Relationship between Dietary Total Antioxidant Capacity and the Prognosis of Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Estimation of DTAC
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Participants According to the Tertile of DTAC
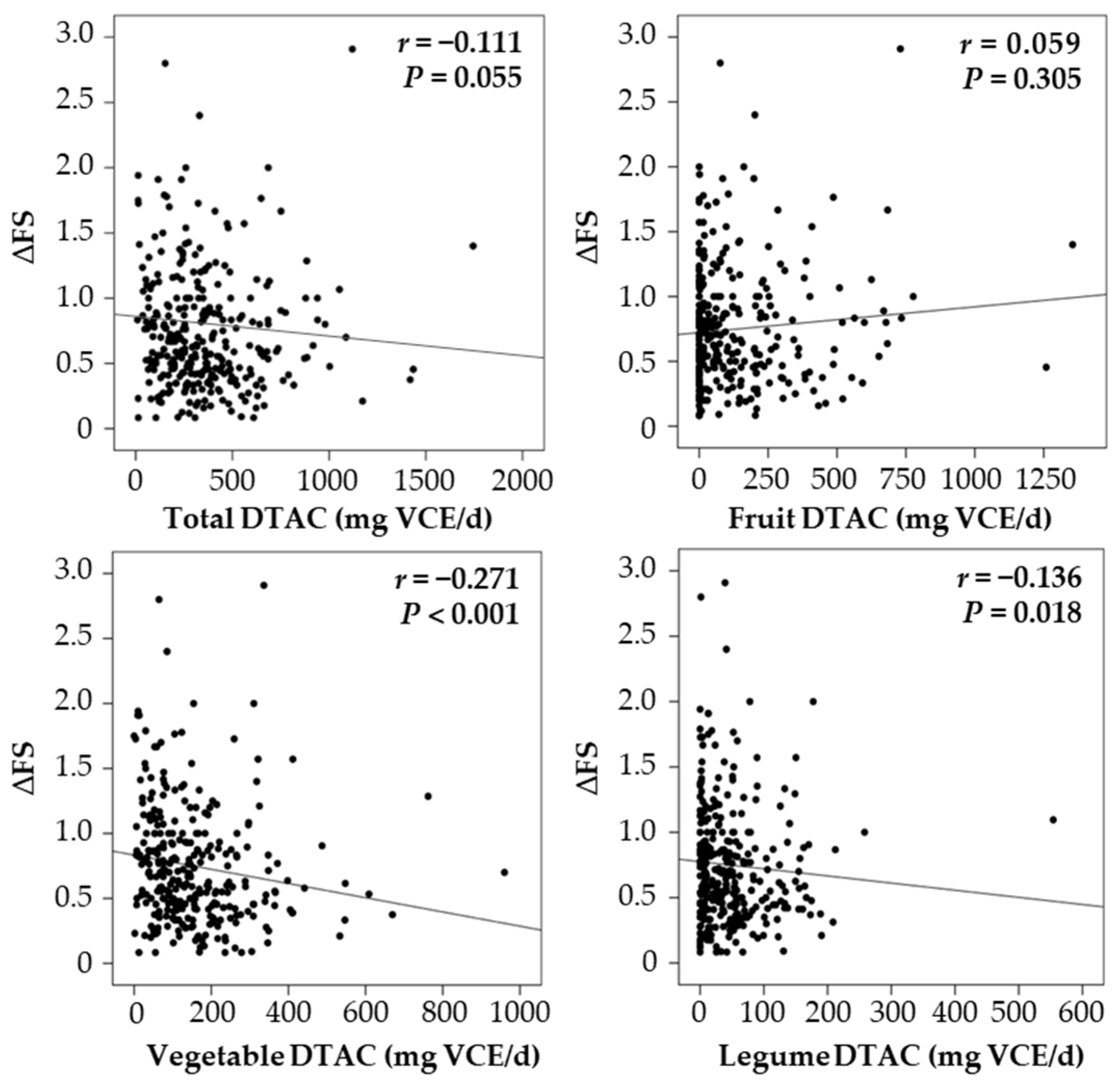
3.2. DTAC and Disease Progression Rate
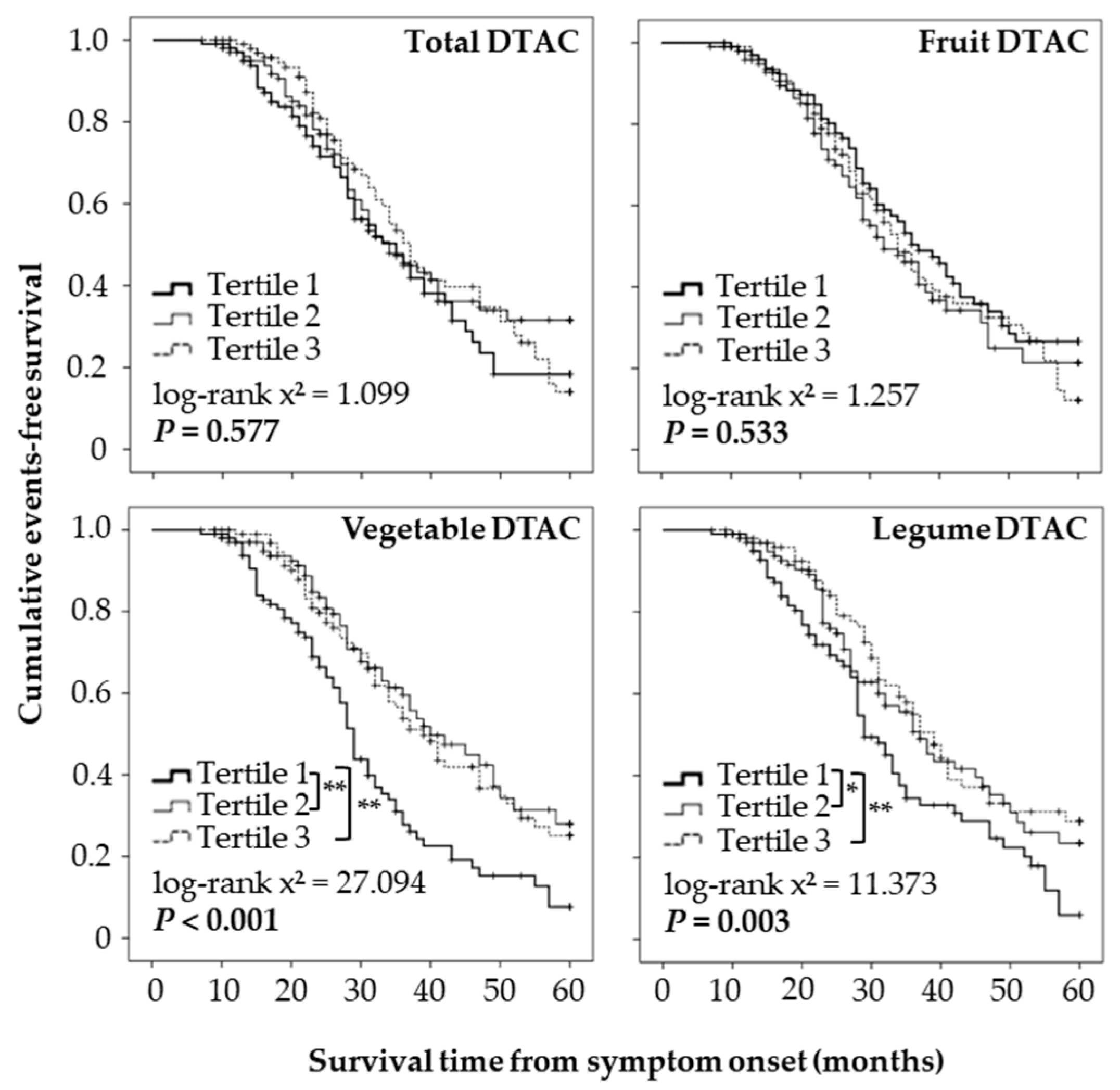
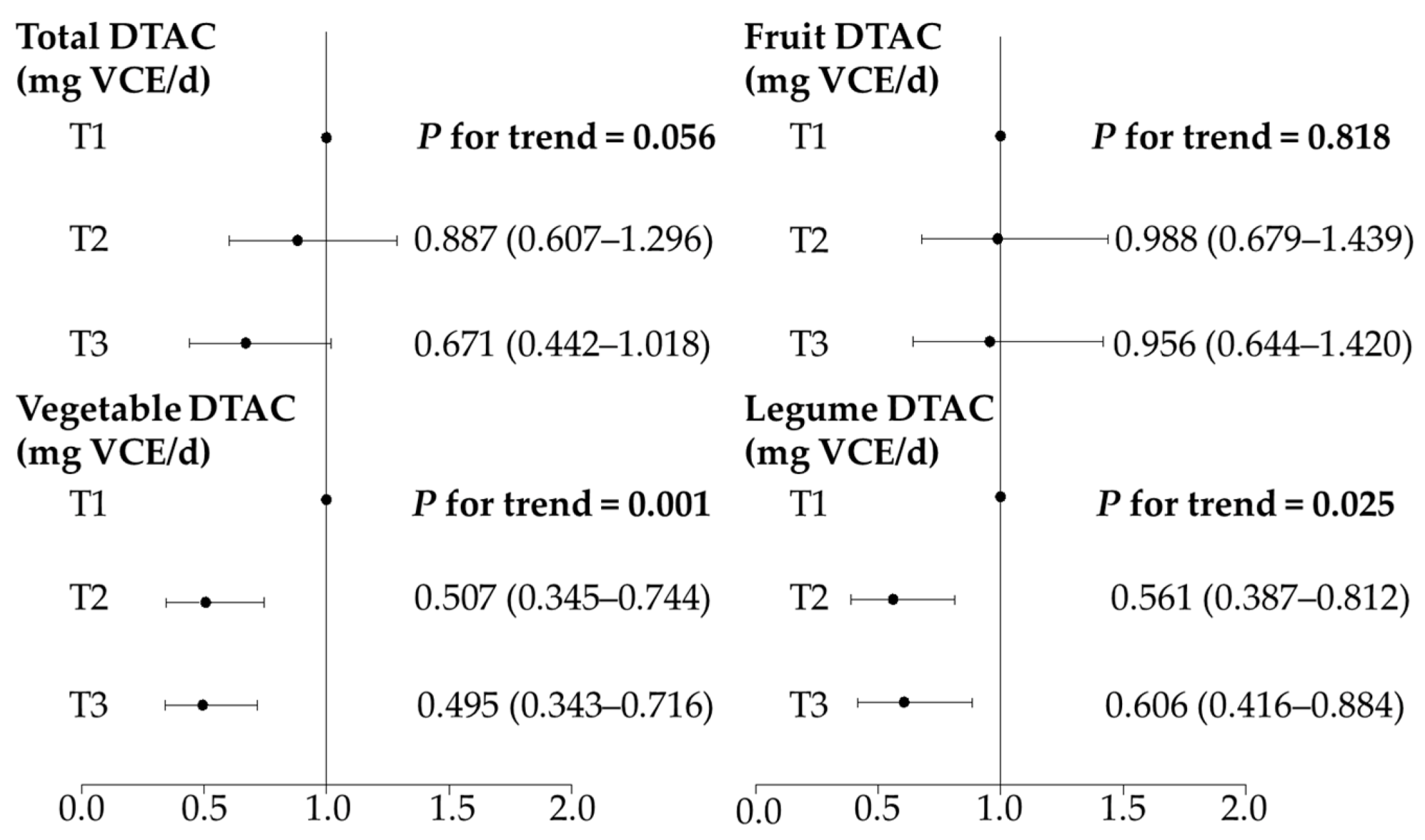
3.3. DTAC and Survival Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.H.; Longo, D.L.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Phukan, J.; Hardiman, O. The management of amyotrophic lateral sclerosis. J. Neurol. 2009, 256, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Oh, K.; Oh, S.I.; Baek, H.; Kim, S.H.; Park, Y. Dietary intake of fruits and beta-carotene is negatively associated with amyotrophic lateral sclerosis risk in Koreans: A case-control study. Nutr. Neurosci. 2014, 17, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Baek, H.; Kim, S.H.; Park, Y. Association between estimated total daily energy expenditure and stage of amyotrophic lateral sclerosis. Nutrition 2017, 33, 181–186. [Google Scholar] [CrossRef]
- Yu, H.; Kim, S.H.; Noh, M.Y.; Lee, S.; Park, Y. Relationship between dietary fiber intake and the prognosis of amyotrophic lateral sclerosis in Korea. Nutrients 2020, 12, 3420. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.; Kim, Y.; Baek, H.; Kim, S.H. Association between nutritional status and disease severity using the amyotrophic lateral sclerosis (ALS) functional rating scale in ALS patients. Nutrition 2015, 31, 1362–1367. [Google Scholar] [CrossRef]
- Kim, B.; Jin, Y.; Kim, S.H.; Park, Y. Association between macronutrient intake and amyotrophic lateral sclerosis prognosis. Nutr. Neurosci. 2020, 23, 8–15. [Google Scholar] [CrossRef]
- Nieves, J.W.; Gennings, C.; Factor-Litvak, P.; Hupf, J.; Singleton, J.; Sharf, V.; Oskarsson, B.; Fernandes Filho, J.A.; Sorenson, E.J.; D’Amico, E.; et al. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 2016, 73, 1425–1432. [Google Scholar] [CrossRef]
- Bond, L.; Bernhardt, K.; Madria, P.; Sorrentino, K.; Scelsi, H.; Mitchell, C.S. A metadata analysis of oxidative stress etiology in preclinical amyotrophic lateral sclerosis: Benefits of antioxidant therapy. Front. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef]
- Bond, L.; Bowen, G.; Mertens, B.; Denson, K.; Jordan, K.; Vidakovic, B.; Mitchell, C.S. Associations of patient mood, modulators of quality of life, and pharmaceuticals with amyotrophic lateral sclerosis survival duration. Behav. Sci. 2020, 10, 33. [Google Scholar] [CrossRef]
- Ascherio, A.; Weisskopf, M.G.; O’Reilly, E.J.; Jacobs, E.J.; McCullough, M.L.; Calle, E.E.; Cudkowicz, M.; Thun, M.J. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 104–110. [Google Scholar] [CrossRef]
- Desnuelle, C.; Dib, M.; Garrel, C.; Favier, A. A double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2001, 2, 9–18. [Google Scholar] [CrossRef]
- Graf, M.; Ecker, D.; Horowski, R.; Kramer, B.; Riederer, P.; Gerlach, M.; Hager, C.; Ludolph, A.C.; Becker, G.; Osterhage, J.; et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: Results of a placebo-controlled double-blind study. J. Neural. Transm. 2005, 112, 649–660. [Google Scholar] [CrossRef]
- Serafini, M.; Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the total antioxidant capacity the right tool? Redox Rep. 2004, 9, 145–152. [Google Scholar] [CrossRef]
- Pellegrini, N.; Salvatore, S.; Valtueña, S.; Bedogni, G.; Porrini, M.; Pala, V.; Del Rio, D.; Sieri, S.; Miglio, C.; Krogh, V.; et al. Development and validation of a food frequency questionnaire for the assessment of dietary total antioxidant capacity. J. Nutr. 2007, 137, 93–98. [Google Scholar] [CrossRef]
- Rautiainen, S.; Larsson, S.; Virtamo, J.; Wolk, A. Total antioxidant capacity of diet and risk of stroke: A population-based prospective cohort of women. Stroke 2012, 43, 335–340. [Google Scholar] [CrossRef]
- Del Rio, D.; Agnoli, C.; Pellegrini, N.; Krogh, V.; Brighenti, F.; Mazzeo, T.; Masala, G.; Bendinelli, B.; Berrino, F.; Sieri, S.; et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J. Nutr. 2011, 141, 118–123. [Google Scholar] [CrossRef]
- Zujko, M.E.; Waśkiewicz, A.; Witkowska, A.M.; Szcześniewska, D.; Zdrojewski, T.; Kozakiewicz, K.; Drygas, W. Dietary total antioxidant capacity and dietary polyphenol intake and prevalence of metabolic syndrome in polish adults: A nationwide study. Oxid. Med. Cell Longev. 2018, 2018, 7487816. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Surkan, P.J.; Azadbakht, L. Dietary total antioxidant capacity and cardiovascular disease risk factors: A systematic review of observational studies. J. Am. Coll. Nutr. 2018, 37, 533–545. [Google Scholar] [CrossRef]
- Parohan, M.; Sadeghi, A.; Khatibi, S.R.; Nasiri, M.; Milajerdi, A.; Khodadost, M.; Sadeghi, O. Dietary total antioxidant capacity and risk of cancer: A systematic review and meta-analysis on observational studies. Crit. Rev. Oncol. Hematol. 2019, 138, 70–86. [Google Scholar] [CrossRef]
- Yang, F.; Wolk, A.; Håkansson, N.; Pedersen, N.L.; Wirdefeldt, K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 2017, 32, 1631–1636. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef]
- Kollewe, K.; Mauss, U.; Krampfl, K.; Petri, S.; Dengler, R.; Mohammadi, B. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J. Neurol. Sci. 2008, 275, 69–73. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Song, W.O.; Fernandez, M.L.; Bruno, R.S.; Koo, S.I.; Chun, O.K. Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int. J. Food Sci. Nutr. 2010, 61, 600–623. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.; Wu, X. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3. 2018. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 14 May 2020).
- Bhagwat, S.; Haytowitz, D.; Holden, J. USDA Database for the Isoflavone Content of Selected Foods, Release 2.1. 2008. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/isoflavone/ (accessed on 14 May 2020).
- Bhagwat, S.; Haytowitz, D.; Holden, J. USDA Database for the Proanthocyanidin Content of Selected Foods, Release 2.1. 2018. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/proanthocyanidin/ (accessed on 14 May 2020).
- Kim, D.; Han, A.; Park, Y. Association of dietary total antioxidant capacity with bone mass and osteoporosis risk in Korean Women: Analysis of the Korea national health and nutrition examination survey 2008–2011. Nutrients 2021, 13, 1149. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. User Guide for the Fourth Korea National Health and Nutrition Examination Survey (KNHANES V). 2016. Available online: https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do (accessed on 10 May 2020).
- Greenland, S.; Pearce, N. Statistical foundations for model-based adjustments. Annu. Rev. Public Health 2015, 36, 89–108. [Google Scholar] [CrossRef]
- Baek, W.; Park, A.; Kim, H.Y.; Kim, S.H. Amyotrophic lateral sclerosis in Korea: Clinical characteristics and prognostic factors. J. Korean Neurol. Assoc. 2011, 29, 16–24. [Google Scholar]
- Kim, S.; Mun, S.; Park, J.; Choi, S.; Lee, S.; Kim, S. Complementary and alternative medicine use in amyotrophic lateral sclerosis cases in South Korea. Evid. Based Complement. Altern. Med. 2019, 2019, 4217057. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Chun, O.K.; Joung, H. Estimation of dietary total antioxidant capacity of Korean adults. Eur. J. Nutr. 2018, 57, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Himali, J.J.; Jacques, P.F.; DeCarli, C.; Satizabal, C.L.; Aparicio, H.; Vasan, R.S.; Beiser, A.S.; Seshadri, S. Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimer’s Dement. 2017, 13, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef]
- Clemens, R.; Drewnowski, A.; Ferruzzi, M.G.; Toner, C.D.; Welland, D. Squeezing fact from fiction about 100% fruit juice. Adv. Nutr. 2015, 6, 236S–243S. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox signaling and advanced glycation endproducts (AGEs) in diet-related diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but bitter: Focus on fructose impact on brain function in rodent models. Nutrients 2020, 13, 1. [Google Scholar] [CrossRef]
- Pupillo, E.; Bianchi, E.; Chiò, A.; Casale, F.; Zecca, C.; Tortelli, R.; Beghi, E.; SLALOM Group; PARALS Group; SLAP Group. Amyotrophic lateral sclerosis and food intake. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 267–274. [Google Scholar] [CrossRef]
- National Center for Health Statistics. 2017–2018 National Health and Nutrition Examination Survey (NHANES). 2018. Available online: https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed on 16 June 2020).
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compos. Anal. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Zeng, C. Effects of different cooking methods on the vitamin C content of selected vegetables. Nutr. Food Sci. 2013, 43, 438–443. [Google Scholar] [CrossRef]
- Nagano, S.; Fujii, Y.; Yamamoto, T.; Taniyama, M.; Fukada, K.; Yanagihara, T.; Sakoda, S. The efficacy of trientine or ascorbate alone compared to that of the combined treatment with these two agents in familial amyotrophic lateral sclerosis model mice. Exp. Neurol. 2003, 179, 176–180. [Google Scholar] [CrossRef]
- Villaverde, P.; Lajous, M.; MacDonald, C.J.; Fagherazzi, G.; Bonnet, F.; Boutron-Ruault, M.C. High dietary total antioxidant capacity is associated with a reduced risk of hypertension in French women. Nutr. J. 2019, 18, 31. [Google Scholar] [CrossRef]
- Karimi, Z.; Bahadoran, Z.; Abedini, S.; Houshyar-Rad, A.; Rashidkhani, B. Dietary total antioxidant capacity and the risk of breast cancer: A case-control study. East Mediterr. Health J. 2015, 21, 564–571. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxid. Med. Cell Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin exerts antioxidant and neuroprotective effects in multiple mutant hSOD1 models of amyotrophic lateral sclerosis by activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Isonaka, R.; Hiruma, H.; Katakura, T.; Kawakami, T. Inhibition of superoxide dismutase selectively suppresses growth of rat spinal motor neurons: Comparison with phosphorylated neurofilament-containing spinal neurons. Brain Res. 2011, 1425, 13–19. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, J.; Li, S.; Li, Z. Neuroprotective effects of genistein in a SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2019, 14, 688–696. [Google Scholar] [CrossRef]
- Essawy, A.E.; Abdou, H.M.; Ibrahim, H.M.; Bouthahab, N.M. Soybean isoflavone ameliorates cognitive impairment, neuroinflammation, and amyloid beta accumulation in a rat model of Alzheimer’s disease. Environ. Sci. Pollut. Res. 2019, 26, 26060–26070. [Google Scholar] [CrossRef]
- Gleason, C.E.; Carlsson, C.M.; Barnet, J.H.; Meade, S.A.; Setchell, K.D.; Atwood, C.S.; Johnson, S.C.; Ries, M.L.; Asthana, S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2009, 38, 86–93. [Google Scholar] [CrossRef]
- Camu, W.; Tremblier, B.; Plassot, C.; Alphandery, S.; Salsac, C.; Pageot, N.; Juntas-Morales, R.; Scamps, F.; Daures, J.P.; Raoul, C. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1198–1205. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 301) | Tertiles of Total DTAC (mg VCE/d) | p-Value 2 | ||
|---|---|---|---|---|---|
| Total T1 (n = 100) ≤228.73 | Total T2 (n = 101) 228.74–396.77 | Total T3 (n = 100) >396.77 | |||
| DTAC (mg VCE/d) | 358.23 ± 256.94 | 127.82 ± 62.29 3a | 307.97 ± 50.22 b | 639.42 ± 239.91 c | <0.001 |
| Type of endpoint, n (%) | 0.539 | ||||
| PEG | 90 (29.9) | 33 (33.0) | 31 (30.7) | 26 (26.0) | |
| Tracheostomy | 47 (15.6) | 17 (17.0) | 14 (13.9) | 16 (16.0) | |
| Death | 37 (12.3) | 9 (9.0) | 12 (11.9) | 16 (16.0) | |
| Age at symptom onset (y) | 54.64 ± 10.43 | 55.69 ± 11.10 | 54.11 ± 10.20 | 54.12 ± 9.98 | 0.384 |
| Sex, male, n (%) | 158 (52.5) | 54 (54.0) | 54 (53.5) | 50 (50.0) | 0.828 |
| Bulbar onset, n (%) | 66 (21.9) | 26 (26.0) | 15 (14.9) | 25 (25.0) | 0.107 |
| Symptom duration (months) 4 | 15.30 ± 4.91 | 16.12 ± 4.63 | 15.16 ± 5.22 | 14.62 ± 4.79 | 0.102 |
| ΔFS 5 | 0.75 ± 0.46 | 0.81 ± 0.47 | 0.73 ± 0.45 | 0.71 ± 0.46 | 0.203 |
| ALSFRS-R score (0–48) | 37.35 ± 6.23 | 35.84 ± 6.70 3a | 37.83 ± 5.70 ab | 38.38 ± 6.01 b | 0.005 |
| Bulbar score (0–12) | 9.97 ± 1.97 | 9.60 ± 2.08 | 10.18 ± 1.88 | 10.12 ± 1.90 | 0.062 |
| BMI (kg/m2) | 22.68 ± 2.88 | 22.14 ± 2.99 | 22.94 ± 2.61 | 22.96 ± 2.96 | 0.068 |
| Exercise, n (%) | 197 (65.4) | 54 (54.0) | 73 (72.3) | 70 (70.0) | 0.012 |
| Sun exposure, n (%) | 0.008 | ||||
| Never | 79 (26.2) | 39 (39.0) | 21 (20.8) | 19 (19.0) | |
| <30 min | 87 (28.9) | 25 (25.0) | 34 (33.7) | 28 (28.0) | |
| ≥30 min | 135 (44.9) | 36 (36.0) | 46 (45.5) | 53 (53.0) | |
| Smoking, n (%) | 109 (36.2) | 33 (33.0) | 41 (40.6) | 35 (35.0) | 0.509 |
| Drinking, n (%) | 144 (47.8) | 52 (52.0) | 50 (49.5) | 42 (42.0) | 0.337 |
| Treated with riluzole, n (%) | 270 (89.7) | 89 (89.0) | 90 (89.1) | 91 (91.0) | 0.391 |
| Treated with edaravone, n (%) | 32 (10.7) | 10 (10.0) | 14 (13.9) | 8 (8.0) | 0.872 |
| Variables | T1 (n = 100) | T2 (n = 101) | T3 (n = 100) | p-Value 2 |
|---|---|---|---|---|
| Fruit DTAC (mg VCE/Day) | ||||
| ≤12.62 | 12.63–126.55 | >126.55 | ||
| Age at symptom onset (year) | 53.86 ± 11.76 | 55.67 ± 10.32 | 54.37 ± 9.05 | 0.447 |
| Sex, male, n (%) | 61 (61.0) | 53 (52.5) | 44 (44.0) | 0.055 |
| Bulbar onset, n (%) | 20 (20.0) | 21 (20.8) | 25 (25.0) | 0.562 |
| Symptom duration (months) 4 | 15.93 ± 4.88 | 15.46 ± 5.06 | 14.51 ± 4.72 | 0.101 |
| ΔFS 5 | 0.68 ± 0.42 3a | 0.82 ± 0.45 b | 0.75 ± 0.51 b | 0.033 |
| ALSFRS-R score (0–48) | 37.85 ± 6.41 | 36.24 ± 6.10 | 37.98 ± 6.07 | 0.086 |
| Bulbar score (0–12) | 10.13 ± 2.03 | 9.80 ± 2.09 | 9.97 ± 1.77 | 0.499 |
| Vegetable DTAC (mg VCE/day) | ||||
| ≤83.92 | 83.93–170.06 | >170.06 | ||
| Age at symptom onset (year) | 54.17 ± 10.18 | 56.27 ± 9.88 | 53.46 ± 11.09 | 0.139 |
| Sex, male, n (%) | 56 (56.0) | 49 (48.5) | 53 (53.0) | 0.564 |
| Bulbar onset, n (%) | 21 (21.0) | 24 (23.8) | 21 (21.0) | 0.959 |
| Symptom duration (months) 4 | 14.62 ± 4.46 | 15.99 ± 5.03 | 15.28 ± 5.15 | 0.132 |
| ΔFS 5 | 0.93 ± 0.49 3a | 0.70 ± 0.41 b | 0.62 ± 0.42 b | <0.001 |
| ALSFRS-R score (0–48) | 35.24 ± 6.78 3a | 37.66 ± 5.59 b | 39.15 ± 5.67 b | <0.001 |
| Bulbar score (0–12) | 9.69 ± 2.01 | 9.88 ± 1.83 | 10.33 ± 2.02 | 0.061 |
| Legume DTAC (mg VCE/day) | ||||
| ≤15.26 | 15.27–53.89 | >53.89 | ||
| Age at symptom onset (year) | 55.97 ± 9.36 | 54.61 ± 11.64 | 53.33 ± 10.07 | 0.202 |
| Sex, male, n (%) | 54 (54.0) | 48 (47.5) | 56 (56.0) | 0.453 |
| Bulbar onset, n (%) | 22 (22.0) | 21 (20.8) | 23 (23.0) | 0.911 |
| Symptom duration (months) 4 | 14.99 ± 4.42 | 15.62 ± 5.06 | 15.28 ± 5.23 | 0.658 |
| ΔFS 5 | 0.83 ± 0.50 3a | 0.76 ± 0.47 ab | 0.65 ± 0.39 b | 0.020 |
| ALSFRS-R score (0–48) | 36.09 ± 7.07 3a | 37.20 ± 6.09 ab | 38.77 ± 5.13 b | 0.016 |
| Bulbar score (0–12) | 9.70 ± 2.18 | 9.91 ± 1.74 | 10.29 ± 1.93 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, J.; Son, B.; Kim, S.H.; Park, Y. Relationship between Dietary Total Antioxidant Capacity and the Prognosis of Amyotrophic Lateral Sclerosis. Nutrients 2022, 14, 3264. https://doi.org/10.3390/nu14163264
Eom J, Son B, Kim SH, Park Y. Relationship between Dietary Total Antioxidant Capacity and the Prognosis of Amyotrophic Lateral Sclerosis. Nutrients. 2022; 14(16):3264. https://doi.org/10.3390/nu14163264
Chicago/Turabian StyleEom, Jihyun, Bugyeong Son, Seung Hyun Kim, and Yongsoon Park. 2022. "Relationship between Dietary Total Antioxidant Capacity and the Prognosis of Amyotrophic Lateral Sclerosis" Nutrients 14, no. 16: 3264. https://doi.org/10.3390/nu14163264
APA StyleEom, J., Son, B., Kim, S. H., & Park, Y. (2022). Relationship between Dietary Total Antioxidant Capacity and the Prognosis of Amyotrophic Lateral Sclerosis. Nutrients, 14(16), 3264. https://doi.org/10.3390/nu14163264






