Adiposity Metabolic Consequences for Adolescent Bone Health
Abstract
1. Introduction
2. Physical Growth and Pubertal Development
2.1. Nutritional Aspects for Growth Optimization
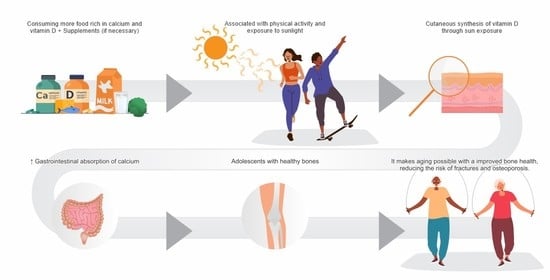
2.2. Nutrients and Bone Mineralization (Impact of Nutrients on Bone Health)
2.3. Calcium Ingestion by Adolescents
3. Nutritional Status of Vitamin D Insufficiency or Deficiency
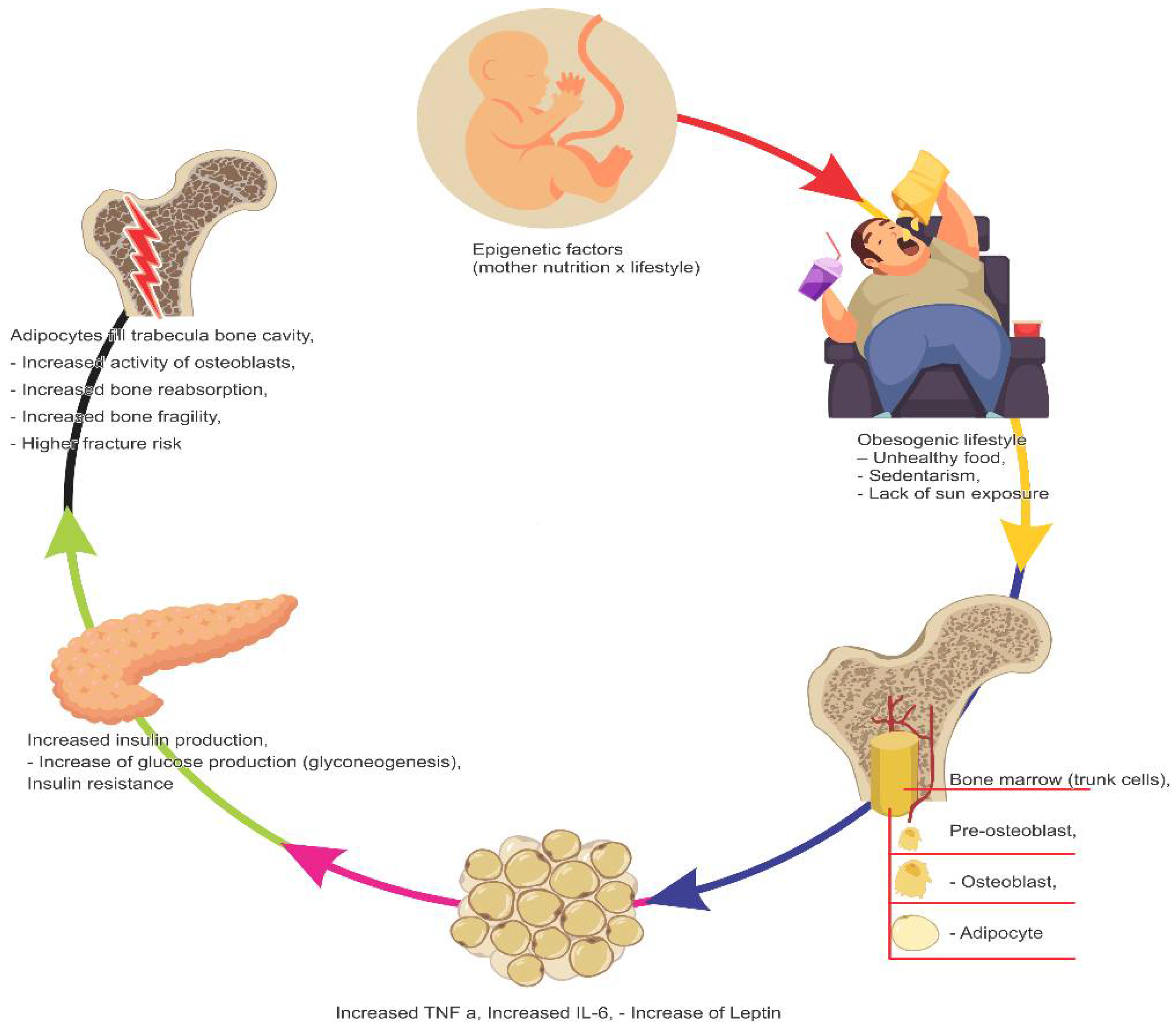
4. Infant–Juvenile Obesity
4.1. Relation between MS and Vitamin D Deficiency in Adolescents
4.2. Relation between Obesity and Bone Health
4.3. Bone Health and Puberty
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Obesity Federation (WOF). Atlas of Childhood Obesity; World Obesity Federation (WOF): London, UK, 2019. [Google Scholar]
- World Health Organization (WHO). Who European Childhood Obesity Surveillance Initiative (COSI). Report on the Fourth Round of Data Collection, 2015–2017; World Health Organization: Copenhagen, Denmark, 2021. [Google Scholar]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index among children and adolescents during the COVID-19 pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef]
- Giorgio, V.; Prono, F.; Graziano, F.; Nobili, V. Pediatric non alcoholic fatty liver disease: Old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatrics 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, W.; Moreno, L.A.; Marild, S.; Molnár, D.; Siani, A.; De Henauw, S.; Böhmann, J.; Günther, K.; Hadjigeorgiou, C.; Iacoviello, L.; et al. IDEFICS consortium. Metabolic syndrome in young children: Definitions and results of the IDEFICS study. Int. J. Obes. 2014, 38, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.S.; Kang, M.J.; Oh, Y.J.; Baek, J.W.; Yang, S.; Hwang, I.T. Association of serum ferritin with insulin resistance, abdominal obesity, and metabolic syndrome in Korean adolescents and adults. The Korean National Health and Nutrition Examination Survey, 2008 to 2011. Medicine 2017, 96, e6179. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, J.H.; Song, M.R. The association between Vitamin D Deficiency and Metabolic Syndrome in Korean Adolescents. J. Pediatr. Nurs. 2018, 38, e7–e11. [Google Scholar] [CrossRef]
- Fu, J.; Han, L.; Zhao, Y.; Li, G.; Zhu, Y.; Li, Y.; Li, M.; Gao, S.; Willi, S.M. Vitamin D levels are associated with metabolic syndrome in adolescents and young adults: The BCAMS study. Clin. Nutr. 2019, 38, 2161–2167. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, C.; Shu, Y.; Xie, Z.; Lu, C.; Mo, X. Serum 25-hydroxyvitamin D is associated with obesity and metabolic parameters in US children. Public Health Nutr. 2020, 23, 1214–1222. [Google Scholar] [CrossRef]
- Xiao, P.; Dong, H.; Li, H.; Yan, Y.; Cheng, H.; Liu, J.; Zhao, X.; Hou, D.; Mi, J. Adequate 25-hydroxyvitamin D levels are inversely associated with various cardiometabolic risk factors in Chinese children, especially obese children. BMJ Open Diab. Res. Care 2020, 8, e000846. [Google Scholar] [CrossRef]
- Bruney, T.S. Childhood obesity: Effects of micronutrients, supplements, genetics, and oxidative stress. J. Nurse Pract. 2011, 7, 647–653. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D deficiency major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.; Kratz, M.; Hoofnagle, A.N. Vitamin D deficiency in obese children and its relationship to insulin resistance and adipokines. J. Obes. 2011, 2011, 495101. [Google Scholar] [CrossRef] [PubMed]
- Motlaghzadeh, Y.; Sayarifard, F.; Allahverdi, B.; Rabbani, A.; Setoodeh, A.; Sayarifard, A.; Abbasi, F.; Haghi-Ashtiani, M.-T.; Rahimi-Froushani, A. Assessment of Vitamin D Status and Response to Vitamin D3 in Obese and Non-Obese Iranian Children. J. Trop. Pediatr. 2016, 62, 269–275. [Google Scholar] [CrossRef]
- Delvin, E.E.; Lambert, M.; Levy, E.; O´Loughlin, J.; Mark, S.; Gray-Donald, K.; Paradis, G. Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J. Nutr. 2010, 140, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Araújo, E.P.S.; Queiroz, D.J.M.; Neves, J.P.R.; Lacerda, L.M.; Gonçalves, M.C.R.; Carvalho, A.T. Prevalence of hypovitaminosis D and associated factors in adolescent students of a capital of northeastern Brazil. Nutr. Hosp. 2017, 34, 1416–1423. [Google Scholar] [CrossRef]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.-C. Obesity is associated with Vitamin D deficiency in Danish children and adolescents. J. Pedriatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- de Assumpção, D.; Dias, M.R.M.G.; Barros, M.B.A.; Fisberg, R.M.; Barros Filho, A.A. Calcium intake by adolescents: A population-based health survey. J. Pediatr. 2016, 92, 251–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nappo, A.; Sparano, S.; Intemann, T.; Kourides, Y.A.; Lissner, L.; Molnar, D.; Moreno, L.A.; Pala, V.; Sioen, I.; Veidebaum, T.; et al. Dietary calcium intake and adiposity in children and adolescents: Cross-sectional and longitudinal results from IDEFICS/I.Family cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 440–449. [Google Scholar] [CrossRef]
- O’Connor, A.; Benelam, B. An update on UK Vitamin D intakes and status, and issues for food fortification and supplementation. Nutr. Bull. 2011, 36, 390–396. [Google Scholar] [CrossRef]
- Trehan, N.; Afonso, L.; Levine, D.L.; Levy, P.D. Vitamin D Deficiency, Supplementation, and Cardiovascular Health. Crit. Pathw. Cardiol. 2017, 16, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 1980–1982. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.A.; Verly, E.; Marchioni, D.M.L.; Fisberg, R.M. Prevalence and correlates of calcium and vitamin D status adequacy in adolescents, adults, and elderly from the Health Survey-São Paulo. Nutrition 2013, 29, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium and obesity: Effect size and clinical relevance. Nutr. Rev. 2011, 69, 333–334. [Google Scholar] [CrossRef]
- Leu, M.; Giovannucci, E. Vitamin D: Epidemiology of cardiovascular risks and events. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 633–646. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Sorice, G.P.; Ajjan, R.; Mezza, T.; Pilz, S.; Prioletta, A.; Scragg, R.; Volpe, S.L.; Witham, M.D.; Giaccari, A. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 81–87. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Garbossa, S.G.; Folli, F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev. Endocr. Metab. Disord. 2017, 18, 243–258. [Google Scholar] [CrossRef]
- Boucher, B.J. Vitamin D insufficiency and diabetes risks. Curr. Drug Targets 2011, 12, 61–87. [Google Scholar] [CrossRef]
- Bueno, A.L.; Czepielewski, M.A. The importance for growth of dietary intake of calcium and vitamin D. J. Pediatr. 2008, 84, 386–394. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A. Committee on nutrition. Optimizing Bone Health in Children and Adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Oda, Y.; Xie, Z. Calcium and 1,25(OH)2D: Interacting drivers of epidermal differentiation. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol.Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Perola, M. Genetics of stature human: Lessons from genome–wide associations studies. Horm. Res. Paedriatr. 2011, 76, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Reichman, B.; Shina, A.; Derazne, E.; Tzur, D.; Yifrach, D.; Wiser, I.; Afek, A.; Shamis, A.; Tirosh, A.; et al. Sex Differences in the impact of Thiness, Overweight, Obesity and Parental Height on Adolescent Height. J. Adolesc. Health 2017, 61, 1–7. [Google Scholar] [CrossRef]
- Tanner, J.M.; Goldstein, H.; Whitehouse, R.H. Standards of children′s height at ages 2–9 years allowing for heights of parents. Arch. Dis. Child. 1970, 45, 755–762. [Google Scholar] [CrossRef]
- Banfi, G.; Lombardi, G.; Colombini, A.; Lippi, G. Bone metabolism-markers in sports medicine. Sports Med. 2010, 40, 697–714. [Google Scholar] [CrossRef]
- Chipkevitch, E. Clinical assessment of sexual maturation in adolescents. J. Pediatr. 2001, 77, s135–s142. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.; Yeste, D.; Moreno-Galdó, A.; Gussinyé, M.; Ferrández, Á.; Clemente, M.; Fernández-Cancio, M. Pubertal growth of 1453 healthy children according to age at pubertal growth spurt onset. The Barcelona longitudinal growth study. Na. Pediatr. 2018, 89, 144–152. [Google Scholar] [CrossRef]
- McCormack, S.E.; Cousminer, D.L.; Chesi, A.; Mitchell, J.A.; Roy, S.M.; Kalkwarf, H.J.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.E.; Shepherd, J.A.; et al. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017, 171, e171769. [Google Scholar] [CrossRef] [PubMed]
- De Leonibus, C.; Marcovecchio, M.L.; Chiarelli, F. Update on statural growth and pubertal development in obese children. Pediatr. Rep. 2012, 4, e35. [Google Scholar] [CrossRef]
- Rivera, J.A.; Hotz, C.; González-Cossío, T.; Neufeld, L.; García-Guerra, A. The effect of micronutrient deficiencies on child growth: A review of results from community-based supplementation trials. J. Nutr. 2003, 133, 4010–4020. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R. Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes: The Essential Guide to Nutrients Requirements; National Academies Press: Washignton, DC, USA, 2006. [Google Scholar]
- Chevalley, T.; Bonjour, J.P.; Audet, M.C.; Merminod, F.; Rietbergen, B.; Rizzoli, R.; Ferrari, S. Prepubertal Impact of Protein Intake and Physical Activity on Weight-Bearing Peak Bone Mass and Strength in Males. J. Clin. Endocrinol. Metab. 2017, 102, 157–166. [Google Scholar] [CrossRef][Green Version]
- Silva, C.C.; Teixeira, A.S.; Goldberg, T.B. The impact of calcium ingestion on the bone mineralization in adolescents. Rev. Nutr. 2004, 17, 351–359. [Google Scholar] [CrossRef]
- Millan, J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef]
- Michigami, T.; Ozono, K. Roles of Phosphate in Skeleton. Front. Endocrinol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Branca, F.; Vatueña, S. Calcium, physical activity and bone health-building bones for a stronger future. Public Health Nutr. 2001, 4, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Black, R.E.; Williams, S.M.; Jones, I.E.; Goulding, A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am. J. Clin. Nutr. 2002, 76, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Martos-Moreno, G.A.; Niklasson, A.; Martinez-Villanueva, J.; Argente, J.; Albertsson- Wikland, K. The pubertal growth spurt is diminished in children with severe obesity. Pediatr. Res. 2020, 90, 184–190. [Google Scholar] [CrossRef]
- Blank, S.K.; McCartney, C.R.; Helm, K.D.; Marshall, J.C. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin. Reprod. Med. 2007, 25, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.K.; McCartney, C.R.; Chhabra, S.; Helm, K.D.; Eagleson, C.A.; Chang, R.J.; Marshall, J.C. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls-implications for regulation of pubertal maturation. J. Clin. Endocrinol. Metab. 2009, 94, 2360–2366. [Google Scholar] [CrossRef]
- Le, S.; Xu, L.; Schumann, M.; Wu, N.; Törmäkangas, T.; Alén, M.; Cheng, S.; Wiklund, P. Does sex hormone-binding globulin cause insulin resistance during pubertal growth? Endocr. Connect. 2019, 8, 510–517. [Google Scholar] [CrossRef]
- Greer, F.R. Issues in establishing vitamin D recommendations for infants and Children. Am. J. Clin. Nutr. 2004, 80, 1759S–1762S. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Spot the silent sufferers: A call for clinical diagnostic criteria for solar and nutritional osteomalacia. J. Steroid Biochem. Mol. Biol. 2019, 188, 141–146. [Google Scholar] [CrossRef]
- Holick, M.F. Environmental factors that influence the cutaneous production of vitamin D. Am. J. Clin. Nutr. 1995, 61, 638–645. [Google Scholar] [CrossRef]
- Murshed, M. Mechanism of bone mineralization. Cold Spring Harb. Perspect Med. 2018, 8, a031229. [Google Scholar] [CrossRef]
- Mak, I.L.; Lavery, P.; Agellon, S.; Rauch, F.; Murshed, M.; Weiler, H.A. Arachidonic acid exacerbates diet-induced obesity and reduces bone mineral contentwithout impacting bone strength in growing male rats. J. Nutr. Biochem. 2019, 73, 108226. [Google Scholar] [CrossRef]
- Corwin, R.L.; Hartman, T.J.; Maczuga, S.A.; Graubard, B.I. Dietary saturated fat intake is inversely associated with bone density in humans: Analysis of NHANES III. J. Nutr. 2006, 136, 159–165. [Google Scholar] [CrossRef]
- Shapses, S.A.; Pop, L.C.; Wang, Y. Obesity is a concern for bone healt with aging. Nutr. Res. 2017, 39, 1–13. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bhattacharya, A.; Fernandes, G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J. Cell Physiol. 2008, 214, 201–209. [Google Scholar] [CrossRef]
- Boeyens, J.C.; Deepak, V.; Chua, W.H.; Kruger, M.C.; Joubert, A.M.; Coetzee, M. Effects of ω3- and ω6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264.7 cells: A comparative in vitro study. Nutrients 2014, 6, 2584–2601. [Google Scholar] [CrossRef]
- Watkins, B.A.; Li, Y.; Lippman, H.E.; Feng, S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 387–398. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef]
- Rouf, A.S.; Sui, Z.; Rangan, A.; Grech, A.; Allman-Farinelli, M. Low calcium intakes among Australian adolescents and young adults are associated with higher consumption of discretionary foods and beverages. Nutrition 2018, 55, 146–153. [Google Scholar] [CrossRef]
- Lappe, J.M.; McMahon, D.J.; Laughlin, A.; Hanson, C.; Desmangles, J.C.; Begley, M.; Schwartz, M. The effect of increasing dairy calcium intake of adolescent girls on changes in body fat and weight. Am. J. Clin. Nutr. 2017, 105, 1046–1053. [Google Scholar] [CrossRef]
- Jurimae, J.; Maestu, E.; Mengel, E.; Remmel, L.; Purge, P.; Tillmann, V. Association between dietary calcium intake and adiposity in male adolescents. Nutrients 2019, 11, 1454. [Google Scholar] [CrossRef]
- Mouratidou, T.; Vicente-Rodriguez, G.; Gracia-Marco, L.; Huybrechts, I.; Sioen, I.; Widhalm, K.; Valtueña, J.; González-Gross, M.; Moreno, L.A. Associations of dietary calcium, vitamin D, milk intakes, and 25-hydroxyvitamin D with bone mass in Spanish adolescents: The HELENA study. J. Clin. Densitom. 2013, 16, 110–117. [Google Scholar] [CrossRef]
- Peters, B.S.E.; Verly Jr, E.; Marchioni, D.M.L.; Fisberg, M.; Martini, L.A. The influence of breakfast and dairy products on dietary calcium and vitamin D intake in postpubertal adolescents and young adults. J. Hum. Nutr. Diet. 2012, 25, 69–74. [Google Scholar] [CrossRef]
- Vogel, K.A.; Martin, B.R.; McCabe, L.D.; Peacock, M.; Warden, S.J.; McCabe, G.P. The effect of dairy intake on bone mass and body composition in earlypubertal girls and boys: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 1214–1229. [Google Scholar] [CrossRef]
- Nezami, M.; Segovia-Siapco, G.; Beeson, W.L.; Sabaté, J. Associations between consumption of dairy foods and anthropometric indicators of health in adolescents. Nutrients 2016, 8, 427. [Google Scholar] [CrossRef]
- De Lamas, C.; De Castro, M.J.; Gil-Campos, M.; Gil, A.; Couce, M.L.; Leis, R. Effects of Dairy Product Consumption on Height and BoneMineral Content in Children: ASystematic Review of Controlled Trials. Adv. Nutr. 2019, 10, S88–S96. [Google Scholar] [CrossRef]
- Marcos-Pasero, H.; Aguilar-Aguilar, E.; De la Iglesia, R.; Espinosa-Salinas, I.; Gómez-Patiño, M.; Colmenarejo, G.; de Molina, A.R.; Reglero, G.; Loria-Kohen, V. Association of calcium and dairy product consumption with childhood obesity and the presence of a Brain Derived Neurotropic Factor-Antisense (BDNF-AS) polymorphism. Clin. Nutr. 2019, 38, 2616–2622. [Google Scholar] [CrossRef]
- Suhett, L.; Silveira, B.; Filgueiras, M.; Peluzio, M.; Hermsdorff, H.; Novaes, J. Inverse association of calcium intake with abdominal adiposity and C-reactive protein in Brazilian children. Public Health Nutr. 2018, 21, 1912–1920. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, A.W.; Kim, W.K.; Kim, S.H. The combined effects of milk intake and physical activity on bone mineral density in korean adolescents. Nutrients 2021, 13, 731. [Google Scholar] [CrossRef]
- Julian, C.; González-Gross, M.; Breidenassel, C.; Mouratidou, T.; Vicente-Rodriguez, G.; Gracia-Marco, L.; Ferrari, M.; Widhalm, K.; Molnár, D.; Kafatos, A.; et al. 25-hydroxyvitamin D is differentially associated with calcium intakes of Northern, Central, and Southern European adolescents: Results from the HELENA study. Nutrition 2017, 36, 22–25. [Google Scholar] [CrossRef]
- Paxton, G.A.; Teale, G.R.; Nowson, C.A.; Mason, R.S.; McGrath, J.J.; Thompson, M.J.; Siafarikas, A.; Rodda, C.P.; Munns, C.F.; Australian and New Zealand Bone and Mineral Society; et al. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: A position statement. Med. J. Aust. 2013, 198, 142–143. [Google Scholar] [CrossRef]
- Khadilkar, A.; Khadilkar, V.; Chinnappa, J.; Rathi, N.; Khadgawat, R.; Balasubramanian, S.; Parekh, B.; Jog, P. Prevention and treatment of vitamin D and calcium deficiency in children and adolescents: Indian Academy of Pediatrics (IAP) Guidelines. Indian Pediatr. 2017, 54, 567–573. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for calcium and vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De Angelis, G.L.; Massari, M.; Miraglia, D.G.E.; Miraglia, D.G.M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Shroff, R.; Wan, M.; Nagler, E.V.; Bakkaloglu, S.; Fischer, D.C.; Bishop, N.; Cozzolino, M.; Bacchetta, J.; Edefonti, A.; Stefanidis, C.J.; et al. European Society for Paediatric Nephrology Chronic Kidney Disease Mineral and Bone Disorders and Dialysis Working Groups. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol. Dial. Transplant. 2017, 32, 1098–1113. [Google Scholar] [CrossRef]
- Society for Adolescent Health and Medicine. Recommended vitamin D intake and management of low vitamin D status in adolescents: A Position Statement of the Society for Adolescent Health and Medicine. Position Statement. J. Adolesc. Health 2013, 52, 801–803. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Wagner, C.L.; Park, Y.; Mirmiran, P.; Hosseinpanah, F. Daily vitamin D3 in overweight and obese children and adolescents: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 2831–2840. [Google Scholar] [CrossRef]
- Fiamenghi, V.I.; Mello, E.D. Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D: Dietary requirements and food fortification as a means of helping achieve. J. Steroid Biochem. Mol. Biol. 2015, 148, 19–26. [Google Scholar] [CrossRef]
- Cureau, F.V.; da Silva, T.L.N.; Bloch, K.V.; Fujimori, E.; Belfort, D.R.; de Carvalho, K.M.B.; de Leon, E.B.; de Vasconcellos, M.T.L.; Ekelund, U.; Schaan, B.D. ERICA: Leisure-time physical inactivity in Brazilian adolescents. Rev. SaúdePública 2016, 50, 4s. [Google Scholar] [CrossRef]
- Peters, B.S.E.; Roque, J.P.; Fisberg, M.; Martini, L.A. There are no association between vitamin D metabolites and blood pressure in adolescents. Arq. Bras. Endocrinol. Metab. 2009, 53, 416–424. [Google Scholar] [CrossRef]
- de Oliveira, C.L.; Cureau, F.V.; Cople-Rodrigues, C.S.; Giannini, D.T.; Bloch, K.V.; Kuschnir, M.C.C.; de Carvalho, M.B.; Schaan, B.D. Prevalence and factors associated with hypovitaminosis D in adolescents from a sunny country: Findings from the ERICA survey. J. Steroid Biochem. Mol. Biol. 2020, 199, 105609. [Google Scholar] [CrossRef]
- Smith, T.J.; Lanham-New, S.; Hart, K.H. Vitamin D in adolescents: Are current recommendations enough? J. Steroid Biochem. Mol. Biol. 2017, 173, 265–272. [Google Scholar] [CrossRef]
- Paes-Silva, R.P.; Gadelha, P.C.F.P.; de Lemos, M.C.C.; de Castro, C.M.M.B.; de Arruda, I.K.D.; Diniz, A.S. Adiposity, inflammation and fat-soluble vitamins in adolescentes. J. Pediatr. 2019, 95, 575–583. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Rodrigues, P.R.M.; Luiz, R.R.; Monteiro, L.S.; Ferreira, M.G.; Gonçalves-Silva, R.M.V.; Pereira, R.A. Adolescents’ unhealthy eating habits are associated with meal skipping. Nutrition 2017, 42, 114–120. [Google Scholar] [CrossRef]
- Voráčová, J.; Badura, P.; Hamrik, Z.; Holubčíková, J.; Sigmund, E. Unhealthy eating habits and participation in organized leisure-time activities in Czech adolescents. Eur. J. Pediatr. 2018, 177, 1505–1513. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Global Burden of Disease (GBD). Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; WHO; World Bank. Joint Child Malnutrition Estimates; UNICEF: New York, NY, USA; World Health Organization: Geneva, Switzerland; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Bjerregaard, L.G.; Jensen, B.W.; Ängquist, L.; Osler, M.; Sørensen, T.I.A.; Baker, J.L. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, K.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Tanrikulu, M.A.; Agirbasli, M.; Berenson, G. Primordial Prevention of Cardiometabolic Risk in Childhood. Adv. Exp. Med. Biol. 2017, 956, 489–496. [Google Scholar] [CrossRef]
- Turer, C.B.; Brady, T.M.; Ferranti, S.D. Obesity, hypertension, and dyslipidemia in childhood are key modificableantecedentes of adult cardiovascular disease: A call to action. Circulation 2018, 137, 1256–1259. [Google Scholar] [CrossRef]
- Koskinen, J.; Juonala, M.; Dwyer, T.; Venn, A.; Thomson, R.; Bazzano, L.; Berenson, G.S.; Sabin, M.A.; Burns, T.L.; Viikari, J.S.A.; et al. Impact of Lipid Measurements in Youth in Addition to Conventional Clinic-Based Risk Factors on Predicting Preclinical Atherosclerosis in Adulthood. Circulation 2018, 137, 1246–1255. [Google Scholar] [CrossRef]
- Fan, H.; Zhu, Q.; Zhang, X. Child Excess Weight Status, Adult Excess Weight Status, and Cardiometabolic Risk Profile. Front. Pediatr. 2020, 8, 301. [Google Scholar] [CrossRef]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfäffle, R.; Kiess, W.; Körner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, Z.; Yang, Z.; Wang, X.; Gao, D.; Dong, Y.; Ma, J.; Ma, Y. Association of high birth weight with overweight and obesity in Chinese students aged 6–18 years: A national, cross-sectional study in China. BMJ Open 2019, 9, e024532. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Jokelainen, J.; Sebert, S.; Auvinen, J.; Järvelin, M.-R.; Keinänen-Kiukaanniemi, S.; Herzig, K.-H. Vitamin D Status and Components of Metabolic Syndrome in Older Subjects from Northern Finland (Latitude 65°North). Nutrients 2019, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N. Serum 25-hidroxyvitamin D is associated with obesity and metabolic parameters in US children. Public Health Nutr. 2020, 23, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Huang, Q.; Rompay, M.I.V.; Chomitz, V.R.; Economos, C.C.; Eliasziw, M.; Gordon, C.M.; Goodman, E. Vitamin D supplementation and cardiometabolic risk factors among diverse schoolchildren: A randomized clinical trial. Am. J. Clin. Nutr. 2022, 115, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 1992, 13, 719–764. [Google Scholar] [CrossRef]
- Harding, M.M.; Kwong, J.; Roberts, D.; Hagler, D.; Reinisch, C. Lewis’s Medical-Surgical Nursing, 11th ed.; Assessment and Management of Clinical Problems; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ellison, D.L.; Moran, H.R. Vitamin D: Vitamin or hormone? Nurs. Clin. N. Am. 2021, 52, 47–57. [Google Scholar] [CrossRef]
- Gannagé-Yared, M.H.; Sabbagh, R.; Chédid, R. Relationship between 25 hydroxyvitamin D and lipid profile in Lebanese school children. J. Endocrinol. Investig. 2018, 41, 1043–1049. [Google Scholar] [CrossRef]
- Filgueiras, M.S.; Suhett, L.G.; Silva, M.A.; Rocha, N.P.; Novaes, J.F. Lower vitamin D intake is associated with low HDL cholesterol and vitamin D insufficiency/deficiency in Brazilian children. Public Health Nutr. 2018, 21, 2004–2012. [Google Scholar] [CrossRef]
- Queiroz, D.J.M.; Silva, A.S.; Dinis, A.S.; Carvalho, A.T.; Araújo, E.P.S.; Neves, J.P.R.; Lacerda, L.M.; Toscano, L.T.; Gonçalves, M.C.R. Vitamin D insufficiency/deficiency and its association with cardiometabolic risk factors in Brazilian adolescents. Nutr. Hosp. 2019, 36, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Salek, S.; Salek, M.; Hashemipour, M.; Movahedian, M. Effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in children with metabolic syndrome: A triple-masked controlled trial. J. Ped. 2014, 90, 28–34. [Google Scholar] [CrossRef]
- Sethuraman, U.; Zidan, M.A.; Hanks, L.; Bagheri, M.; Ashraf, A. Impact of vitamin D treatment on 25 hydroxy vitamin D levels and insulin homeostasis in obese African American adolescents in a randomized trial. J. Clin. Transl. Endocrinol. 2018, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, D.B.D.L.; Adikaram, S.G.S.; Atapattu, N.; Kendaragama, K.M.D.L.D.; Senevirathne, J.T.N.; Jayasekera, H.D.; Wickramasinghe, V.P. Vitamin D supplementation in obese Sri Lankan children: A randomized controlled trial. BMC Pediatr. 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Lacombe, J. Regulation of energy metabolism by skeleton: Osteocalcin and beyond. Arch. Biochem. Biophys. 2014, 561, 137–146. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Koditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Kessler, J.; Koebnick, C.; Smith, N.; Adams, A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. OrthopaedRelat Res. 2013, 471, 1199–1207. [Google Scholar] [CrossRef]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The bones of Children with Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.N.; Goldberg, T.B.L.; Silva, V.N.; Kurokawa, C.S.; Rizzo, A.C.B.; Silva, C.C.; Teixeira, A.S.; Corrente, J.E. The impact of excess body fat on bone remodeling in adolescents. Osteoporos. Int. 2016, 28, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Gállego-Suarez, C.; Singer, B.H.; Gebremariam, A.; Lee, J.M.; Singer, K. The relationship between adiposity and bone density in U. S. children and adolescents. PLoS ONE 2017, 12, e0181587. [Google Scholar] [CrossRef] [PubMed]
- Rokoff, L.B.; Rifas-Shiman, S.L.; Switkowski, K.M.; Young, J.G.; Rosen, C.J.; Oken, E.; Fleisch, A.F. Body composition and Bone Mineral Density in Childhood. Bone 2019, 121, 9–15. [Google Scholar] [CrossRef]
- Liang, J.; Chen, Y.; Zhang, J.; Ma, B.; Hu, Y.; Liu, Y.; Lin, S.; Zhang, Z.; Song, Y. Associations of Weight-Adjusted Body Fat and Fat Distribution with Mineral Bone Density in Chinese Children Aged 6–8 Years. Int. J. Environ. Res. Public Health. 2020, 17, 1763. [Google Scholar] [CrossRef]
- Jeddi, M.; Dabbaghmanesh, M.H.; Omrani, G.R.; Ayatollahi, S.M.T.; Bagheri, Z.; Bakhshayeshkaram, M. Relative Importance of Lean and Fat Mass on Bone Mineral Density in Iranian Children and Adolescents. Int. J. Endocrinol. Metab. 2015, 13, e25542. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jung, H.W.; Hong, H.; Kim, J.H.; Shin, C.H.; Yang, S.W.; Lee, Y.A. The role of Overweight and Obesity on Bone Health in Korean Adolescents with a Focus on Lean and Fat Mass. J. Koren. Med. Sci. 2017, 32, 1633–1641. [Google Scholar] [CrossRef]
- Soininen, S.; Sidoroff, V.; Lindi, V.; Mahonen, A.; Kroger, L.; Kroger, H.; Jaaskelainen, J.; Atalay, M.; Laaksonen, D.E.; Laitinen, T.; et al. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6–8 years of age—The Physical Activity and Nutrition in Children (PANIC) study. Bone 2018, 108, 106–114. [Google Scholar] [CrossRef]
- Kouda, K.; Ohara, K.; Nakamura, H.; Fujita, Y.; Jaalkhorol, M.; Iki, M. Fat mass is positively associated with bone mass acquisition in children with small or normal lean mass: A three-year follow-up study. Bone 2018, 107, 222–227. [Google Scholar] [CrossRef]
- Ulbricht, L.; Campos, M.F.; Esmanhoto, E.; Ripka, W.L. Prevalence of excessive body fat among adolescents of a South Brazilian metropolitan region and State capital, associated risk factors, and consequences. BMC Public Health 2018, 18, 312. [Google Scholar] [CrossRef]
- Mengel, E.; Tillmann, V.; Remmel, L.; Kool, P.; Purge, P.; Lätt, E.; Jürimäe, J. Extensive BMI Gain in Puberty is Associated with Lower Increments in Bone Mineral Density in Estonian Boys with Overweight and Obesity: A 3-Year Longitudinal Study. Calcif. Tissue Int. 2017, 101, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K. Childhood obesity, bone development, and cardiometabolic risk factors. Mol. Cell. Endocrinol. 2015, 410, 52–63. [Google Scholar] [CrossRef]
- Streeter, A.J.; Hosking, J.; Metcalf, B.S.; Jeffery, A.N.; Voss, L.D.; Wilkin, T.J. Body fat in children does not adversely influence bone development: A 7-year longitudinal study (EarlyBird 18). Pediatric Obes. 2013, 8, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-S.; Kim, H.-K.; Kim, S. Effects of adolescents’ lifestyle habits and body composition on bone mineral density. Int. J. Environ. Res. Public Health 2021, 18, 6170. [Google Scholar] [CrossRef] [PubMed]
- Sopher, A.B.; Fennoy, I.; Oberfield, S.E. An update on childhood bone health: Mineral accrual, assessment and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 35–40. [Google Scholar] [CrossRef]
- Mosca, L.N.; Silva, V.N.; Goldberg, T.B.L. Does excess weight interfere with bone mass accumulattion during adolescence? Nutrients 2013, 5, 2047–2061. [Google Scholar] [CrossRef]
- Tritos, N.A.; Klibanski, A. Effects of growth hormone on bone. Prog. Mol. Biol. Transl. Sci. 2016, 138, 193–211. [Google Scholar] [CrossRef]
- Sopher, A.B.; Jean, A.M.; Zwany, S.K.; Winston, D.M.; Pomeranz, C.B.; Bell, J.J.; McMahon, D.J.; Hassoun, A.; Fennoy, I.; Oberfield, S.E. Bone age advancement in prepuberal children with obesity and premature adrenarche: Possible potentiating factors. Obesity 2011, 19, 1259–1264. [Google Scholar] [CrossRef]
- Silva, H.M.B.S.; Oliveira, C.C.; Souza, A.L.C.; Aguiar, L.B.V. The relation between adolescents’ body mass index and bone age. Nutr. Hosp. 2019, 36, 1037–1042. [Google Scholar] [CrossRef]
- Busch, A.S.; Hϕjgaard, B.; Hagen, C.P.; Teilmann, G. Obesity is associated with earlier pubertal onset in boys. J. Clin. Endocrinol. Metab. 2020, 105, e1667–e1672. [Google Scholar] [CrossRef]
- Diaz, A.; Bhandari, S.; Sison, C.; Vogiatzi, M. Characteristics of children with premature pubarche in the New York metropolitan area. Horm. Res. 2008, 70, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O.; Newfield, R.S.; Hassink, S.G. Bone maturation along the spectrum from normal weight to obesity: A complex interplay of sex, growth factors and weight gain. J. Pediatr. Endocrinol. Metab. 2016, 29, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Kim, S.; Lee, J.; Lee, M.S.; Kim, Y.J.; Kang, K.S. Factors associated with Advanced Bone Age in Overweight and Obese Children. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definitions, mechanisms, and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Weilner, S.; Skalick, S.; Salzer, B.; Keider, V.; Wagner, M.; Hildner, F.; Gabriel, C.; Dovjak, P.; Pietschmann, P.; Grillari-Voglauer, R.; et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 2015, 79, 43–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Cai, I.; Li, F.; Lou, Y.; Xu, N.; Kang, Y.; Yang, H. MicroRNA-221 is involved in the regulation of osteoporosis through regulates RUNX2 protein expression and osteoblast differentiation. Am. J. Transl. Res. 2017, 9, 126–135. [Google Scholar]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Dong, S.; Yang, B.; Guo, H.; Kang, F. MicroRNAs regulate osteogenesis and chondrogenesis. Biochem. Biophys. Res. Commun. 2012, 418, 587–591. [Google Scholar] [CrossRef]
- Bocheva, G.; Boyadjieva, N. Epigenetic regulation of fetal bone development and placental transfer of nutrients: Progress for osteoporosis. Interdiscip. Toxicol. 2011, 4, 167–172. [Google Scholar] [CrossRef]
- Petersen, S.B.; Rasmussen, M.A.; Olsen, S.F.; Vestergaard, P.; Molgaard, C.; Halldorsson, T.I.; Strom, M. Maternal dietary patterns during pregnancy in relation offspring forearm fractures: Prospective study from the Danish National Birth Cohort. Nutrients 2015, 7, 2382–2400. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, K.G.; Rodrigues, E.L.; da Silva Lopes, M.R.; do Nascimento, V.A.; Pott, A.; Guimarães, R.d.C.A.; Pegolo, G.E.; Freitas, K.d.C. Adiposity Metabolic Consequences for Adolescent Bone Health. Nutrients 2022, 14, 3260. https://doi.org/10.3390/nu14163260
Lopes KG, Rodrigues EL, da Silva Lopes MR, do Nascimento VA, Pott A, Guimarães RdCA, Pegolo GE, Freitas KdC. Adiposity Metabolic Consequences for Adolescent Bone Health. Nutrients. 2022; 14(16):3260. https://doi.org/10.3390/nu14163260
Chicago/Turabian StyleLopes, Kátia Gianlupi, Elisana Lima Rodrigues, Mariana Rodrigues da Silva Lopes, Valter Aragão do Nascimento, Arnildo Pott, Rita de Cássia Avellaneda Guimarães, Giovana Eliza Pegolo, and Karine de Cássia Freitas. 2022. "Adiposity Metabolic Consequences for Adolescent Bone Health" Nutrients 14, no. 16: 3260. https://doi.org/10.3390/nu14163260
APA StyleLopes, K. G., Rodrigues, E. L., da Silva Lopes, M. R., do Nascimento, V. A., Pott, A., Guimarães, R. d. C. A., Pegolo, G. E., & Freitas, K. d. C. (2022). Adiposity Metabolic Consequences for Adolescent Bone Health. Nutrients, 14(16), 3260. https://doi.org/10.3390/nu14163260