Abstract
Increasing evidence supports the importance of the gut microbiota (GM) in regulating multiple functions related to host physical health and, more recently, through the gut–brain axis (GBA), mental health. Similarly, the literature on the impact of physical activity (PA), including exercise, on GM and GBA is growing. Therefore, this narrative review summarizes and critically appraises the existing literature that delves into the benefits or adverse effects produced by PA on physical and mental health status through modifications of the GM, highlighting differences and similarities between preclinical and human studies. The same exercise in animal models, whether performed voluntarily or forced, has different effects on the GM, just as, in humans, intense endurance exercise can have a negative influence. In humans and animals, only aerobic PA seems able to modify the composition of the GM, whereas cardiovascular fitness appears related to specific microbial taxa or metabolites that promote a state of physical health. The PA favors bacterial strains that can promote physical performance and that can induce beneficial changes in the brain. Currently, it seems useful to prioritize aerobic activities at a moderate and not prolonged intensity. There may be greater benefits if PA is undertaken from a young age and the effects on the GM seem to gradually disappear when the activity is stopped. The PA produces modifications in the GM that can mediate and induce mental health benefits.
Keywords:
cognitive functions; anxiety; depression; microbiome; overweight; elderly; athletes; sports; overtraining; fitness 1. Introduction
In recent years, research on the interaction between gut microbiota (GM) and health has expanded considerably. High-throughput sequencing has favored and facilitated research on GM in multiple aspects [1]. Indeed, humans have specific microbial profiles at the oral, skin, reproductive organ, gastrointestinal and fecal levels [2,3]. In particular, the human gastrointestinal (GI) tract has a microbial diversity of up to 100 trillion microorganisms, predominantly bacteria but also archaea, viruses, and parasites. The GM encodes more than three million genes that produce hundreds of metabolites [4]. This vast ecosystem is susceptible to adaptive changes based on multiple factors, external and internal, such as genetics, age, diet, drugs, stress, physical activity (PA), including exercise [5,6]. Only recently, the effect of the PA on the GM has been more thoroughly investigated, although considerable difficulties persist in structuring research protocols capable of limiting the various confounding elements as much as possible.
Most evidence shows that a sedentary lifestyle is associated with a higher incidence of chronic diseases [7,8,9], on which PA can play a preventive and therapeutic role through different mechanisms. Recently, the potential effects produced on GM have been proposed as another modality through which PA can perform beneficial functions on host health [10]. In fact, the proliferation of some bacterial strains is also possible through PA, but it seems that a basic GM remains stable from adulthood to elderhood [3,4], while it would appear that between 20–60% of bacterial diversity could be influenced by diet and PA [11,12,13,14].
Although there is no gold standard for gut health, and its definition remains uncertain [15,16,17], the interaction between host PA can favor the growth of beneficial bacteria and gut cells that maintain the integrity of the intestinal surface, which acts as the first defense against pathogens. In return, the selected bacteria synthesize molecules capable of modifying the metabolism and immune functions of the host [18]. Furthermore, disruption of the gut barrier is considered one of the mechanisms of major depression and other cognitive and behavioral alterations, with the gut–brain axis (GBA) playing a role in mediating these processes [19]. In this context, PA seems able to influence this two-way communication interaction through the GBA [20].
This review aims to summarize the current evidence about the effects that PA has on GM and how GM, in turn, can influence physical performance and cognitive functionality, providing an overview of the currently most promising aspects in this field of research. We first discuss the extent to which GM can affect the physiological condition of its host and its influence on the onset of certain pathologies when an imbalance state occurs. Next, we analyze the evidence from preclinical studies concerning different PA modalities, the effects of PA on young or elderly subjects, as well as the interaction between PA and GM-mediated physical performance and overtraining. We then review the influence of PA on the human intestinal microbiota, specifically the role of physical fitness, the impact of endurance activity, and the effect that PA can exert on the microbiota of the elderly population; lastly, we discuss the relationship between PA and GM in the overweight subjects. Finally, we look at how and to what degree PA may exert certain effects on the GBA and indirectly on cognitive function.
2. Materials and Methods
This narrative review was carried out following the Narrative Review checklist [21]. Comprehensive research of Medline In-Process and other Non-Indexed Citations was conducted (prior to 31 March 2022), using MEDLINE(PubMed), Web of Science, and Google Scholar to retrieve relevant articles. The literature search was made using medical subject headings (MeSH) and Boolean syntax. Controlled terms were used to search for studies (“Physical Activity” OR “Physical Exercise” AND “Microbiota” OR “Microbiome”)/(“Sport” OR “Athletes” AND “Microbiota” OR “Microbiome”)/(“Gut-brain” OR “GBA” AND “Physical activity” OR “Physical exercise” AND “Microbiota” OR “Microbiome”). Following filters were used: Full text, pre-clinic or human studies, and English language. After candidate articles were collected, further identification was conducted based on inclusion and exclusion criteria.
Studies Selection
The inclusion criteria were only English-language original peer-reviewed articles, randomized and non-randomized studies, and observational and pilot studies published from January 2008 to March 2022. Excluded records were review articles, meta-analyses, practical guidelines, unpublished studies, editorials, letters to the editor, and essays, although they were used as an added measure to ensure search comprehensiveness and used as references.
We included pre-clinic (murine models) studies except for those involving nutritional supplementation to enhance exercise performance or pre/pro-biotics and/or antibiotics (in the 2 months before the intervention), and human studies with the exception of those that included subjects who take or have taken (in the 2-months before the intervention) pre/pro-biotics and/or antibiotics, as well as those that provided a specific sports supplementation intervention since evidence suggests that these conditions may lead to significant changes in the composition of gut microbiota. The search was not restricted to the frequency, intensity, type, or time (FITT) of exercise, gender, age, clinical condition, sample size, or length of follow-up.
Since human and animal studies analyzing the involvement of the gut microbiota in various responses to PA and exercise are still a young field of research, especially about the interaction between these and the GBA, we did not use restrictive criteria for selecting the papers for this narrative review.
Two team authors independently extracted relevant information from included studies: author, year of publication, study design, number and age of participants, species, type of PA carried out, protocol and diet assessment, duration of intervention, and main outcomes. Any disagreements were resolved by consensus. The characteristics of the studies were summarized, and data on the effects of PA on the metabolic variables of GM were synthesized.
3. Results
3.1. Identification of Studies
At the end of the selection process, 1907 articles were extracted, of which n = 823 from Web of Science, n = 727 from PubMed, and n = 357 from Google Scholar. Each title and abstract were screened for relevance, removing review articles, unpublished studies, meta-analyses, practical guidelines, editorials, letters to the editor, and essays (n = 876). Thereafter, the search strategy was based on the assessment of the full text of the remaining 121 articles to verify their eligibility. Lastly, on a total of 46 research articles selected, 19 focused on pre-clinic (murine) models and 27 on humans, specifically focusing on GM responses to PA, were included (Figure 1).
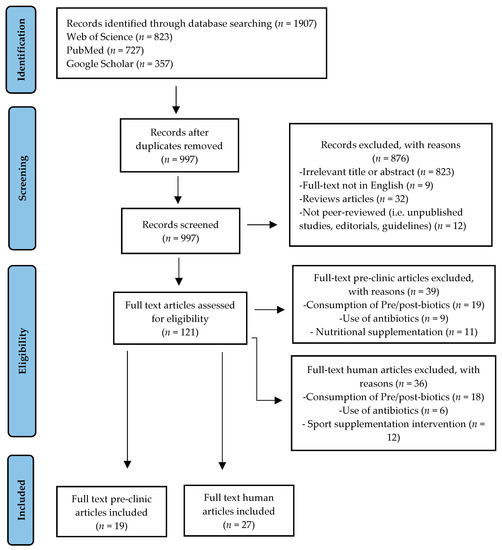
Figure 1.
Study selection and eligibility screening flow diagram.
3.2. Studies Characteristics
Thus, this narrative review provides an evaluation of 46 studies [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] inquiring in-depth about the PA-induced changes on GM, and the resulting physical and cognitive effects, in pre-clinic [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] and human models [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Most of them (n = 26) [23,24,26,28,31,34,38,39,41,49,50,51,52,53,54,55,57,58,59,60,61,62,64,65,66,67] regarded GM modifications following aerobic PA, while other works (n = 9) [29,32,33,35,36,46,48,56,63] focused on changes occurring after an aerobic endurance protocol, few studies (n = 3) [40,44,47] focus on the changes induced by concurrent training or (n = 3) [37,43,45] separate interventions of resistance and aerobic exercises, lastly, further works (n = 5) [22,25,27,30,42] investigated the effects produced on the GM following the practice of specific sports. Table 1 and Table 2 summarize the characteristics of the human and pre-clinic studies included.

Table 1.
Summary characteristics of reviewed human studies.

Table 2.
Summary characteristics of reviewed pre-clinic studies.
4. Discussion
4.1. The Gut Microbiota
Over millennia, human beings evolved symbiotically in environments where there was enormous microbic diversity [68]. The endosymbiosis theory even suggests that today’s eukaryotic cells were formed through the union of simpler cells, symbiotic bacteria, capable of performing certain specific functions on their own, such as extracting energy from organic compounds through aerobic respiration mechanisms. These conditions meant that over time, the bacteria themselves became completely dependent on the cell that had incorporated them, and similarly, the latter obtained an extra supply of energy in return [69,70,71].
Only in recent decades our knowledge of microbes cohabiting with humans has advanced to such an extent that we are able to make more concrete hypotheses about their functions and interactions with the host, as well as the mechanisms through which they are able to produce their effects. Since 2001, when Joshua Lederberg [72] came up with the term microbiome, studies on this subject have proliferated thanks also to new technologies capable of investigating this area. Especially since 2006, when Gill et al. [73] succeeded in sequencing the genome of human bacteria for the first time, demonstrating the presence of an interaction with lifestyle, diet, age, and other factors with the human intestinal bacterial composition. We now know that our bodies, and in particular our digestive tracts, are populated by a huge number of bacteria (microbiota) with which we have a symbiotic relationship that has a profound effect on our quality of life and whose gene expression (microbiome) is a second set of genes. The evolution of scientific knowledge has led us to look at our bodies no longer as simple organisms but rather as super-organisms that functions thanks to the perfect balance of a mixed genetic component, composed partly of human genes and partly of bacterial genes. Every time a part of these bacteria is destroyed, the relative balances within this ecosystem are altered and remain altered, sometimes for months or years. This results in a series of serious physiological imbalances that, in the long run, can give rise to the so-called diseases of progress [74].
The GM has important roles in the training of host immunity, digesting food, and endocrine and neurological functions, producing numerous compounds that influence the host. Thus, it appears that a considerable part of the environmental influence on human health and disease risk may be mediated or modified by microbial communities [75]. Alterations in the gut environment can affect GM community composition and function [76,77]. Through these pathways, the GM communicates with the host, affects host systems and organs, and modulates the host’s physiological functions, including glucose metabolism and liver function. Alteration in the GM composition can potentially be associated with various chronic diseases, such as allergic asthma, inflammatory bowel disease (IBD), major depression, and obesity [78].
New sequence technologies, together with measures of host physiology and mechanistic experiments in humans, animals, and cells, hold potential as initial steps in the identification of potential molecular mechanisms behind reported associations [79].
4.2. Physical Activity and Gut Microbiota in Preclinic Studies
It is now quite clear that, in animal models, exercise initiates significant changes in the GM. Matsumoto et al. [49], in a first study, investigated the effect of exercise on the gut microbiota, showing that after five weeks, mice given free access to the exercise wheel (VWR) had an altered microbiota composition compared with the control group of sedentary mice (SED); this was accompanied by the detection of changes in two species of butyrate-producing bacteria (SM7/11 and T2-87) with a relative twofold increase in butyrate concentration induced by exercise. This is important because butyrate, a short-chain fatty acid (SCFA), is the preferred energy source for epithelial cells lining the colon and has been shown to have multiple beneficial functions on gut function, including regulation of satiety, insulin sensitivity, and inflammation [80].
Allen et al. [55] found that voluntary exercise attenuates and forced exercise increases intestinal inflammation in a model of colitis, hypothesizing that these different exercise patterns may differentially influence GM. Choi et al. [50] evaluated the effects of six weeks of VWR on the microbiota by comparing healthy mice and mice given polychlorinated biphenyls (PCBs), carcinogenic toxins with adverse modulatory effects on gut function [81,82], some of which are currently banned due to their dioxin-like toxicological properties and risk of bioaccumulation in humans [83]. They found that exercise significantly attenuated the changes produced in the GM by PCBs, as demonstrated by a greater bacterial diversity in mice given PCBs after voluntary exercise for five weeks than in the group of sedentary mice that still received PCBs. Specifically, 67 taxa, groups of bacteria with similar morphological, biochemical, and genetic aspects, were found only in mice that exercised. Of these, half are from the Lactobacillales order, which demonstrates beneficial properties for colon health and immune function [84,85] as well as improved protective function in intestinal disorders [86,87]. These results could be related to experiments in germ-free (GF) mice showing that GM can regulate the expression of cytochrome P450, an enzyme involved in the metabolism of various foreign substances, including chemical pollutants [88].
Kang et al. [52] demonstrated that forced exercise (FWR), required to precisely control training volume, alters several groups of bacteria in both mice fed a normal diet and those on a high-fat diet (HFD), the latter of which is known to induce changes in the bacterial community and metabolic state of the host [89]. Similar results were found by Evans et al. [53], who sought to investigate whether exercise (VWR) can alter the GM in HFD-fed subjects. Specifically, this work observed the modification of multiple classes and families of bacteria following exercise, even in the group given an HFD. At a more general taxonomic level, exercise has shown the ability to increase the ratio of Bacteroidetes:Firmicutes, the main phyla in humans [90], where Bacteroidetes are directly associated with lean mass, and therefore, in the case of obesity, there is a reduction, and in parallel when obese subjects lose weight there is an increase [91]. In addition, according to Matsumoto et al. [49], Evans et al. [53] found a significant growth of butyrate-producing bacteria (e.g., Bacteroidales S24-7, Clostridiaceae, Lachnospiraceae, and Ruminococcaceae). Similar conclusions were reached by Campbell et al. [59] using a 12-week protocol and randomly assigning experimental subjects to four groups: lean sedentary (LS), sedentary (OS) with diet-induced obesity (DIO), lean active (LX) and DIO active (OX), with controlled caloric intake. They were able to observe that exercise (FWR) appears to influence the microbiota independently of diet. In this case, however, GM changes were analyzed at the genus level rather than at the phylum, class, or family level, highlighting the presence in active mice of bacteria related to Fecalibacterium prausnitzii, which may provide intestinal protection.
In agreement with Kang et al. [52], Denou et al. [60] also found an increased bacterial diversity in DIO mice fed an HFD following forced treadmill running (FTR); a proliferation of Bacteroidetes was observed in the cecum, colon, and feces with a consequent reduction in the Firmicutes:Bacteroidetes ratio. In contrast to Kang et al. [52], using a high-intensity interval training (HIIT) protocol did not result in a reduction in body mass, suggesting the possibility of different bacterial changes based on the exercise modality used. The data from this study suggest that exercise can counterbalance the changes made to the GM by obesity, but how this may occur is not entirely clear. Increased intestinal motility and reduced colonic blood flow during exercise are some of the possible modalities investigated.
Ribeiro et al. [64] highlighted that the use of a medium-low intensity exercise (50% of maximal velocity) induces insignificant changes in the GM to counteract the effects of a prolonged HFD.
4.3. Voluntary or Forced Exercise?
It has been found [55] that FWR results in a significant up-regulation of the Ruminococcus gnavus species, a bacterium capable of degrading the intestinal mucosa and thus penetrating its inner and outer layers, exposing intestinal epithelial cells to immunogenic bacterial proteins and thus exacerbating intestinal inflammation [92]. However, the regulation of mucin, the glycoprotein that makes up intestinal muscle, and the bacterial dynamics involved in relation to exercise have been little investigated. In the case of VWR, distinctive changes are induced both in the entire bacterial community and in individual taxa present in the GM of mice. Of note is the increase in the Anaerotruncus genus, a butyrate-producing bacterial group that colonizes the outer layer of the colonic mucosa and is phylogenetically related to Fecalibacterium prausnitzii, a known butyrate producer in humans [93,94]. These bacteria often feed on lactate, acetate, or other intermediates produced by other bacterial strains, and so it is possible that the activity-induced changes in so-called lactate producers (e.g., lactobacilli) and butyrate producers (e.g., Anaerotruncus) are related by a cross-feeding phenomenon.
Kellermayer et al. [95] also found a complete abolition of the Turicibacter population in so-called knockout mice, genetically modified not to express a given gene, for Toll-like receptor-2 (TLR-2), transmembrane molecules expressed in a number of intestinal cells (epithelial, B and T lymphocytes, macrophages) involved in the complex network of microbial pattern recognition associated with chronic intestinal inflammation disorders, although its role as a regulator of intestinal inflammation is still debated. Both papers thus suggest an immune and disease regulatory capacity of Turicibacter. This is related to exercise by the previously cited work of Evans et al. [53], which shows that VWR significantly reduces Turicibacter species populations in feces and caecum, indicating a possible immune-regulatory role of exercise through changes in the GM.
4.4. Exercise and Host Age
The period of life in which exercise is carried out would also appear to be important, thus representing age as a determining factor in the regulation of the GM. Indeed, some research has shown that the impact of GM on host physiology can be age-dependent; using GF mice, an early sensitive period has been identified during which the absence of an intact GM reflects physiological consequences such as the exaggerated activity of the hypothalamic-pituitary-adrenal (HPA) axis that can only be partly normalized by the introduction of Bifidobacteria infantis if administered during the early period of life [96].
Just as early exposure to certain microorganisms (e.g., Bifidobacteria species) can program the future immune system, it has recently been shown, in mice, that administration of antibiotics in the early period of life leads to an increase in total body mass as well as fat mass in adulthood [97]. This evidence reveals that the framework of GM during development can strongly influence the health of the host throughout life. Furthermore, just as physiological systems during development are remarkably malleable and sensitive to change, likewise the GM is more plastic in the early stages of life [98,99] and, consequently, the microbial ecosystem present during the early period of life may be more sensitive to environmental changes due in part to its lower stability and diversity than in adulthood. Thus, manipulations of environmental factors could have a more important impact on the GM framework if implemented early in life. Obviously, PA ranks well among those modifiable environmental factors, and so Mika et al. [58] hypothesize and note that exercise undertaken during early life (EL) may have a greater impact on GM than that undertaken in adulthood, corroborated recently by another study [100]. This is highlighted by comparison at the phylum level, where changes in phyla are only observed in young rats, and at the genus level, where a more important change in the microbial genus is observed in young than in adults. In terms of phylum, exercise in EL subjects increases the presence of Bacteroidetes, associated with a lean phenotype [101], while decreasing that of Firmicutes, which are correlated to obesity [52]. In this work, therefore, microbial patterns reflecting a lean phenotype were only observed in EL subjects indicating that the developmental stage during which exercise is performed may be important in determining this adaptive change in phyla. The absence of changes under the phylum profile in adult subjects, however, appears to contrast with the observation by Clarke et al. [22] in humans, Petriz et al. [54], and Evans et al. [53] in rats. Regarding the genus, six modified genera are identified in EL subjects and three in adults. In young mice, there is an increase in species Bifidobacteria and Methanosphaera, the latter belonging to the Archaea domain, which, by using hydrogen as an energy source [102], may help to make carbohydrate fermentation more efficient and less problematic. This study, therefore, suggests that although six weeks of exercise initiated in adulthood may alter the abundance of some bacterial genera, if the same activity is carried out in early life, there is a greater capacity to influence the overall structure of the microbial ecosystem. Furthermore, these changes in phylum and genus appear to be consistent with phenotypic changes in the body composition of EL subjects, who report an increase in lean mass, unlike adult rats, which agrees with the observations of Shindo et al. [103]. Nevertheless, it is emphasized that this effect might simply be the direct result of exercise, although the reported data set suggests that GM composition in young rats might play a role in promoting and maintaining gains in lean mass.
Measurement of α-diversity measures the variation of microbes in a single sample, i.e., the number of species (taxa) present (richness) and whether certain species are homogeneous or dominant (diversity) in the sample. Through this assessment, Mika et al. [58] observe a lower species richness in juvenile than in adult rats. A similar condition was observed in humans by Yatsunenko et al. [104], supporting the idea that it is precisely this condition of the young GM that encourages greater changes within it, unlike in the adult GM where the greater microbial complexity may make it more resistant to changes induced by environmental factors. In addition, they observe a lower Shannon value, which indicates a lower homogeneity in the microbial structure; the Shannon evenness index measures how homogeneously the bacteria are distributed in a specific sample without considering the number of species, so the lower this value is, the less homogeneous the microbial population will be. This, together with lower species richness, indicates that exercise in EL subjects can affect the uniformity of the bacterial community by selecting specific taxa. However, it should be noted that these results contrast with the findings of Clarke et al. [22] in humans and Petriz et al. [54] in several rat species. Furthermore, although this is the first work that attempts to investigate a relationship between exercise, GM, and age, the measurement of bacterial composition only in stool samples, the failure to control for the caloric intake of subjects, and the use of separate cohorts represent caveats that do not allow a more concrete causality to be extrapolated from the simple correlation.
4.5. Exercise, Performance and Overtraining Mediated by Gut Microbiota
Hsu et al. [56] sought to establish a relationship between performance and physical fatigue, antioxidant enzyme levels, and GM by comparing three groups: GF mice, GF mice colonized by a single genus of bacterium Bacteroides fragilis (BF), which is also normally present in the GM of the human colon, and pathogen-specific deprived (SPF) mice with an intact microbiota. By administering the endurance swimming test, commonly used to assess the degree of fatigue in animal models, the best time was obtained by SPF mice, with complete microbiota, followed by BF and finally GF mice. At the same time, a significant reduction in the activity of antioxidant enzymes was observed in GF mice, suggesting the influence of GM on the latter, which in turn can influence physical performance, as similarly reported by other studies [105,106]. A lower activity of glutathione peroxidase (GPx) and catalase (CAT), antioxidant enzymes that may contribute to counteracting the onset of fatigue, was found in GF mice compared to SPF and BF mice; therefore, the absence or limited presence of a bacterial community could be associated with an under-regulation of GPx and CAT activity, thus affecting physical performance.
Queipo-Ortuño et al. [51] evaluated differences in GM composition in rats during different nutritional conditions and PA and identified their relationship with leptin and ghrelin levels. The protocol involved dividing subjects into four groups: calorie restriction combined with voluntary PA (ABA), calorie restriction in the absence of PA (control ABA), ad libitum feeding with exercise (EG), and ad libitum feeding without PA (AL). Thus, there was a significant increase in Proteobacteria, Bacteroides, Clostridium, Enterococcus, Prevotella, and M.smithii and a consequent reduction in Actinobacteria, Firmicutes, and B. coccoides—E. rectale, Lactobacillus, and Bifidobacterium in the groups subjected to calorie restriction compared to those on ad libitum feeding. In particular, the increase in Prevotella species could be related to the degradation and reduction of mucin, which is essential for the integrity of the intestinal mucosa. On the other hand, in the EG group, Lactobacillus and Bifidobacterium genera increased, both capable of producing lactate, which is then converted into butyrate by specific GM bacteria. Thus, the potential negative impact on the number of bacteria beneficial to the health of the host and the increase in those potentially related to the destruction of the gastrointestinal barrier in subjects on calorie restriction accompanied by PA is highlighted. Although the researchers used male rats of the same weight from the same farm to reduce GM variation, the different types of diet during the protocol remain an important confounder when trying to extrapolate conclusions about the impact of PA on GM. Concordant, Petriz et al. [54], using a training protocol with a slightly shorter duration, four weeks, detected an increase in the Lactobacillus genus in obese rats following exercise. Recalling that this type of bacteria (LAB) in the gastrointestinal tract would appear to induce beneficial effects by influencing the intestinal microbiota, protecting the mucosa, and competing with pathogens [107], it is suggested that exercise can modify the GM at the level of the bacterial genus, with possible consequent beneficial effects, particularly in obese subjects.
Liu et al. [57], instead, sought to understand whether PA (VWR) could have an impact on the GM in ovariectomized (OVX) female rats fed with HFD. Specifically, as aerobic capacity is largely genetically predetermined [108], rats genetically selected to express high (HCR) or low (LCR) running capacity were used to assess how intrinsic fitness status might influence the impact of exercise on GM. Subjects were ovariectomized to reproduce a pattern like that established in post-menopausal women. The higher caloric intake and greater distance traveled found in the HCR group are associated with a relative abundance of Christensenellaceae and a reduced abundance of Clostridiaceae and Desulfovibrionaceae. In contrast to Turnbaugh et al. [89], HCR subjects show reduced α-diversity compared to the LCR group, while similar to Petriz et al. [54], an increase in the relative abundance of Firmicutes and reduction of Proteobacteria in HCR rats after exercise is noted. In the LCR group, as already observed [53], the opposite situation occurs. However, the relative abundance of Bacteroidetes appears to be unchanged within the different groups, contrary to the work of Petriz et al. [54] and Evans et al. [53]. It was observed that eleven weeks of VWR changed the relative abundance of different taxa at the family and genus level and that the type of this change depended on the genetic line (HCR vs. LCR). Due to the multiple variables considered to which altered hormonal balance is added, it becomes difficult to extrapolate conclusions regarding the exclusive impact of exercise on GM.
Li et al. [67] note that HFDs, by reducing microbial diversity, enhancing the proliferation of a pro-inflammatory microbiota, and increasing intestinal permeability (IP), can induce an increase in circulating Lipopolysaccharide (LPS) levels, endotoxins present on the membrane of certain bacteria, which are related to the onset of osteoarthritis [109]. PA (VWR) seems able to remodel the GM by increasing its diversity and consequently reducing LPS levels in the blood and synovial fluid. According to this work, PA, in addition to reducing body weight, may have a protective effect on cartilage by modifying the GM and reducing serum LPS levels.
Leigh et al. [66], in agreement with Lamoureux et al. [61] and Ribeiro et al. [64], after four weeks of moderate treadmill exercise, found no relevant changes in the overall composition of the GM. Specifically, when attempting to investigate the relationship between exercise and GM composition in rats exposed to two types of diet, they observed slight changes in the microbiota of those fed a standard chow diet but no changes to counteract the changes induced by a ‘cafeteria diet’ (CAF), a 10% sucrose solution along with commercial products such as biscuits and salty foods. These results may also be due in part to the use of this type of diet (CAF), which results in greater metabolic changes than the classic purified HFD used in the other studies.
Yuan et al. [63] set out to assess the negative effects of excessive exercise on the immunity system, energy metabolism, and GM. After four weeks of the protocol (excessive ES swimming), they found reduced α-diversity and β-diversity compared with the control group. In terms of phylum, the ES group showed an abundance of Bacteroidetes and Firmicutes at the ileocecal level and a reduction in Proteobacteria. In the families, there is a lower presence of Bacteroidales: S24-7 and Lachnospiraceae and an increase in Helicobacteraceae. At the genus level, there is an increase in Helicobater and Bacteroides and a reduction in Odoribater. It is thus observed that certain disease-related bacteria appear to be present to a greater extent in the ES group than in the control group. However, due to the limited sample, these differences do not have statistical significance.
4.6. Physical Activity and Gut Microbiota in Human Studies
Clarke et al. [22] performed the first study on athletes. Expecting to find greater bacterial diversity in athletes than in sedentary subjects, they set up the study by recruiting 40 male rugby players with an average BMI of 29.1 and, due to their size, two control groups were composed of sedentary subjects, one with a BMI ≤ 25 (LBG) and the other with a BMI > 28 (HBG). Stool and blood samples were collected from all participants. Thus, greater richness and α-diversity were observed in the athletes than in the control groups, in agreement with Mörkl et al. [25]. Microbial diversity is positively associated with protein intake, suggesting that exercise and diet are drivers of gut bacterial diversity. In the athletes’ group, an increase in butyrate-producer phylum (Firmicutes) and genus (F. prausnitzii) was observed [93,94]. Furthermore, athletes and LBG show increased Akkermansiaceae family and Akkermansia genus, associated with metabolic disorders [110]. Despite these interesting correlations between exercise and GM, the research appears confounded by its nature as a cross-sectional study and no control of confounders. Three years later, researchers involved in this work conducted a new study [30] through which they try to understand whether the taxonomic differences noted in their previous work [22] are reflected at the functional level. They re-examined the GM of professional rugby players during pre-season training, investigating the metagenome. They observed, in athletes, the strengthening of certain pathways, which express a beneficial potential for the host, such as those for amino acids (AA) biosynthesis and carbohydrate metabolism. Finally, there is a high concentration of SCFAs in the feces.
Bressa et al. [24] found no modification in GM richness and diversity (α and β) in people who perform 30 min of moderate-intensity PA three days a week [111]. A food frequency questionnaire (FFQ) is administered for nutritional assessment, and stool samples are collected for microbial assessment. No changes in the Bacteroidetes:Firmicutes ratio were found. A lower abundance of the family Turicibacteraceae and genus Turicibacter is observed in the active group, as already found in animal studies [53,55,112], showing the first correlation between certain bacterial taxa and the introduction of exercise in humans. A significant abundance of Coprococcus genus and certain species (R. hominis, A. muciniphila and F. prausnitzii) in the active group compared to the sedentary group was observed, species associated with positive effects on health [113,114,115]. Although macronutrient and caloric intake were similar between the groups, different intakes of fruit/vegetables and animal products that can alter the GM [116] may confound the results.
Castellanos et al. [39], using data collected through their previous observational study [117] from a cohort of 109 volunteers (18–40 years old, BMI 20–30) classified as active, at least 3 h of activity per week, or sedentary, no minimum PA as defined by the World Health Organization (WHO) [111], analyzed the GM to identify the relevant bacteria involved in the reorganization of the microbial network. They identified bacterial taxa considered to be ‘key’ microorganisms for the structure of the GM since the pathogenicity of certain bacteria is not always and only related to their abundance but also to other factors such as interactions with other microorganisms. Roseburia fecis species, considered a health marker, Rikenellaceae and Erysipelotrichaceae families, whose role is not yet clear, were found in active subjects, while unclassified species of Sutterella genus, associated with impairment of cognitive and immune system function, were found in the sedentary group, suggesting that the GM of active subjects have higher efficiency.
No changes were found after three weeks of HIIT protocol, indicating that short-term HIIT protocol does not impact the fecal bacterial community and that progress in the cardiorespiratory fitness (CRF) does not lead to modification of GM in the short term [41], similarly to Moitinho-Silva [45] after six weeks of exercise
4.7. Influence of Physical Fitness on the Gut Microbiota
Estaki et al. [23] analyzed the composition of the GM as a function of the CRF, the overall capacity of the cardiovascular and respiratory systems that contributes to the ability to perform prolonged and strenuous exercise. To isolate, as far as possible, the influence of physical fitness on GM diversity, stool samples of subjects with different fitness levels, assessed by means of the cycle test for VO2peak, and a comparable diet were analyzed. After recruiting 39 healthy subjects (18–35 years) and interviewing them about their dietary habits (24 h dietary recall), they found that in individuals with higher CRF levels, bacterial diversity (α and β) is higher, regardless of diet. No single bacterial taxon or group of taxa showed significant variation in relation to CRF levels, but the functionality of the GM appears to favor an increase in chemotaxis-related genes, which are useful for bacterial nutritional processes, and a reduction in LPS biosynthesis pathways, indicating that microbial diversity and aerobic fitness are related. A study conducted by Yang et al. [26] on 71 premenopausal women (19–49 years), using a cycle ergometer to measure VO2max and analyzing stool samples, came to similar conclusions. Low aerobic fitness correlates positively with a higher abundance of Eubacterium rectale–Clostridium coccoides (EreC), present in obese subjects [118], Enterobacteria, and a decrease in Bacteroides. However, these results lose relevance due to the various limitations present, first and foremost the percentage of fat mass of subjects with low aerobic fitness. These, in fact, have a higher BMI than subjects with a higher CRF, and once the results are corrected for this factor, the observed differences disappear.
A year later, Durk et al. [34] looked for a new correlation between CRF and GM composition in young (22–32 years) and healthy subjects. CFR is assessed by the Symptom-Limited Maximal Grade Treadmill Exercise Test to measure VO2max, while the dietary aspect was asked to follow the usual diet for seven days by tracking intake through MyFitnessPal.com. They observed a correlation between VO2max and the Firmicutes:Bacteroidetes ratio. Although limitations like the collection of information on different variables, such as caloric intake, through subjective reports from participants, the results of this study support other similar work in both animal models and humans [23,26,55], indicating the PA as a factor that can positively affect the human GM. Similarly, Paulsen et al. [28], in a longitudinal study of breast cancer survivors using submaximal treadmill testing, found that CRF was associated with increased β-diversity, as also found by Estaki et al. [23] where, however, β-diversity was not explained by VO2max but by protein intake. The nature of a pilot study and thus the small sample size is the main limitation.
Bycura et al. [43] examined the changes in the GM following a separate double intervention for the duration of eight weeks characterized by cardiorespiratory exercise (CRE) or resistance training exercise (RTE). A total of 28 subjects (18–26 years old) were assigned to the CRE group and 28 subjects (18–33 years old) to the RTE group. In contrast to Quiroga et al. [40], aerobic and resistance exercises were analyzed separately, observing that only aerobic activity causes an initial significant change within the GM that subsequently decreases until it becomes irrelevant.
4.8. Endurance Activities and Gut Microbiota
A first pilot study conducted by Petersen et al. [27], on 22 professional cyclists and 11 amateurs at a competitive level, investigated whether a difference in GM composition between professionals and amateurs can be detected through an analysis of the metagenome (representative of the species present) and the metatranscriptome (representative of the functions expressed in a specific environment). There was a significant correlation between the presence of the genus Prevotella, with concomitant upregulation of BCAAs, and the time spent training (>11 h/week) in both professionals and amateurs. A decrease in Bacteroides and, among 30 cyclists, an increase in the genus Akkermansia was noted, as already observed [22]. It was also observed that the increased presence of Prevotella correlates with certain carbohydrate and AAs metabolic pathways, including the biosynthesis of BCAAs [119]. However, as no FFQs were administered for dietary analysis, the abundance of Prevotella may be due, at least in part, to the high calorie and carbohydrate intake typical of a cyclist’s diet, coupled with the high weekly hours spent on training. In professional athletes, the metatranscriptomic analysis showed a high percentage of M.smithii compared to their amateur counterparts, associated with energy efficiency [120,121].
A second work by Scheiman et al. [35] was conducted on 15 marathon runners during the Boston Marathon and 10 sedentary subjects. A relative abundance of the species Veillonella atypica was observed, which can positively influence running performance through the conversion of lactate, produced by muscle activity, into propionate (SCFA), which is used for energy purposes. It has been shown that propionate can increase heart rate, VO2max, and influence blood pressure in mice [122,123] as well as increase resting energy expenditure and lipid oxidation in fasted humans [124]. While it was shown for the first time that GM could significantly contribute to physical performance and its benefits, how this may occur remains uncertain. It could be that the high lactate environment in the athlete results in a selective advantage for colonization by lactate metabolizing bacteria such as those of the Veillonella species.
Keohane et al. [36] investigated the GM response of four ultra-endurance athletes during an ocean crossing (33 days). During this event, a change in α-diversity was detected independently of changes in CRF, withVO2Max similar pre- and post-race. Changes in taxonomic composition were observed, including an increase in butyrate-producing species (Roseburia hominis and members of the genus Subdoligranulum) and species associated with improved insulin sensitivity (Dorea longicatena). Bacterial species that have shown increased gene expression include Prevotella, as already observed by Petersen et al. [27]. Many of these changes, moreover, persisted in the following three months of follow-up. It was observed [29] that significantly increased species richness after endurance running within a single day (half marathon) in 20 healthy amateur runners in the families of Coriobacteriaceae e Succinivibrionaceae involved in the metabolism of steroids and activation of dietary polyphenols in the human gut [125]. Although α-diversity remains substantially stable, this finding suggested that long-distance running can promote, throughout the GM, the regulation of energy and hormone levels to maintain the homeostasis of electrolyte and gut environment.
Morishima et al. [46] investigated the relationship between intensive exercise and the GM status, in female elite endurance runners (ER), compared with the non-athletic healthy control group. They found that in the ER group, some bacteria associated with gut inflammation (Haemophilus, Rothia, and Ruminococcus ganvus) were more abundant. Counterintuitively, Fecalibacterium, known as a beneficial butyrate producer, was also more abundant in the ER group. This could be explained by the fact that an abnormal intestinal environment prompts the Fecalibacterium to produce succinate, a risk factor for diarrhea and loose stools [126], and not butyrate. This suggests that prolonged high-intensity exercise may lead to a form of dysbiosis in the athlete.
4.9. Physical Activity and Bacterial Changes in the Elderly Population
Morita et al. [37], in a non-randomized comparative study, investigated the effects of an aerobic exercise protocol on the GM composition of healthy elderly women and found a relative abundance of Bacteroides, associated with improvement in metabolic disease [91,127] in subjects who performed the aerobic activity, which was positively correlated with an improvement in the 6 min walking test. One study [33] attempted to assess whether endurance activity can modulate GM in elderly men and whether these changes are associated with specific cardiometabolic conditions in the host. While genus Oscillospira, associated with reduced BMI [128,129], increases, species Clostridioides difficile decreases. Furthermore, changes in these taxa were correlated with changes in several cardio-metabolic risk factors such as systolic and diastolic blood pressure. Researchers found that after six months of combined training, seven high-intensity and eight moderate-intensity exercises [47], Oscillospira, Bifidobacterium, and Anaerostipes, health-related genus [130,131], were increased.
Fart et al. [42] focused on identifying GM profiles related to healthy aging through GM analysis of senior orienteering athletes and found that 18% of the total fecal GM consists of F. prausnitzii, but no increase in microbial diversity was found, and a lower abundance of Parasutterella excrementihominis and Bilophila wadsworthia associated with decreased intestinal health [132,133,134]. An observational study [48] tried to assess the impact of endurance exercise performed four times per week, throughout life, in elderly athletes (EA), on the GM diversity and structure, compared to the control group that only met the PA recommendation for older adults [135]. Although no significant differences were found, there was a lower presence of the Bacteroides genus and an increased abundance of the Prevotella genus, as already observed in previous studies [22,40]. The small sample size is the main limitation. At least it was found that eight weeks of combined exercise protocol in untrained older women [44] led to a decrease in Firmicutes phylum, Bacteroidaceae family, and Clostridioides Escherichia genera, related to detrimental outcomes [136,137].
4.10. Physical Activity, Gut Microbiota and Overweight
Among the few longitudinal studies, Allen et al. [31] sought to investigate the effects of exercise on GM. Thirty-two sedentary, normal-weight (BMI < 25) and obese (BMI > 30) adult subjects (20–45 years) were recruited. After two weeks of baseline testing, they were administered an aerobic activity protocol (30 to 60 min, three sessions per week, moderate to intense 60–75% HRR) lasting six weeks, followed by an equal number of weeks during which the subjects did not exercise (washout). Participants completed a food diary for seven days prior to the intervention; a dietician then drew up an eating plan based on the diary, which the participants had to follow for the three days prior to the collection of stool samples, while on the remaining days they were asked to follow their usual eating patterns including the use of caffeine, alcohol, and supplements normally taken. Thus, no significant differences in β-diversity were found after the exercise protocol and maintained similarity even after the washout period. Aerobic activity induced a significant increase in SCFAs concentration in the feces, supporting what has previously been observed in both animals and humans [23,49], but only in normal-weight subjects; however, this effect disappeared during the washout period. The exercise seemed able to alter the abundance of certain microbial species in a BMI-dependent manner: with normal-weight subjects experiencing an increase in Fecalibacterium and a decrease in Bacteroides genus, while obese subjects experienced the exact opposite. Together with the concentration of SCFAs, many of the bacterial taxa increased with exercise and decreased following the washout period, suggesting that the effect of exercise on the GM is transient and reversible.
Munukka et al. [32] sought to determine whether an endurance protocol could affect the gut metagenome in previously sedentary overweight subjects. In order to determine individual aerobic and anaerobic thresholds, a submaximal incremental endurance test is administered, and then the 6-week (3 × week) intervention protocol is applied, with increasing intensity from light to moderate. It thus appears that this endurance protocol, in agreement with Allen et al. [31], is capable of modestly impacting the GM framework. As already observed [22,24,27], exercise increased the genus Akkermansia, which is inversely associated with body weight [63]. Furthermore, increased Proteobacteria, which may adversely affect human health [138,139], was found.
To establish whether a combined strength and endurance protocol can exert an effect on the GM of obese children (7–12 years), Quiroga et al. [40] prepared a 12-week (2 × week) concurrent training protocol through which it was found that obese subjects exhibit a bacterial profile associated with this condition. Subsequently, this protocol was shown to alter GM composition and function in obese subjects by significantly reducing the phylum Proteobacteria, corroborating the results obtained by Munukka et al. [32] and the class Gammaproteobacteria. Furthermore, an increasing trend of some bacterial genera as part of the phylum Firmicutes was observed, which made the GM of obese subjects like that of normal-weight control subjects. These results, therefore, suggest the presence of a negative bacterial profile related to the state of obesity that can be positively modified by PA. However, the need for further studies with a larger sample size is highlighted to increase the statistical power of these findings.
Finally, in the longest randomized controlled study to date, Kern et al. [38] set out to assess whether exercise alters the diversity, composition, and functionality of the GM in overweight or obese subjects. Specifically, the effects of regular aerobic training carried out with different modalities and intensities, but with similar energy expenditure, on the GM of 88 subjects (20–45 years old) divided into four groups: commuters traveling by non-motorized bicycles (BIKE), moderate-intensity PA (MOD) or vigorous PA (VIG) and the control group leading their usual lives (CON) are investigated over a six-month period using a specific protocol (ACTIWE) [140]. In all groups that performed PA, changes in β-diversity were observed, while low heterogeneity was found in the VIG group. In the MOD group, changes in α-diversity were noted. These discrepancies between studies may be due to the lack of a non-exercising control group and the small sample size used in previous studies. Similarly, since the primary objective of the researchers in this study is to evaluate the impact of prescribing exercise to overweight or obese subjects in a daily life context, the dietary intake was not voluntarily standardized, which makes it difficult to determine whether the observed results are due to the exercise per se or its effect on the subjects’ diet.
4.11. The Gut–Brain Axis Functional Basis and the Impact of Physical Activity
Only recently a connection between GM and host brain function has been identified. This relationship seems to be the result of the ability of different bacterial species to exert an influence on brain activity [141] through neuroendocrine pathways probably developed initially to connect the gut and the areas of the brain responsible for stimuli and the control of hunger/satiety and, subsequently, extended to the influence on emotions linked to different eating behaviors [142] mediated by a particular neurotrophic factor such as Brain-Derived Neurotrophic Factor (BDNF), whose concentration can be modulated by the GM [143]. This two-way communication pathway between the enteric (ENS) and central nervous systems (CNS) is known as the gut–brain axis (GBA). Several papers in recent years have highlighted the importance of this relationship as reported by Erny et al. [144], who note that host bacteria regulate the maturation and function of microglia, immune defense cells in the CNS and that impairment of these cells can be rebalanced to some extent by a diverse and complex microbiota. Furthermore, it seems that the microbiota is necessary for the normal development of the amygdala both structurally and functionally and may contribute to several brain functions dependent on this nucleus, from pain sensitivity to social behavior via emotion regulation [145].
Recent evidence suggested that PA, particularly of an aerobic nature, improving the diversity and abundance of certain bacterial genera may have positive effects on the gut and neurology [20]. The bi-directional communication between the gut and the brain has embryogenic origins as the CNS and the ENS, or the metasympathetic system, have identical tissue derivation [146]. Communication between these two systems is achieved through neuroendocrine, immune, and neuronal regulation [147] and, in particular, is mediated through signals traveling via the vagus nerve and the hypothalamic-pituitary-adrenal (HPA) axis [148]. Through these pathways, alterations in the gut microbiota can influence the communication that takes place between the gut and the brain [20]. Indeed, several molecules produced by the GM, such as SCFAs, bile acids, and tryptophan, interact with enteroendocrine cells by propagating a long-distance signal and activating the vagus nerve [149]. For example, if the information received centrally from the vagus nerve indicates an imbalance in the composition of the GM, the CNS decides what action to take, usually adverse to the health of the host. Conversely, supplementation with specific probiotics has been shown to improve brain function by promoting an anxiolytic effect, which is lessened by vagotomy [143]. Similarly, GM via LPS can damage the intestinal mucosa, which promotes the release of pro-inflammatory cytokines capable of hyper-stimulating the HPA [150], a condition associated with psychological disorders [151]. Several behaviors appear to be influenced by GM, which depend on a properly functioning serotonergic neurotransmission; it should be noted that 90% of serotonin, known as the main cognitive and mood regulator [152], can be synthesized and regulated by specific gut bacteria [149].
4.12. Physical Activity, Gut Microbiota and Cognitive Ability
According to Gubert et al. [153], considering the concomitant intestinal dysbiosis present in several neurodegenerative diseases and the impact that PA or exercise can have on the intestinal microbiome and neuronal degeneration, a triangulation between these aspects seems plausible to assess whether PA can contribute to the modulation of neurodegeneration through GM. Among the few studies that tried to find a correlation between PA and GM in humans, none have investigated this neural aspect in-depth, whereas some studies in animal models have shown some initial evidence.
Kang et al. [52] showed that mice subjected to a 16-week training protocol reported an improvement in memory associated with the increase in the Firmicutes:Bacteroidetes ratio induced by exercise. They also point out that one hour per day of exercise can increase the relative abundance of the family Lachnospiraceae, which is negatively correlated with anxiogenic behavior and is capable of producing butyrate (SCFA), a molecule that over-regulates BDNF expression in the hippocampus and frontal cortex, supporting the survival of existing neurons and stimulating the formation of new neurons and synapses [154]. Thus, according to the authors, this association between induced changes in certain GM phyla and families and memory improvement could be used as a biomarker for exercise-induced effects at a cognitive level.
Feng et al. [62] considered a condition known as postoperative cognitive dysfunction (POCD), whereby the memory and learning of individuals undergoing surgery decline, particularly in the elderly [155]. Applying a treadmill training protocol during the six weeks prior to surgery, performed five days a week, prevented POCD, assessed through the Morris Maze Test, along with an increase in microbial diversity (α and β) and improvement in the Firmicutes:Bacteroidetes ratio in favor of the former, indicating an improvement in dysbiosis following surgery. Concomitantly, the ability of exercise to reduce the postoperative neuroinflammatory state is observed. Thus, the authors suggested that GM contributed to the modulation of inflammatory status through a pathway mediated by the exercise performed previously since a low GM diversity appears associated with increased inflammation [156]. According to Gubert et al. [153], experimental data have shown that GM modifications induced by an aerobic activity, specifically characterized by an increase in certain bacteria (e.g., Lactobacillus plantarum and Streptococcus thermophile), are capable of inducing the synthesis of serotonin, a molecule that protects against symptoms of anxiety and depression [157].
Abraham et al. [65] extrapolated findings that seem to indicate the ability of exercise to improve cognitive function and some markers of Alzheimer’s disease (AD), neurodegenerative disease for which there is currently no cure. They hypothesize that regular exercise combined with nutritional intervention may reduce the incidence of AD. Using transgenic mice (APP/PS1), which mimic a model of AD, subjected to a 20-week treadmill exercise protocol, they note a significant improvement in the Morris Maze Test, which assesses spatial memory, and a reduction in β-amyloid plaques, one of the main aspects involved in AD [158], supporting what was previously observed by Lin et al. [159]. Near these plaques, an increase in microglia, important for brain development by providing structural and metabolic support to neurons and involved in neuroplasticity and regulation of neural repair [160], is found, underlining the neuroprotective effect of exercise. This appears to be associated with the abundance of some bacterial strains (Eubacteria, Roseburia) and the reduction of others, so these results suggest that the cognitive effects of exercise may be mediated through GM alteration, reducing the levels of microbes involved in disease exacerbation and promoting the abundance of those bacteria capable of producing SCFAs that appear beneficial. Similar bacterial changes were highlighted in a meta-analysis that sought to identify a relationship between GM and Parkinson’s disease (PD). The ecological imbalance of the GM can induce IP, impaired SCFA production, and altered immunity function. Therefore, alteration of the GM could be considered an environmental trigger of the pathological process of PD and contribute to its development [161]. This would seem to support the so-called dual-hit hypothesis that PD is due to peripheral dysregulations of the GM, known as dysbiosis. This makes it possible to speculate that the benefits of exercise observed in PD may be partly due to its ability to restore GM [162].
5. Limitations and Future Directions
Despite the promising future and interest in this research area, multiple aspects make the development of adequate study protocols complicated, first and foremost the not easy dissociation between PA and nutrition. Furthermore, in preclinical studies, the same type of diet (LFD, HFD, LC, HC) provided to animals may interfere differently with the GM depending on the type of diet used, from the more classic standard chow, which has manufacturer-dependent variability, to the purified diet. The studies carried out on humans appear to be more difficult to interpret, first of the difficulty of dietary control also due to the instruments used for this purpose that are imprecise, starting from the most accurate modality, the food diary, that can psychologically lead subjects to eat better, to the most used instruments, the FFQ, subject to a high degree of uncertainty. Different types of bacterial genome sequencing methods and different types of PA used in the studies make it difficult to extrapolate guidelines. Future research should isolate more confounding elements, first and foremost the dietary aspect, with a greater focus on young and elderly populations, as well as on resistance training protocols where evidence is scarce. Specific attention should be paid to the development of research in humans to investigate the concrete impact of PA on the GBA.
6. Conclusions
Although the literature analyzing GM is steadily increasing, the effects of PA on bacterial flora remain uncertain. A PA or exercise performed voluntarily appears to attenuate intestinal inflammation, in contrast to a forced activity that instead increases this condition in animal models. Vigorous endurance exercise can negatively affect the GM framework in humans; furthermore, only aerobic activities can alter the GM structure, probably because of the positive correlation between CRF and microbial diversity.
PA can stimulate bacterial community richness by altering SCFAs-producing species, as well as favoring the colonization of health and athletic performance-promoting strains (e.g., A. muciniphila and Veillonella). The ability to promote a bacterial composition capable of protecting the intestinal mucosa from possible permeability and counteracting HFD-induced changes in the GM is observed.
Overall, PA can increase the relative abundance of phylum Bacteroidetes and reduce that of Firmicutes as well as may exert greater and more lasting benefits if undertaken from an early age, but the effects on GM seem to gradually disappear when the PA is no longer practiced.
PA seems able to regulate cognitive conditions (e.g., anxiety and depression) and functionality (e.g., Alzheimer’s and Parkinson’s Disease) through modifications of microbial composition and subsequently the production of certain protective molecules, to date in animal models.
Author Contributions
Conceptualization, S.C. and L.P.; methodology, G.G., A.P. and A.D.B.; software, F.N.Ş., A.P. and G.G.; validation, F.N.Ş., L.S. and B.G.; resources, L.S., A.B. and B.G.; writing—original draft preparation, S.C. and L.P.; writing—review and editing, G.G., A.B. and A.D.B.; supervision, F.F.; project administration, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, G.; Zhao, N.; Zhang, C.; Lam, Y.Y.; Zhao, L. Guild-based analysis for understanding gut microbiome in human health and diseases. Genome Med. 2021, 13, 22. [Google Scholar] [CrossRef]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Zhu, X.; Moan, E.; Murff, H.J.; Ness, R.M.; Seidner, D.L.; Sun, S.; Yu, C.; Dai, Q.; Fodor, A.A.; et al. Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci. Rep. 2018, 8, 4139. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Ballini, A.; Boccellino, M.; Scacco, S.; Lovero, R.; Charitos, I.A.; Santacroce, L. The Intestinal Microbiota May Be a Potential Theranostic Tool for Personalized Medicine. J. Pers. Med. 2022, 12, 523. [Google Scholar] [CrossRef]
- González, K.; Fuentes, J.; Márquez, J.L. Physical Inactivity, Sedentary Behavior and Chronic Diseases. Korean J. Fam. Med. 2017, 38, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Laye, M.J.; Lees, S.J.; Rector, R.S.; Thyfault, J.P. Reduced physical activity and risk of chronic disease: The biology behind the consequences. Eur. J. Appl. Physiol. 2008, 102, 381–390. [Google Scholar] [CrossRef]
- Zhao, R.; Bu, W.; Chen, Y.; Chen, X. The Dose-Response Associations of Sedentary Time with Chronic Diseases and the Risk for All-Cause Mortality Affected by Different Health Status: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.E.; Farnleitner, A.H. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Hanage, W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014, 512, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.W.; Priya, S.; Blekhman, R.; Bordenstein, S.R. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018, 16, e2006842. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, F.; Liu, S.; Liu, G.; Yang, X.; Gao, W.; Fan, K.; Zhao, H.; Ma, J. Significance of gastrointestinal tract in the therapeutic mechanisms of exercise in depression: Synchronism between brain and intestine through GBA. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 103, 109971. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Narrative Review Checklist. Available online: https://www.elsevier.com/__data/promis_misc/ANDJ%20Narrative%20Review%20Checklist.pdf (accessed on 29 April 2022).
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar]
- Mörkl, S.; Lackner, S.; Müller, W.; Gorkiewicz, G.; Kashofer, K.; Oberascher, A.; Painold, A.; Holl, A.; Holzer, P.; Meinitzer, A.; et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int. J. Eat. Disord. 2017, 50, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Supportive Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef] [PubMed]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Keohane, D.M.; Woods, T.; O’Connor, P.; Underwood, S.; Cronin, O.; Whiston, R.; O’Sullivan, O.; Cotter, P.; Shanahan, F.; Molloy, M.G.M. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport 2019, 22, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.; Blond, M.B.; Hansen, T.H.; Rosenkilde, M.; Quist, J.S.; Gram, A.S.; Ekstrøm, C.T.; Hansen, T.; Stallknecht, B. Structured exercise alters the gut microbiota in humans with overweight and obesity-A randomized controlled trial. Int. J. Obes. 2020, 44, 125–135. [Google Scholar] [CrossRef]
- Castellanos, N.; Diez, G.G.; Antúnez-Almagro, C.; Bressa, C.; Bailén, M.; González-Soltero, R.; Pérez, M.; Larrosa, M. Key Bacteria in the Gut Microbiota Network for the Transition between Sedentary and Active Lifestyle. Microorganisms 2020, 8, 785. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Cree, J.; Adams, S.E.; MacRae, C.; Skidmore, P.; Cameron-Smith, D.; Gant, N.; Blenkiron, C.; Merry, T.L. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp. Physiol. 2020, 105, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Fart, F.; Rajan, S.K.; Wall, R.; Rangel, I.; Ganda-Mall, J.P.; Tingö, L.; Brummer, R.J.; Repsilber, D.; Schoultz, I.; Lindqvist, C.M. Differences in Gut Microbiome Composition between Senior Orienteering Athletes and Community-Dwelling Older Adults. Nutrients 2020, 12, 2610. [Google Scholar] [CrossRef] [PubMed]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wen, X.; Yang, M.; Lai, H.Y.; Momma, H.; Cheng, L.; Sun, X.; Nagatomi, R.; Huang, C. Effect of an 8-week Exercise Training on Gut Microbiota in Physically Inactive Older Women. Int. J. Sports Med. 2021, 42, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Moitinho-Silva, L.; Wegener, M.; May, S.; Schrinner, F.; Akhtar, A.; Boysen, T.J.; Schaffer, E.; Hansen, C.; Schmidt, T.; Ruhlemann, M.C.; et al. Short-term physical exercise impacts on the human holobiont obtained by a randomised intervention study. BMC Microbiol. 2021, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Morishima, S.; Aoi, W.; Kawamura, A.; Kawase, T.; Takagi, T.; Naito, Y.; Tsukahara, T.; Inoue, R. Intensive, prolonged exercise seemingly causes gut dysbiosis in female endurance runners. J. Clin. Biochem. Nutr. 2021, 68, 253–258. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Liu, J.; Johnson, R.; Dillon, S.; Jankowski, C.M.; Kroehl, M.; Robertson, C.E.; Frank, D.N.; Tuncil, Y.; Higgins, J.; et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 2021, 8, 20499361211027067. [Google Scholar] [CrossRef] [PubMed]
- Šoltys, K.; Lendvorský, L.; Hric, I.; Baranovičová, E.; Penesová, A.; Mikula, I.; Bohmer, M.; Budiš, J.; Vávrová, S.; Grones, J.; et al. Strenuous Physical Training, Physical Fitness, Body Composition and Bacteroides to Prevotella Ratio in the Gut of Elderly Athletes. Front. Physiol. 2021, 12, 670989. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Berg Miller, M.E.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.W.; Park, Y.M.; Holscher, H.D.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K.S. Physical Activity Differentially Affects the Cecal Microbiota of Ovariectomized Female Rats Selectively Bred for High and Low Aerobic Capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLoS ONE 2015, 10, e0125889. [Google Scholar]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, E.V.; Grandy, S.A.; Langille, M. Moderate Exercise Has Limited but Distinguishable Effects on the Mouse Microbiome. mSystems 2017, 2, e00006-17. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Uchida, Y.; Koch, L.; Britton, S.; Hu, J.; Lutrin, D.; Maze, M. Exercise Prevents Enhanced Postoperative Neuroinflammation and Cognitive Decline and Rectifies the Gut Microbiome in a Rat Model of Metabolic Syndrome. Front. Immunol. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Ribeiro, C.; Ana Claudia, M.G.; Castro, A.P.; Almeida, J.A.; Franco, O.L.; Petriz, B.A. Limited Effects of Low-to-Moderate Aerobic Exercise on the Gut Microbiota of Mice Subjected to a High-Fat Diet. Nutrients 2019, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Leigh, S.J.; Kaakoush, N.O.; Escorihuela, R.M.; Westbrook, R.F.; Morris, M.J. Treadmill exercise has minimal impact on obesogenic diet-related gut microbiome changes but alters adipose and hypothalamic gene expression in rats. Nutr. Metab. 2020, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, A.; Zong, W.; Dai, L.; Liu, Y.; Luo, R.; Ge, S.; Dong, G. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J. Biol. Sci. 2021, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Hwang, I. Understanding the evolution of endosymbiotic organelles based on the targeting sequences of organellar proteins. New Phytol. 2021, 230, 924–930. [Google Scholar] [CrossRef]
- Zachar, I.; Boza, G. Endosymbiosis before eukaryotes: Mitochondrial establishment in protoeukaryotes. Cell. Mol. Life Sci. CMLS 2020, 77, 3503–3523. [Google Scholar] [CrossRef] [PubMed]
- Gabaldón, T. Relative timing of mitochondrial endosymbiosis and the “pre-mitochondrial symbioses” hypothesis. IUBMB Life 2018, 70, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Piccini, F. Alla Scoperta del Microbioma Umano: Flora Batterica, Nutrizione e Malattie del Progresso; Amazon: London, UK, 2017; ISBN 9781521884195. [Google Scholar]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.; De Vos, P.; Dekker, J.; et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci. Rep. 2019, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, G.; Robertson, L.W. Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett. 2013, 334, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Rude, K.M.; Pusceddu, M.M.; Keogh, C.E.; Sladek, J.A.; Rabasa, G.; Miller, E.N.; Sethi, S.; Keil, K.P.; Pessah, I.N.; Lein, P.J.; et al. Developmental exposure to polychlorinated biphenyls (PCBs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ. Pollut. 2019, 253, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Norström, K.; Czub, G.; McLachlan, M.S.; Hu, D.; Thorne, P.S.; Hornbuckle, K.C. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int. 2010, 36, 855–861. [Google Scholar] [CrossRef]
- Boesten, R.J.; de Vos, W.M. Interactomics in the human intestine: Lactobacilli and Bifidobacteria make a difference. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. S3), S163–S167. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. Role of intestinal bacteria in nutrient metabolism. J. Parenter. Enter. Nutr. 1997, 21, 357–365. [Google Scholar] [CrossRef]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. American journal of physiology. Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Claus, S.P.; Ellero, S.L.; Berger, B.; Krause, L.; Bruttin, A.; Molina, J.; Paris, A.; Want, E.J.; de Waziers, I.; Cloarec, O.; et al. Colonization-induced host-gut microbial metabolic interaction. mBio 2011, 2, e00271-10. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Lay, C.; Sutren, M.; Rochet, V.; Saunier, K.; Doré, J.; Rigottier-Gois, L. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 2005, 7, 933–946. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Tailford, L.E.; Owen, C.D.; Walshaw, J.; Crost, E.H.; Hardy-Goddard, J.; Le Gall, G.; de Vos, W.M.; Taylor, G.L.; Juge, N. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat. Commun. 2015, 6, 7624. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Kellermayer, R.; Dowd, S.E.; Harris, R.A.; Balasa, A.; Schaible, T.D.; Wolcott, R.D.; Tatevian, N.; Szigeti, R.; Li, Z.; Versalovic, J.; et al. Colonic mucosal DNA methylation, immune response, and microbiome patterns in Toll-like receptor 2-knockout mice. FASEB J. 2011, 25, 1449–1460. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Ryan, C.A.; Hussey, S.; Murphy, B.; Fitzgerald, G.F.; Stanton, C. Role of gut microbiota in early infant development. Clinical medicine. Pediatrics 2009, 3, 45–54. [Google Scholar]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- McNamara, M.P.; Singleton, J.M.; Cadney, M.D.; Ruegger, P.M.; Borneman, J.; Garland, T. Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice. J. Exp. Biol. 2021, 224 Pt 4, jeb239699. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Wilson, M. Microbial Inhabitants of Humans: Their Ecology and Role in Health and Disease; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Shindo, D.; Matsuura, T.; Suzuki, M. Effects of prepubertal-onset exercise on body weight changes up to middle age in rats. J. Appl. Physiol. 2014, 116, 674–682. [Google Scholar] [CrossRef][Green Version]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef]
- Klaenhammer, T.; Altermann, E.; Arigoni, F.; Bolotin, A.; Breidt, F.; Broadbent, J.; Cano, R.; Chaillou, S.; Deutscher, J.; Gasson, M.; et al. Discovering lactic acid bacteria by genomics. Antonie Van Leeuwenhoek 2002, 82, 29–58. [Google Scholar]
- Bouchard, C.; Lesage, R.; Lortie, G.; Simoneau, J.A.; Hamel, P.; Boulay, M.R.; Pérusse, L.; Thériault, G.; Leblanc, C. Aerobic performance in brothers, dizygotic and monozygotic twins. Med. Sci. Sports Exerc. 1986, 18, 639–646. [Google Scholar] [CrossRef]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nature reviews. Rheumatology 2016, 12, 123–129. [Google Scholar]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2010. [Google Scholar]
- Mahizir, D.; Briffa, J.F.; Wood, J.L.; Anevska, K.; Hill-Yardin, E.L.; Jefferies, A.J.; Gravina, S.; Mazzarino, G.; Franks, A.E.; Moritz, K.M.; et al. Exercise improves metabolic function and alters the microbiome in rats with gestational diabetes. FASEB J. 2020, 34, 1728–1744. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Castellanos, N.; Diez, G.G.; Antúnez-Almagro, C.; Bailén, M.; Bressa, C.; González Soltero, R.; Pérez, M.; Larrosa, M. A Critical Mutualism—Competition Interplay Underlies the Loss of Microbial Diversity in Sedentary Lifestyle. Front. Microbiol. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Cella, V.; Migliaccio, S.; Paoli, A. Microbiota intestinale ed esercizio fisico: Nuova possibile area di intervento? L’Endocrinologo 2020, 21, 338–343. [Google Scholar] [CrossRef]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Hansen, E.E.; Manchester, J.K.; Coutinho, P.M.; Henrissat, B.; Fulton, R.; Latreille, P.; Kim, K.; Wilson, R.K.; Gordon, J.I. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. USA 2007, 104, 10643–10648. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Clavel, T.; Lepage, P.; Charrier, C. The family coriobacteriaceae. In The Prokaryotes, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 201–238. [Google Scholar]
- Tsukahara, T.; Ushida, K. Succinate accumulation in pig large intestine during antibiotic-associated diarrhea and the constitution of succinate-producing flora. J. Gen. Appl. Microbiol. 2002, 48, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tims, S.; Derom, C.; Jonkers, D.M.; Vlietinck, R.; Saris, W.H.; Kleerebezem, M.; de Vos, W.M.; Zoetendal, E.G. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013, 7, 707–717. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Hold, G.L.; Duncan, S.H.; Gruhl, B.; Collins, M.D.; Lawson, P.A.; Flint, H.J.; Blaut, M. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 2002, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Shekoohi, N.; Covic, A.; Mikhailidis, D.P.; Banach, M. Adverse Impact of Desulfovibrio spp. and Beneficial Role of Anaerostipes spp. on Renal Function: Insights from a Mendelian Randomization Analysis. Nutrients 2020, 12, 2216. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, H.; Wu, S.D.; Lu, N.; Wang, Y.T.; Liu, H.N.; Dong, L.; Liu, T.T.; Shen, X.Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef]
- Baron, E.J. Bilophila wadsworthia: A unique Gram-negative anaerobic rod. Anaerobe 1997, 3, 83–86. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Kreznar, J.H.; Keller, M.P.; Traeger, L.L.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Zhao, W.; Vivas, E.I.; Yandell, B.S.; Broman, A.T.; et al. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Rep. 2017, 18, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.R.; Pike, C.M.; Parsons, R.J.; Rivera, A.J.; Foley, M.H.; McLaren, M.R.; Montgomery, S.A.; Theriot, C.M. Clostridioides difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota. Nat. Commun. 2021, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, L.E.; Penders, J.; Mbakwa, C.A.; Thijs, C.; Mommers, M.; Arts, I.C. The intestinal microbiota composition and weight development in children: The KOALA Birth Cohort Study. Int. J. Obes. 2015, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.; Petersen, M.B.; Gram, A.S.; Quist, J.S.; Winther, J.; Kamronn, S.D.; Milling, D.H.; Larsen, J.E.; Jespersen, A.P.; Stallknecht, B. The GO-ACTIWE randomized controlled trial—An interdisciplinary study designed to investigate the health effects of active commuting and leisure time physical activity. Contemp. Clin. Trials 2017, 53, 122–129. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e6093. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Cowan, C.; Hoban, A.E.; Ventura-Silva, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. BioEssays News Rev. Mol. Cell. Dev. Biol. 2018, 40, 1700172. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Junges, V.M.; Closs, V.E.; Nogueira, G.M.; Gottlieb, M. Crosstalk between Gut Microbiota and Central Nervous System: A Focus on Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.J.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nature reviews. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar]
- Royes, L. Cross-talk between gut and brain elicited by physical exercise. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165877. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Kotekar, N.; Shenkar, A.; Nagaraj, R. Postoperative cognitive dysfunction—Current preventive strategies. Clin. Interv. Aging 2018, 13, 2267–2273. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Ceccarelli, M.; D’Andrea, G.; Tirone, F. Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain Res. Bull. 2018, 143, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Lin, T.W.; Shih, Y.H.; Chen, S.J.; Lien, C.H.; Chang, C.Y.; Huang, T.Y.; Chen, S.H.; Jen, C.J.; Kuo, Y.M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Zapanta, K.; Schroeder, T.; Fisher, B. Rethinking Parkinson Disease: Exploring Gut-Brain Interactions and the Potential Role of Exercise. Phys. Ther. 2022, 102, pzac022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).