Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propolis Sample and Bacterial Strain
2.2. Preparation of PEO
2.3. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis of PEO
2.4. Antibacterial Activity Assessment
2.4.1. Determination of Inhibition Zone Diameter (DIZ)
2.4.2. Determination of the Minimal Inhibitory Concentration (MIC)
2.4.3. Determination of the Minimum Bactericidal Concentration (MBC)
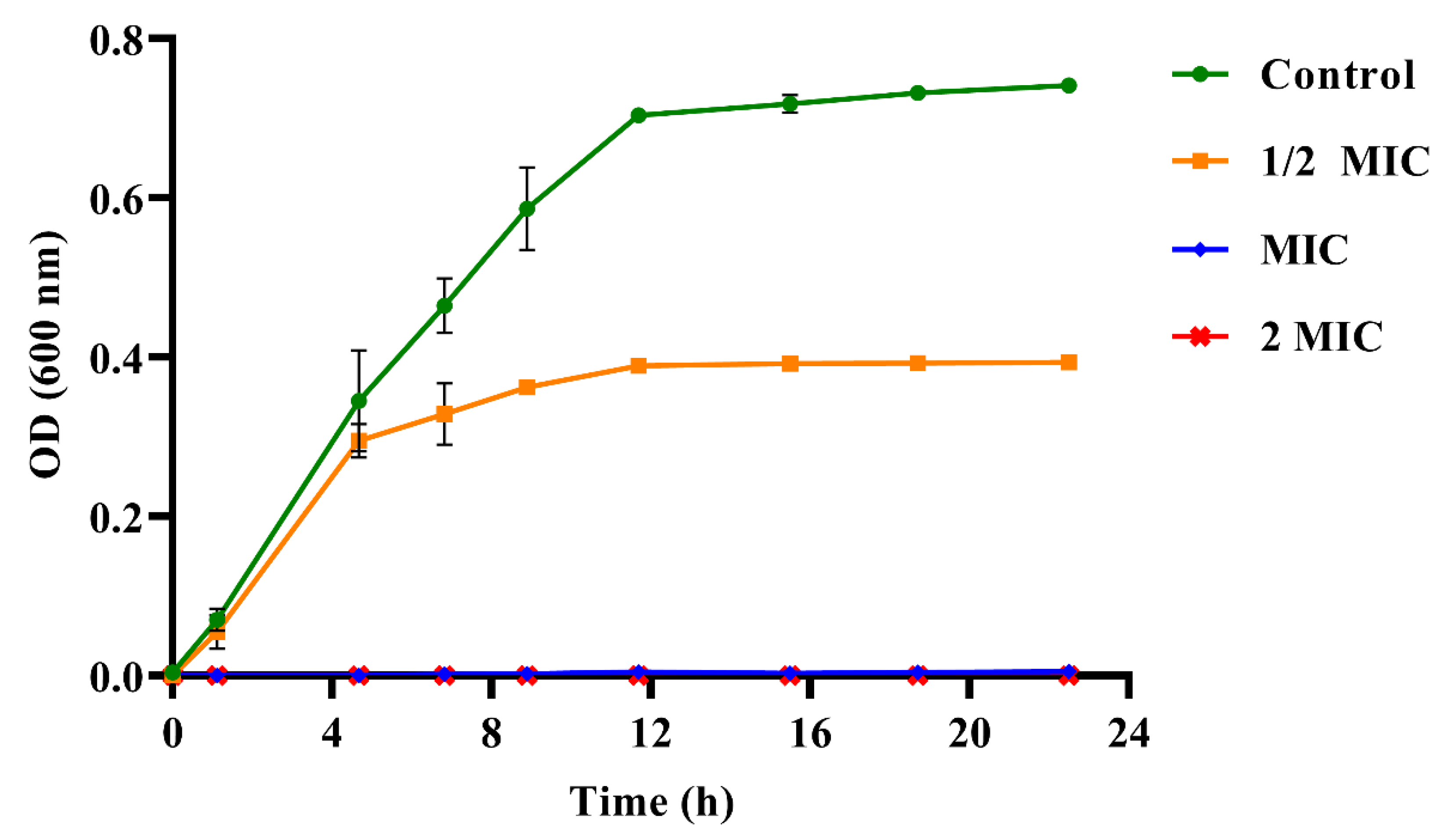
2.4.4. Determination of Growth Curve
2.5. Biofilm Assessment
2.5.1. Determination of Total Biofilm Biomasses
2.5.2. Determination of the Cell Activity within Biofilm
2.5.3. Live/Dead Bacteria Staining
2.5.4. Scanning Electron Microscopy (SEM) Observation of Biofilm
2.6. Cell Damage Assay
2.6.1. Determination of Lactic Dehydrogenase (LDH) Activity
2.6.2. Leakage of Calcium Ion
2.7. Determination of Bacterial Adhesion
2.8. Extracellular Polysaccharides (EPSs) Production Assay
2.9. The Extraction and Determination of GTFs
2.10. Determination of PEO Cytotoxicity to HOEC Cells
2.11. Statistical Analysis
3. Results
3.1. The Main Constituents of PEO
3.2. The Antibacterial Activities of PEO against S. mutans
3.3. Effect of PEO on Biofilm of S. mutans
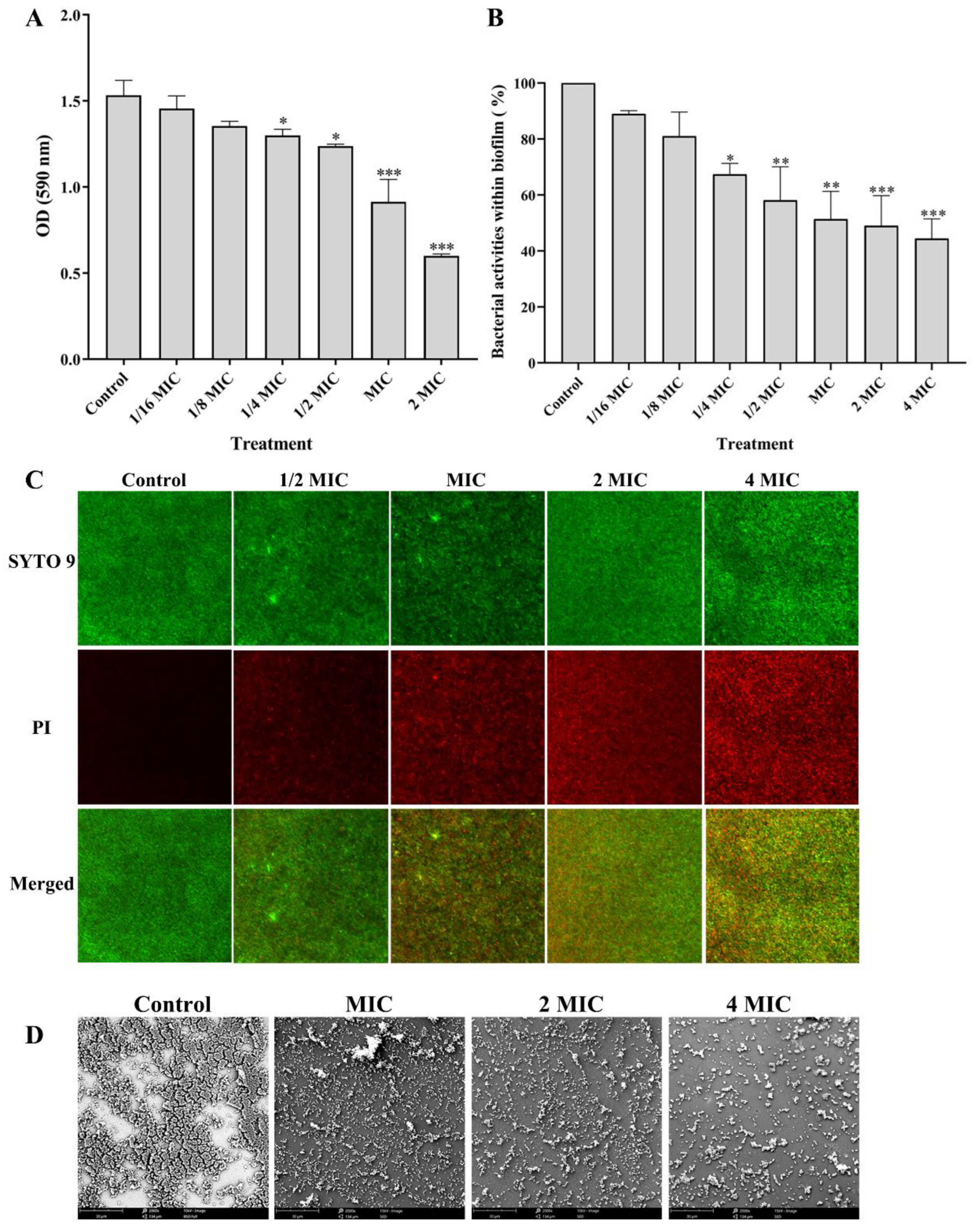
3.3.1. Effect of PEO on the Biofilm Biomasses
3.3.2. Effects of PEO on Bacterial Activity within Biofilm
3.3.3. Effects of PEO on the Bacterial Viability within Biofilm
3.3.4. Effect of PEO on the Biofilm Structure
3.4. Effects of PEO on Cell Damage of S. mutans
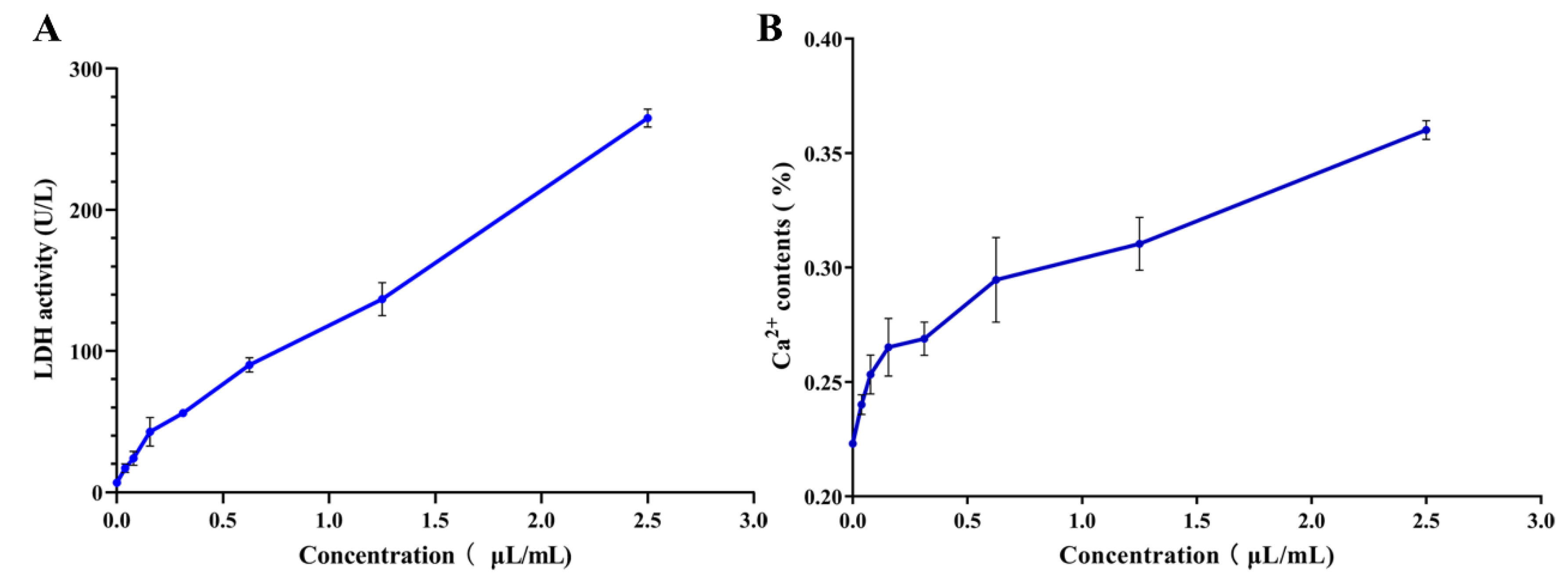
3.4.1. Effects of PEO on LDH Activity
3.4.2. Effects of PEO on Ca2+ Leakage
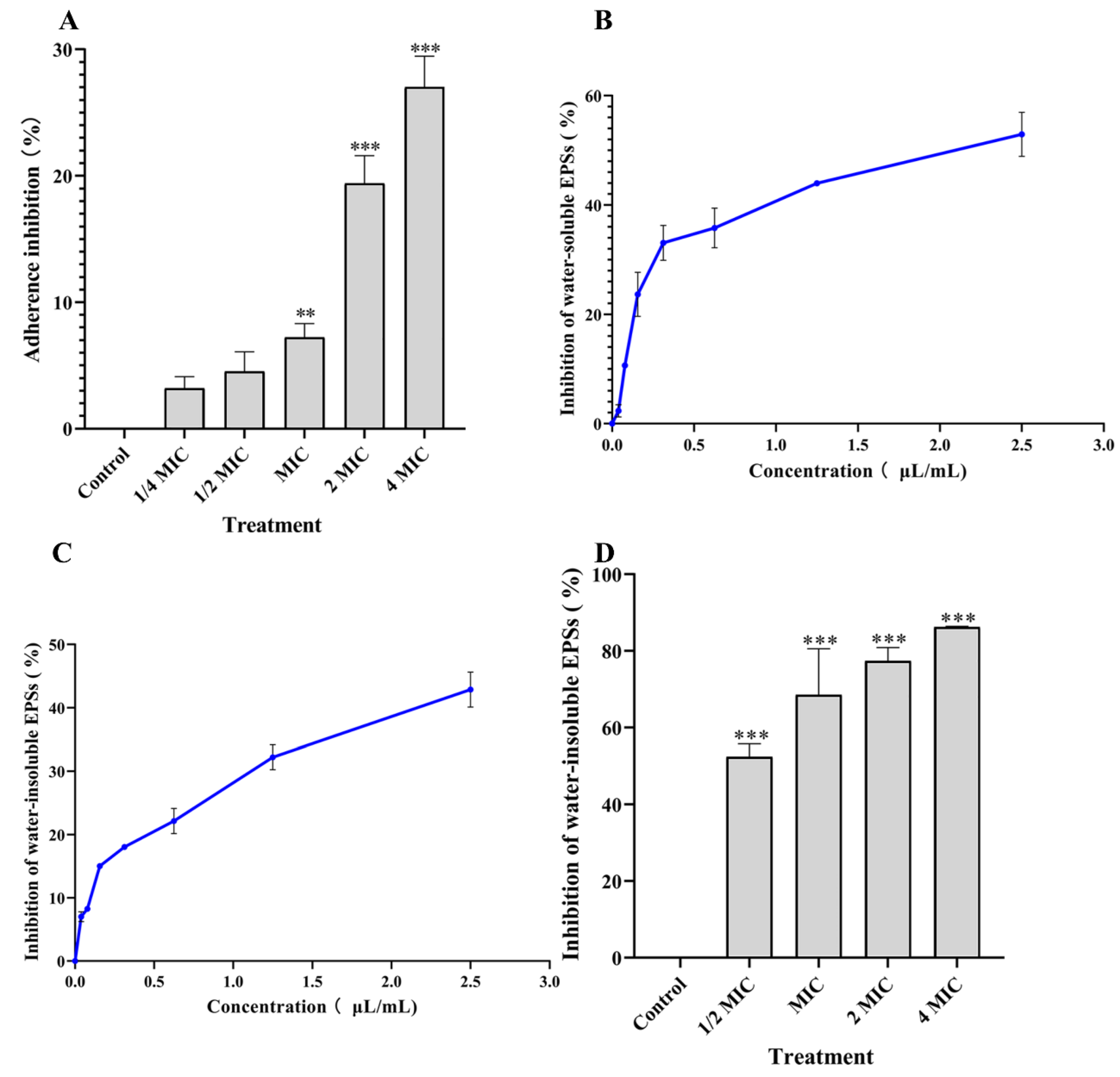
3.5. Effects of PEO on Cell Adhesion
3.6. Effects of PEO on EPSs Content
3.7. Effects of PEO on GTFs Activity
3.8. Toxicity Analysis of PEO on HOECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ham, S.Y.; Kim, H.S.; Cha, E.; Lim, T.; Byun, Y.; Park, H.D. Raffinose Inhibits Streptococcus mutans Biofilm Formation by Targeting Glucosyltransferase. Microbiol. Spectr. 2022, 10, e0207621. [Google Scholar] [CrossRef] [PubMed]
- Laudenbach, J.M.; Simon, Z. Common dental and periodontal diseases: Evaluation and management. Med. Clin. N. Am. 2014, 98, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Mandava, K.; Batchu, U.R.; Kakulavaram, S.; Repally, S.; Chennuri, I.; Bedarakota, S.; Sunkara, N. Design and study of anticaries effect of different medicinal plants against S.mutans glucosyltransferase. BMC Complementary Altern. Med. 2019, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Koga, T.; Ooshima, T. Virulence factors of Streptococcus mutans and dental caries prevention. J. Dent. Res. 1984, 63, 407–411. [Google Scholar] [CrossRef]
- Yan, T.; Rukayadi, Y.; Kim, K.H.; Hwang, J.K. In vitro anti-biofilm activity of macelignan isolated from Myristica fragrans Houtt. against oral primary colonizer bacteria. Phytother. Res. 2008, 22, 308–312. [Google Scholar]
- Kim, D.; Barraza, J.P.; Arthur, R.A.; Hara, A.; Lewis, K.; Liu, Y.; Scisci, E.L.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA. 2020, 117, 12375–12386. [Google Scholar] [CrossRef]
- Autio-Gold, J. The Role of Chlorhexidine in Caries Prevention. Oper. Dent. 2008, 33, 710–716. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Liu, Y.C.; Xu, Y.J.; Song, Q.H.; Wang, F.; Sun, L.G.; Liu, L.; Yang, X.G.; Yi, J.W.; Bao, Y.L.; Ma, H.F. Anti-biofilm Activities from Bergenia crassifolia Leaves against Streptococcus mutans. Front. Microbiol. 2017, 8, 1738. [Google Scholar] [CrossRef]
- Wang, F.; Wei, F.Y.; Song, C.X.; Jiang, B.; Tian, S.Y.Y.; Yi, J.W.W.; Yu, C.L.L.; Song, Z.B.B.; Sun, L.G.G.; Bao, Y.L.L. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crops Prod. 2017, 109, 358–366. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Sarveswari, H.B.; Vasudevan, S.; Shanmugam, K.; Solomon, A.P.; Neelakantan, P. Baicalein Inhibits Streptococcus mutans Biofilms and Dental Caries-Related Virulence Phenotypes. Antibiotics 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, H.; Liu, X.Y.; Hao, S.Y.; Zhang, Z.H.; Xuan, H.Z. Chinese Poplar Propolis Inhibits MDA-MB-231 Cell Proliferation in an Inflammatory Microenvironment by Targeting Enzymes of the Glycolytic Pathway. J. Immunol. Res. 2021, 2021, 6641341. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Roles of Honey, Royal Jelly, and Propolis in Suppressing Nephrotoxicity Induced by Doxorubicin in Male Albino Rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.Z.; Zhang, C.P.; Hu, F.L. Molecular Mechanisms Underlying the In Vitro Anti-Inflammatory Effects of a Flavonoid-Rich Ethanol Extract from Chinese Propolis (Poplar Type). Evid. Based Complementary Alter. Med. 2013, 2013, 127672. [Google Scholar]
- Wang, K.; Hu, L.; Jin, X.L.; Ma, Q.X.; Marcucci, M.C.; Netto, A.A.L.; Sawaya, A.C.H.F.; Huang, S.; Ren, W.K.; Conlon, M.A.; et al. Polyphenol-rich propolis extracts from China and Brazil exert anti-inflammatory effects by modulating ubiquitination of TRAF6 during the activation of NF-kappa B. J. Funct. Foods 2015, 19, 464–478. [Google Scholar] [CrossRef]
- Hsiao, F.S.H.; Artdita, C.A.; Hua, K.F.; Tsai, C.J.; Chien, Y.H.; Chen, Y.W.; Cheng, Y.H.; Yu, Y.H. Optimization of Emulsification Conditions on Ethanol Extract of Taiwanese Green Propolis Using Polysorbate and Its Immunomodulatory Effects in Broilers. Animals 2022, 12, 446. [Google Scholar] [CrossRef]
- Trusheva, B.; Ivanova, D.; Popova, M.; Bankova, V. Insights into the Essential Oil Compositions of Brazilian Red and Taiwanese Green Propolis. Nat. Prod. Commun. 2017, 12, 197–200. [Google Scholar] [CrossRef]
- Quintino, R.L.; Reis, A.C.; Fernandes, C.C.; Martins, C.H.G.; Colli, A.C.; Crotti, A.E.M.; Squarisi, L.S.; Ribeiro, A.B.; Tavares, D.C.; Miranda, M.L.D. Brazilian Green Propolis: Chemical Composition of Essential Oil and Their In Vitro Antioxidant, Antibacterial and Antiproliferative Activities. Braz. Arch. Biol. Technol. 2020, 63, e20190408. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.A.; Wu, F.H.; Zhu, M.; Zhang, Y.; Cheng, N.; Xue, X.F.; Wu, L.M.; Cao, W. Molecular Mechanism of Mature Honey Formation by GC-MS- and LC-MS-Based Metabolomics. J. Agric. Food Chem. 2021, 69, 3362–3370. [Google Scholar] [CrossRef]
- Naik, R.R.; Shakya, A.K.; Oriquat, G.A.; Katekhaye, S.; Paradkar, A.; Fearnley, H.; Fearnley, J. Fatty Acid Analysis, Chemical Constituents, Biological Activity and Pesticide Residues Screening in Jordanian Propolis. Molecules 2021, 26, 5076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; He, B.; Zhou, J.; He, D.H.; Deng, J.D.; Zeng, R.H. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind. Crops Prod. 2016, 83, 607–613. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Li, J.; Zhang, W.; Jiang, B.; Xuan, H. Australian propolis ethanol extract exerts antibacterial activity against methicillin-resistant Staphylococcus aureus by mechanisms of disrupting cell structure, reversing resistance, and resisting biofilm. Braz. J. Microbiol. 2021, 52, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Bai, J.R.; Zhong, K.; Huang, Y.N.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef]
- Huang, R.J.; Li, M.Y.; Gregory, R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur. J. Oral Sci. 2012, 120, 319–325. [Google Scholar] [CrossRef]
- Navarro-Perez, M.L.; Vadillo-Rodriguez, V.; Fernandez-Babiano, I.; Perez-Giraldo, C.; Fernandez-Calderon, M.C. Antimicrobial activity of a novel Spanish propolis against planktonic and sessile oral Streptococcus spp. Sci. Rep. 2021, 11, 23860. [Google Scholar] [CrossRef]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020, 1131, 827–855. [Google Scholar]
- Habluetzel, A.; Schmid, C.; Carvalho, T.S.; Lussi, A.; Eick, S. Impact of honey on dental erosion and adhesion of early bacterial colonizers. Sci. Rep. 2018, 8, 10936. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F. Scientific note: Often quoted, but not factual data about propolis composition. Apidologie 2021, 52, 312–314. [Google Scholar] [CrossRef]
- Bouchelaghem, S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Ristivojevic, P.; Dimkic, I.; Guzelmeric, E.; Trifkovic, J.; Knezevic, M.; Beric, T.; Yesilada, E.; Milojkovic-Opsenica, D.; Stankovic, S. Profiling of Turkish propolis subtypes: Comparative evaluation of their phytochemical compositions, antioxidant and antimicrobial activities. LWT 2018, 95, 367–379. [Google Scholar] [CrossRef]
- Bridi, R.; Montenegro, G.; Nunez-Quijada, G.; Giordano, A.; Moran-Romero, M.F.; Jara-Pezoa, I.; Speisky, H.; Atala, E.; Lopez-Alarcon, C. International Regulations of Propolis Quality: Required Assays do not Necessarily Reflect their Polyphenolic-Related In Vitro Activities. J. Food Sci. 2015, 80, C1188–C1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Fang, J.K.; Yang, J.Y.; Gao, X.L.; Dong, L.Y.; Zheng, X.; Sun, L.J.; Xia, B.; Zhao, N.; Ma, Z.Y.; et al. Streptococcus mutans-associated bacteria in dental plaque of severe early childhood caries. J. Oral. Microbiol. 2022, 14, 2046309. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
| No | Compounds | RI 1 | RT (min) 2 | PA (%) 3 |
|---|---|---|---|---|
| 1 | Ethyl benzenecarboxylate | 1170 | 16.833 | 2.4 |
| 2 | α-Cedrene | 1408 | 26.33 | 1.14 |
| 3 | α-Bergamotene | 1426 | 27.305 | 4.5 |
| 4 | (E)-β-Famesene | 1438 | 28.15 | 1.14 |
| 5 | β-Himachalene | 1490 | 29.108 | 13.94 |
| 6 | α-Curcumene | 1493 | 29.247 | 11.28 |
| 7 | β-Bisabolene | 1505 | 30.187 | 3 |
| 8 | Sesquicineole | 1517 | 30.396 | 4.35 |
| 9 | Cadina-1(10) | 1527 | 30.712 | 2.28 |
| 10 | α-Copaen-11-ol | 1539 | 31.226 | 1.67 |
| 11 | Guaiol | 1595 | 33.547 | 2.06 |
| 12 | γ-Eudesmol | 1630 | 34.8 | 2.2 |
| 13 | β-Eudesmol | 1645 | 35.468 | 2.91 |
| 14 | α-Eudesmol | 1652 | 35.58 | 1.99 |
| 15 | Bulnesol | 1666 | 36.015 | 1.03 |
| 16 | Bisabolol | 1680 | 36.615 | 1.01 |
| Strain | DIZ (mm) 1 | MIC (μL/mL) | MBC (μL/mL) | ||||
|---|---|---|---|---|---|---|---|
| Control | PEO | Gentamycin Sufate (10 μg/mL) | Ampicillin, Sodium Salt (10 μg/mL) | Vancomycin Hydrochloride (10 μg/mL) | |||
| S. mutans | 6 | 24.5 ± 0.71 | 22.5 ± 2.12 | 11.0 ± 1.41 | 8.5 ± 0.71 | 0.625 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Yuan, W.; Guo, Y.; Wu, Q.; Wang, F.; Xuan, H. Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans. Nutrients 2022, 14, 3290. https://doi.org/10.3390/nu14163290
Yuan J, Yuan W, Guo Y, Wu Q, Wang F, Xuan H. Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans. Nutrients. 2022; 14(16):3290. https://doi.org/10.3390/nu14163290
Chicago/Turabian StyleYuan, Jie, Wenqin Yuan, Yuyang Guo, Qian Wu, Fei Wang, and Hongzhuan Xuan. 2022. "Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans" Nutrients 14, no. 16: 3290. https://doi.org/10.3390/nu14163290
APA StyleYuan, J., Yuan, W., Guo, Y., Wu, Q., Wang, F., & Xuan, H. (2022). Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans. Nutrients, 14(16), 3290. https://doi.org/10.3390/nu14163290





