandra, A.C.; Scholtens, D.M.; Wolever, T.M.S.; Josefson, J.L.; Layden, B.T. Maternal short-chain fatty acids are associated with metabolic parameters in Mothers and Newborns. Transl. Res. 2015, 164, 153–157. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Borengasser, S.J.; Barbour, L.A.; Friedman, J.E. Microbial transmission from mothers with obesity or diabetes to infants: An innovative opportunity to interrupt a vicious cycle. Diabetologia 2016, 59, 895–906. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhuang, X.; Luo, M.; Yin, W.; Xiong, L. The propionic acid and butyric acid in serum but not in feces are increased in patients with diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Akolekar, R.; Chelliah, A.; Mandal, R.; Dong, E.; Kruger, M.; Wishart, D.S.; Nicolaides, K. Metabolomic analysis for first-trimester trisomy 18 detection. Am. J. Obstet. Gynecol. 2013, 209, 65.e1–65.e9. [Google Scholar] [CrossRef] [PubMed]
- Sparvoli, L.G.; Cortez, R.V.; Daher, S.; Padilha, M.; Sun, S.Y.; Nakamura, M.U.; Taddei, C.R. Women’s multisite microbial modulation during pregnancy. Microb. Pathog. 2020, 147, 104230. [Google Scholar] [CrossRef]
- Taddei, C.R.; Cortez, R.V.; Mattar, R.; Torloni, M.R.; Daher, S. Microbiome in normal and pathological pregnancies: A literature overview. Am. J. Reprod. Immunol. 2018, 80, e12993. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2013, 150, 470–480. [Google Scholar] [CrossRef]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting iron dose supplementation in pregnancy for greater effectiveness on mother and child health: Protocol of the ECLIPSES randomized clinical trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Jardí, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; Study Group, T.E. Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients 2019, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- De Catalunya, G. Classificació Catalana D’ocupacions (CCO-2011). 2011. Available online: https://www.idescat.cat/serveis/biblioteca/docs/cat/cco2011.pdf (accessed on 24 August 2022).
- Edwards, M.K.; Loprinzi, P.D. Affective Responses to Acute Bouts of Aerobic Exercise, Mindfulness Meditation, and Combinations of Exercise and Meditation: A Randomized Controlled Intervention. Psychol. Rep. 2019, 122, 465–484. [Google Scholar] [CrossRef]
- Fagerström, K. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight, (s.f.). Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 August 2022).
- Institute of Medicine (US); National Research Council (US); Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K., Yaktine, A., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Rodríguez, I.; Ballart, J.; Pastor, G.; Jordà, E.; Arija-Val, V. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Favier, J.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Répertoire Général des Aliments: Tables de Composition; Technique & Documentation; INRA: Paris, France, 1995. [Google Scholar]
- Mataix, J.; García-Diz, L.; Mañas, M.; Martinez de Vitoria, E.; Llopis, J. Tablas de Composición de Alimentos, 5th ed.; Editorial Universidad de Granada: Granada, Spain, 2009. [Google Scholar]
- Norte Navarro, A.I.; Ortiz Moncada, R. Calidad de la dieta española según el índice de alimentación saludable. Nutr. Hosp. 2011, 26, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Cao, H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1083, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd ed; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2010.
- Martín-Grau, C.; Deulofeu, R.; Orus, N.S.; Arija, V. Trimester-specific reference ranges for saturated, monounsaturated and polyunsaturated fatty acids in serum of pregnant women: A cohort study from the eclipses group. Nutrients 2021, 13, 4037. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome during Pregnancy. MCN Am. J. Matern. Nurs. 2017, 42, 310–316. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Grace-farfaglia, P.; Frazier, H.; Iversen, M.D. Essential Factors for a Healthy Microbiome: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8361. [Google Scholar] [CrossRef] [PubMed]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Summary Statistics |
|---|---|
| Age (years) a | 30.6 ± 5.0 |
| Country of origin, Spain (%) | 84.1 |
| Primipara (%) | 37.5 |
| Gestational age at T1 (weeks) a | 12 ± 0.5 |
| Gestational age at birth (weeks) a | 39.8 ± 1.1 |
| BMI (kg/m2) at first trimester (%) | |
| 18.5–24.9 (normal weight) | 62.2 (22.1 ± 1.7) a |
| 25.0–29.9 (overweight) | 25.3 (27.3 ± 1.3) a |
| ≥30 (obesity) | 12.5 (33.3 ± 2.9) a |
| Gestational weight gain (kg) a | 10.8 ± 3.6 |
| Educational level (%) | |
| Low (primary or less) | 30.1 |
| Medium (high school) | 38.3 |
| High (university or more) | 31.6 |
| Occupation (%) | |
| Student | 2.4 |
| Employed | 87.1 |
| Unemployed | 10.5 |
| Smoking status (%) | |
| Smoker | 15.3 |
| Non-Smoker | 69.5 |
| Ex-Smoker | 15.3 |
| Alcohol consumption (%) | 14 |
| Physical Activity (METs min/week) at first trimester (%) | |
| Low (<600) | 56.4 |
| Moderate (≥600–2999) | 39.4 |
| High (≥3000) | 4.2 |
| SQDI (score) at first trimester a | 9.7 ± 2.6 |
| Range | Reference Interval | Percentile | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short Chain Fatty Acid | Min | Mean ± SD | Max | Percentiles (2.5–97.5%) | 2.5 | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 95 | 97.5 | |
| Acetic acid (C2:0) | T1 | 4.37 | 49.0 ± 21.4 | 172.8 | 16.4–103.8 | 16.4 | 21.4 | 29.2 | 34.5 | 38.4 | 42.0 | 45.7 | 49.7 | 53.9 | 60.7 | 72.2 | 84.9 | 103.8 |
| T3 | 13.9 | 48.5 ± 18.2 | 130.4 | 23.3–108.1 | 23.3 | 26.8 | 30.8 | 35.0 | 38.8 | 42.5 | 45.0 | 48.6 | 52.6 | 59.1 | 68.9 | 89.3 | 108.1 | |
| Propionic acid (C3:0) | T1 | 1.60 | 3.54 ± 0.87 | 6.55 | 2.1–5.8 | 2.1 | 2.3 | 2.5 | 2.8 | 3.1 | 3.3 | 3.5 | 3.7 | 3.9 | 4.2 | 4.6 | 4.9 | 5.8 |
| T3 | 1.63 | 3.52 ± 1.03 | 7.81 | 2.1–6.5 | 2.1 | 2.2 | 2.4 | 2.8 | 3.0 | 3.1 | 3.3 | 3.5 | 3.8 | 4.1 | 5.0 | 5.6 | 6.5 | |
| Isobutyric acid (C4:0) | T1 | 0.19 | 0.47 ± 0.19 | 1.35 | 0.16–1.01 | 0.16 | 0.21 | 0.26 | 0.33 | 0.38 | 0.41 | 0.45 | 0.48 | 0.53 | 0.57 | 0.66 | 0.83 | 1.01 |
| T3 | 0.24 | 0.45 ± 0.24 | 1.83 | 0.14–1.19 | 0.14 | 0.18 | 0.24 | 0.28 | 0.32 | 0.36 | 0.40 | 0.44 | 0.49 | 0.56 | 0.68 | 0.92 | 1.19 | |
| Butyric acid (C4:0) | T1 | 0.33 | 0.79 ± 0.33 | 1.98 | 0.32–1.67 | 0.32 | 0.37 | 0.43 | 0.52 | 0.60 | 0.67 | 0.73 | 0.82 | 0.89 | 1.05 | 1.26 | 1.48 | 1.67 |
| T3 | 0.41 | 0.91 ± 0.42 a | 2.70 | 0.37–2.09 | 0.37 | 0.42 | 0.49 | 0.58 | 0.66 | 0.74 | 0.81 | 0.90 | 1.03 | 1.16 | 1.52 | 1.70 | 2.09 | |
| Short Chain Fatty Acids (µmol/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid (C2:0) | Propionic Acid (C3:0) | Isobutyric Acid (C4:0) | Butyric Acid (C4:0) | |||||||||
| T1 (n = 454) | T3 (n = 454) | p | T1 (n = 449) | T3 (n = 449) | p | T1 (n = 450) | T3 (n = 450) | p | T1 (n = 457) | T3 (n = 457) | p | |
| Maternal Factors | Mean ± SD | Mean ± SD | T1–T3 | Mean ± SD | Mean ± SD | T1–T3 | Mean ± SD | Mean ± SD | T1–T3 | Mean ± SD | Mean ± SD | T1–T3 |
| All | 49.0 ± 21.4 | 48.5 ± 18.2 | 0.661 | 3.54 ± 0.87 | 3.52 ± 1.03 | 0.701 | 0.47 ± 0.19 | 0.45 ± 0.24 | 0.103 | 0.79 ± 0.33 | 0.91 ± 0.42 | <0.001 * |
| Age (years) | ||||||||||||
| <25 | 52.6 ± 22.7 | 43.8 ± 12.7 | 0.023 * | 3.52 ± 0.83 | 3.39 ± 0.94 | 0.351 | 0.46 ± 0.18 | 0.40 ± 0.17 | 0.101 | 0.81 ± 0.32 | 0.86 ± 0.38 | 0.181 |
| 25–34.9 | 49.4 ± 21.6 | 49.1 ± 18.9 | 0.731 | 3.55 ± 0.87 | 3.53 ± 1.05 | 0.872 | 0.47 ± 0.19 | 0.46 ± 0.26 | 0.182 | 0.79 ± 0.33 | 0.92 ± 0.44 | <0.001 * |
| ≥35 | 46.6 ± 20.8 | 49.6 ± 19.4 | 0.197 | 3.55 ± 0.91 | 3.54 ± 1.05 | 0.954 | 0.45 ± 0.19 | 0.46 ± 0.25 | 0.726 | 0.79 ± 0.35 | 0.87 ± 0.37 | 0.040 * |
| BMI (kg/m2) at T1 | ||||||||||||
| 18.5–24.9 (NW) | 49.3 ± 22.0 | 47.6 ± 18.1 | 0.305 | 3.53 ± 0.87 | 3.41 ± 0.96 | 0.134 | 0.48 ± 0.20 | 0.44 ± 0.23 | 0.071 | 0.81 ± 0.34 | 0.90 ± 0.41 | <0.001 * |
| 25.0–29.9 (OW) | 48.6 ± 19.6 | 50.5 ± 18.7 | 0.387 | 3.59 ± 0.92 | 3.61 ± 1.00 | 0.985 | 0.45 ± 0.18 | 0.47 ± 0.28 | 0.677 | 0.80 ± 0.32 | 0.87 ± 0.40 | 0.086 |
| ≥30 (O) | 48.9 ± 25.0 | 47.3 ± 19.2 | 0.732 | 3.65 ± 0.82 | 3.86 ± 1.34 a | 0.243 | 0.46 ± 0.19 | 0.43 ± 0.26 | 0.474 | 0.74 ± 0.30 | 0.92 ± 0.45 | 0.015 * |
| Gestational weight gain (kg) | ||||||||||||
| Insufficient | 49.9 ± 20.1 | 47.9 ± 18.2 | 0.411 | 3.54 ± 0.92 | 3.46 ± 1.07 | 0.786 | 0.47 ± 0.2 | 0.44 ± 0.24 | 0.331 | 0.81 ± 0.36 | 0.86 ± 0.38 | 0.148 |
| adequate | 48.8 ± 22.3 | 48.7 ± 17.2 | 0.937 | 3.64 ± 0.91 | 3.56 ± 1.00 | 0.450 | 0.48 ± 0.20 | 0.42 ± 0.21 | 0.011 | 0.77 ± 0.32 | 0.91 ± 0.42 | 0.003 * |
| Excessive | 48.8 ± 18.7 | 45.7 ± 19.1 | 0.353 | 3.51 ± 0.70 | 3.51 ± 1.19 | 0.892 | 0.47 ± 0.17 | 0.43 ± 0.27 | 0.133 | 0.77 ± 0.27 | 0.91 ± 0.48 | 0.024 * |
| Social class | ||||||||||||
| Low | 50.7 ± 19.6 | 48.3 ± 17.2 | 0.464 | 3.71 ± 0.82 | 3.76 ± 1.15 | 0.543 | 0.51 ± 0.24 | 0.42 ± 0.22 | 0.047 | 0.83 ± 0.34 | 0.93 ± 0.50 | 0.123 |
| Medium | 49.0 ± 20.9 | 48.1 ± 18.4 | 0.548 | 3.54 ± 0.86 | 3.50 ± 1.01 | 0.469 | 0.46 ± 0.17 | 0.44 ± 0.25 | 0.361 | 0.81 ± 0.33 | 0.91 ± 0.42 | <0.001 * |
| High | 49.7 ± 23.7 | 51.1 ± 19.4 | 0.782 | 3.47 ± 0.95 | 3.53 ± 1.04 | 0.764 | 0.47 ± 0.20 | 0.48 ± 0.26 | 0.884 | 0.74 ± 0.31 | 0.90 ± 0.38 | 0.004 * |
| Smoking during pregnancy | ||||||||||||
| No | 50.0 ± 22.5 | 48.9 ± 18.4 | 0.414 | 3.59 ± 0.88 | 3.54 ± 1.01 | 0.440 | 0.47 ± 0.19 | 0.46 ± 0.25 | 0.282 | 0.79 ± 0.32 | 0.92 ± 0.42 | <0.001 * |
| Yes | 45.0 ± 13.6 | 47.2 ± 17.8 | 0.311 | 3.32 ± 0.71 a | 3.41 ± 1.11 | 0.333 | 0.44 ± 0.18 | 0.38 ± 0.22 a | 0.172 | 0.81 ± 0.39 | 0.81 ± 0.36 a | 0.752 |
| Parity | ||||||||||||
| Primiparous | 50.8 ± 24.6 | 47.2 ± 17.5 | 0.127 | 3.53 ± 0.88 | 3.39 ± 1.00 | 0.307 | 0.48 ± 0.20 | 0.45 ± 0.25 | 0.302 | 0.82 ± 0.35 | 0.92 ± 0.43 | 0.003 * |
| Multiparous | 48.2 ± 19.0 | 49.6 ± 18.9 | 0.396 | 3.57 ± 0.85 | 3.61 ± 1.04 a | 0.677 | 0.46 ± 0.19 | 0.44 ± 0.24 | 0.254 | 0.78 ± 0.33 | 0.90 ± 0.41 | <0.001 * |
| Alcohol consumption | ||||||||||||
| No | 49.3 ± 21.5 | 48.7 ± 18.3 | 0.687 | 3.55 ± 0.87 | 3.58 ± 1.03 | 0.786 | 0.46 ± 0.19 | 0.44 ± 0.25 | 0.145 | 0.80 ± 0.34 | 0.91 ± 0.42 | <0.001 * |
| Yes | 46.4 ± 5.38 | 34.3 ± 3.6 | 0.037 | 3.90 ± 0.36 | 3.00 ± 0.59 | 0.219 | 0.53 ± 0.09 | 0.34 ± 0.08 | 0.117 | 0.77 ± 0.10 | 0.66 ± 0.24 | 0.959 |
| Physical Activity (METs/week) at T1 | ||||||||||||
| Low (<600) | 48.7 ± 21.2 | 48.4 ± 19.0 | 0.716 | 3.64 ± 0.92 | 3.58 ± 1.12 | 0.382 | 0.48 ± 0.20 | 0.48 ± 0.27 | 0.757 | 0.79 ± 0.31 | 0.89 ± 0.42 | <0.001 * |
| Moderate (600–2999) | 50.6 ± 21.6 | 49.2 ± 18.1 | 0.571 | 3.46 ± 0.82 | 3.49 ± 0.92 | 0.623 | 0.45 ± 0.18 | 0.42 ± 0.22 a | 0.119 | 0.82 ± 0.37 | 0.95 ± 0.42 | 0.001 * |
| High (≥3000) | 46.7 ± 18.3 | 46.3 ± 11.1 | 0.940 | 3.19 ± 0.53 a | 3.40 ± 0.93 | 0.401 | 0.48 ± 0.19 | 0.36 ± 0.13 | 0.050 * | 0.74 ± 0.24 | 0.89 ± 0.41 | 0.092 |
| SQDI (score) in T1 or T3 | ||||||||||||
| Low (0–6 pts) | 54.1 ± 20.3 | 47.6 ± 17.3 | 0.115 | 3.67 ± 0.90 | 3.54 ± 1.26 | 0.553 | 0.48 ± 0.19 | 0.46 ± 0.29 | 0.672 | 0.85 ± 0.37 | 0.86 ± 0.47 | 0.928 |
| Moderate (7–10 pts) | 49.0 ± 21.7 | 46.5 ± 17.5 | 0.239 | 3.59 ± 0.87 | 3.53 ± 1.07 | 0.547 | 0.48 ± 0.19 | 0.45 ± 0.23 | 0.237 | 0.81 ± 0.33 | 0.90 ± 0.43 | 0.026 * |
| High(11–18 pts) | 47.9 ± 22.1 | 47.3 ± 16.4 | 0.770 | 3.48 ± 0.87 | 3.53 ± 1.05 | 0.686 | 0.45 ± 0.19 | 0.41 ± 0.21 | 0.053 | 0.76 ± 0.31 | 0.89 ± 0.39 | 0.002 * |
| Fiber (g/d) in T1 or T3 | ||||||||||||
| t1 (<10 g/d) | 49.0 ± 21.8 | 47.4 ± 15.3 | 0.504 | 3.59 ± 0.88 | 3.56 ±1.07 | 0.803 | 0.46 ± 0.17 | 0.43 ± 0.22 | 0.222 | 0.80 ± 0.33 | 0.90 ± 0.41 | 0.031 * |
| t2 (10–14 g/d) | 49.3 ± 20.7 | 45.5 ±16.4 | 0.110 | 3.48 ± 0.87 | 3.39 ± 1.07 | 0.436 | 0.46 ± 0.19 | 0.42 ± 0.19 | 0.062 | 0.77 ± 0.32 | 0.85 ± 0.39 | 0.049 * |
| t3 (>14 g/d) | 49.6 ± 22.8 | 47.9 ±19.0 | 0.534 | 3.60 ± 0.88 | 3.63 ± 1.08 | 0.821 | 0.49 ± 0.21 | 0.46 ± 0.27 | 0.310 | 0.83 ± 0.33 | 0.91 ± 0.44 | 0.073 |
| Proteins (g/d) in T1 or T3 | ||||||||||||
| t1 (<49g/d) | 47.8 ± 22.1 | 47.7 ± 15.5 | 0.960 | 3.47 ± 0.86 | 3.57 ± 1.03 | 0.419 | 0.45 ± 0.19 | 0.43 ± 0.22 | 0.335 | 0.79 ± 0.34 | 0.92 ± 0.43 | 0.007 * |
| t2 (49–61g/d) | 50.3 ± 22.4 | 46.9 ± 17.8 | 0.194 | 3.55 ± 0.87 | 3.54 ± 1.11 | 0.953 | 0.49 ± 0.20 | 0.44 ± 0.23 | 0.064 | 0.76 ± 0.30 | 0.92 ± 0.44 | 0.001 * |
| t3 (>61 g/d) | 49.9 ± 20.7 | 46.3 ± 17.6 | 0.142 | 3.67 ± 0.90 | 3.49 ± 1.10 | 0.152 | 0.48 ± 0.19 | 0.44 ± 0.24 | 0.248 | 0.84 ± 0.34 | 0.83 ± 0.38 | 0.824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Grau, C.; Díaz-López, A.; Aparicio, E.; Arija, V. Short-Chain Fatty Acid Reference Ranges in Pregnant Women from a Mediterranean Region of Northern Spain: ECLIPSES Study. Nutrients 2022, 14, 3798. https://doi.org/10.3390/nu14183798
Martín-Grau C, Díaz-López A, Aparicio E, Arija V. Short-Chain Fatty Acid Reference Ranges in Pregnant Women from a Mediterranean Region of Northern Spain: ECLIPSES Study. Nutrients. 2022; 14(18):3798. https://doi.org/10.3390/nu14183798
Chicago/Turabian StyleMartín-Grau, Carla, Andrés Díaz-López, Estefania Aparicio, and Victoria Arija. 2022. "Short-Chain Fatty Acid Reference Ranges in Pregnant Women from a Mediterranean Region of Northern Spain: ECLIPSES Study" Nutrients 14, no. 18: 3798. https://doi.org/10.3390/nu14183798
APA StyleMartín-Grau, C., Díaz-López, A., Aparicio, E., & Arija, V. (2022). Short-Chain Fatty Acid Reference Ranges in Pregnant Women from a Mediterranean Region of Northern Spain: ECLIPSES Study. Nutrients, 14(18), 3798. https://doi.org/10.3390/nu14183798







