Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Total Antioxidant Capacity Determination
2.2.1. Antioxidant Activity Determined via Iron Reduction (FRAP)
2.2.2. Antioxidant Potential Determined via 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Reduction
2.3. CHO-K1 Cellular Studies
2.3.1. In Vitro Model
2.3.2. Treatments
2.4. Antioxidant Capacity of CoQ10 and Zn in CHO-K1 Cells
2.4.1. Protective Effect against Arsenic (As) Treatments
2.4.2. Violet Crystal Colorimetric Assay
2.4.3. Aqueous Hydroperoxide Determination
2.4.4. Determination of Intracellular ROS
2.5. Effect of Treatments on Cell Viability
2.5.1. MTT Reduction
2.5.2. Alamar Blue® Reduction
2.6. Statistical Analysis
3. Results
3.1. Total Antioxidant Capacity of CoQ10 and Zn
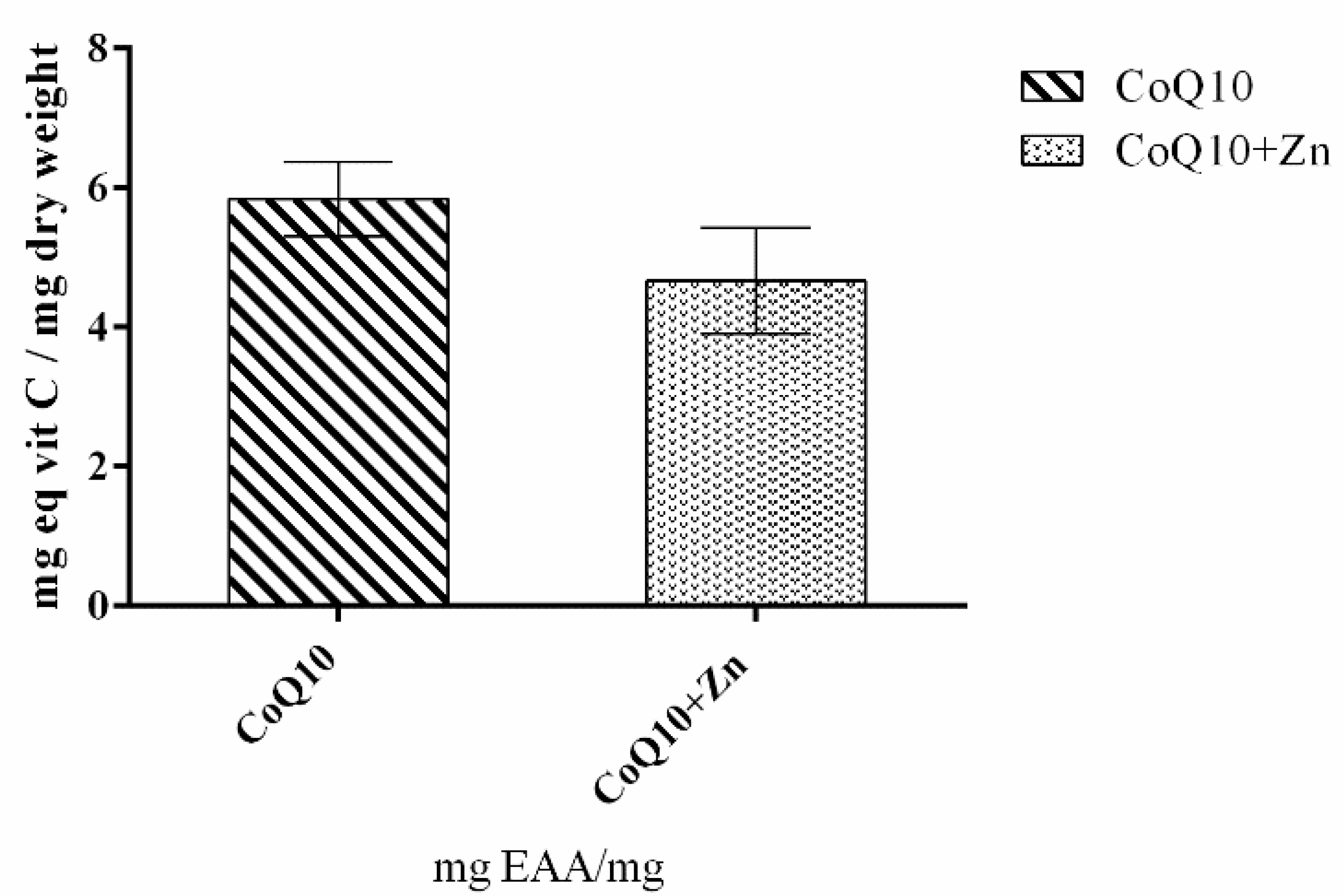
3.1.1. FRAP
3.1.2. DPPH Method
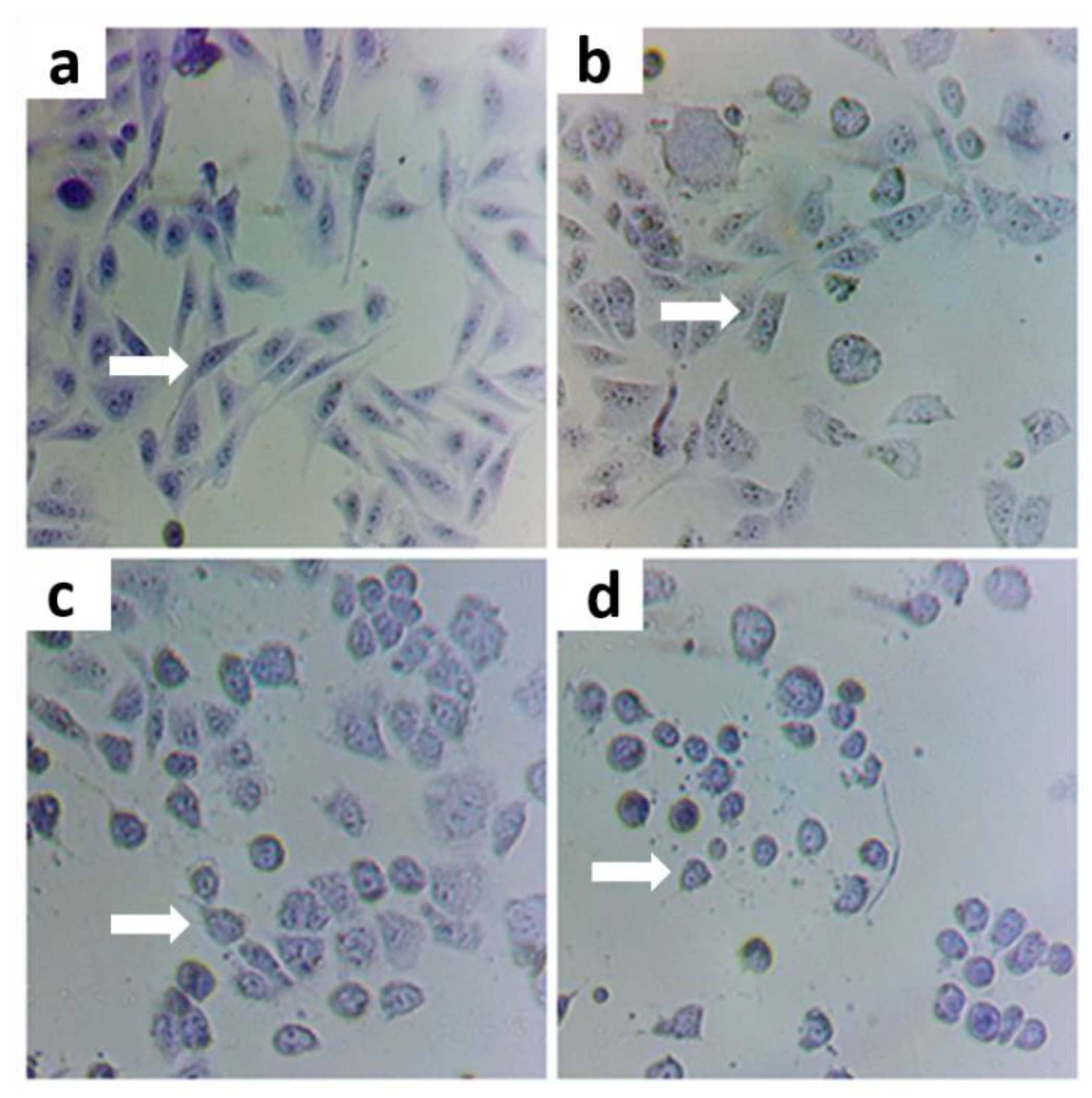
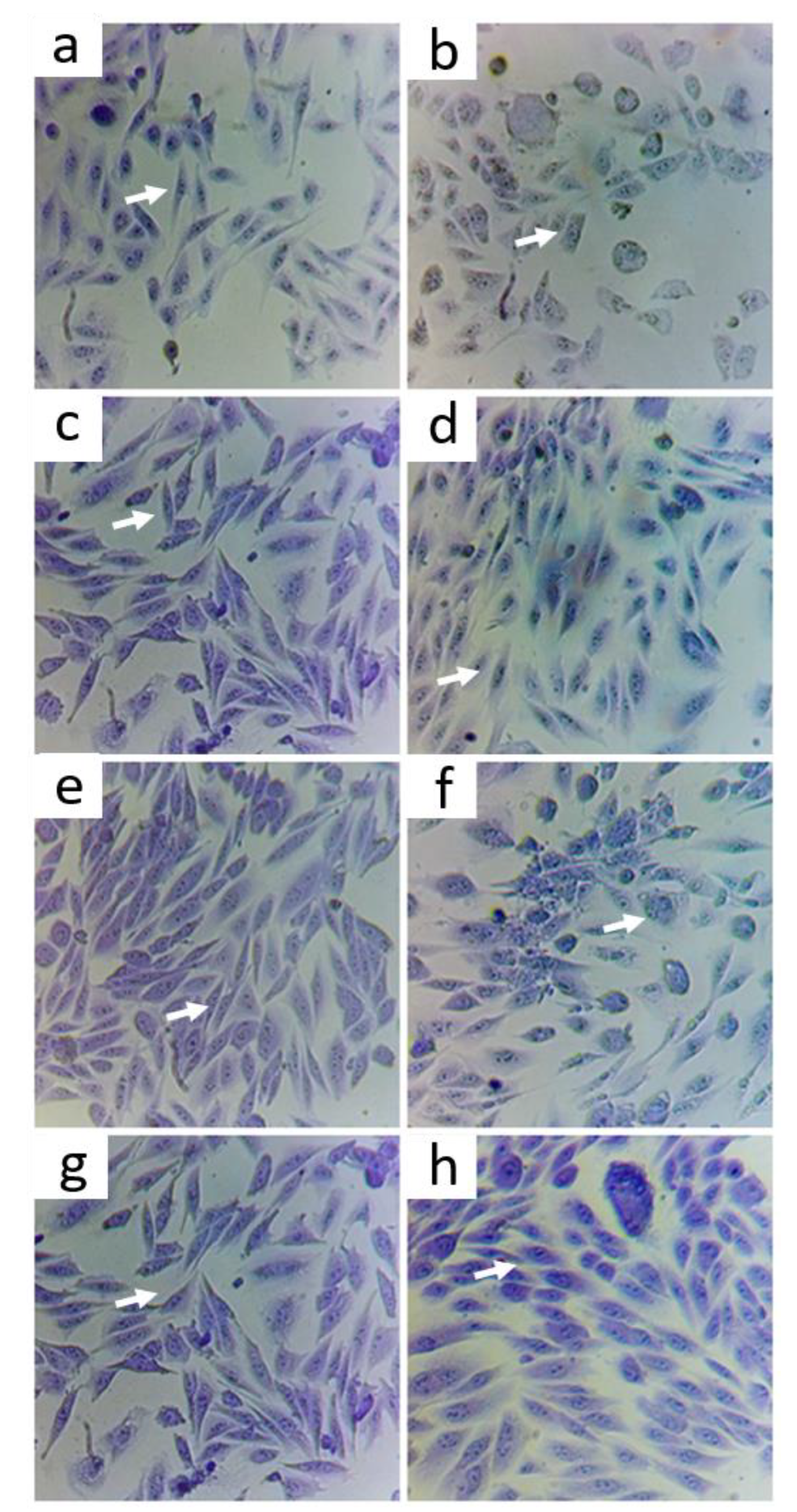
3.2. Protective Effect of Treatments on Cells Exposed to As
Morphological State with Violet Crystal
3.3. Antioxidant Potential with Reduction of Oxidative Stress by Aqueous Hydroperoxide
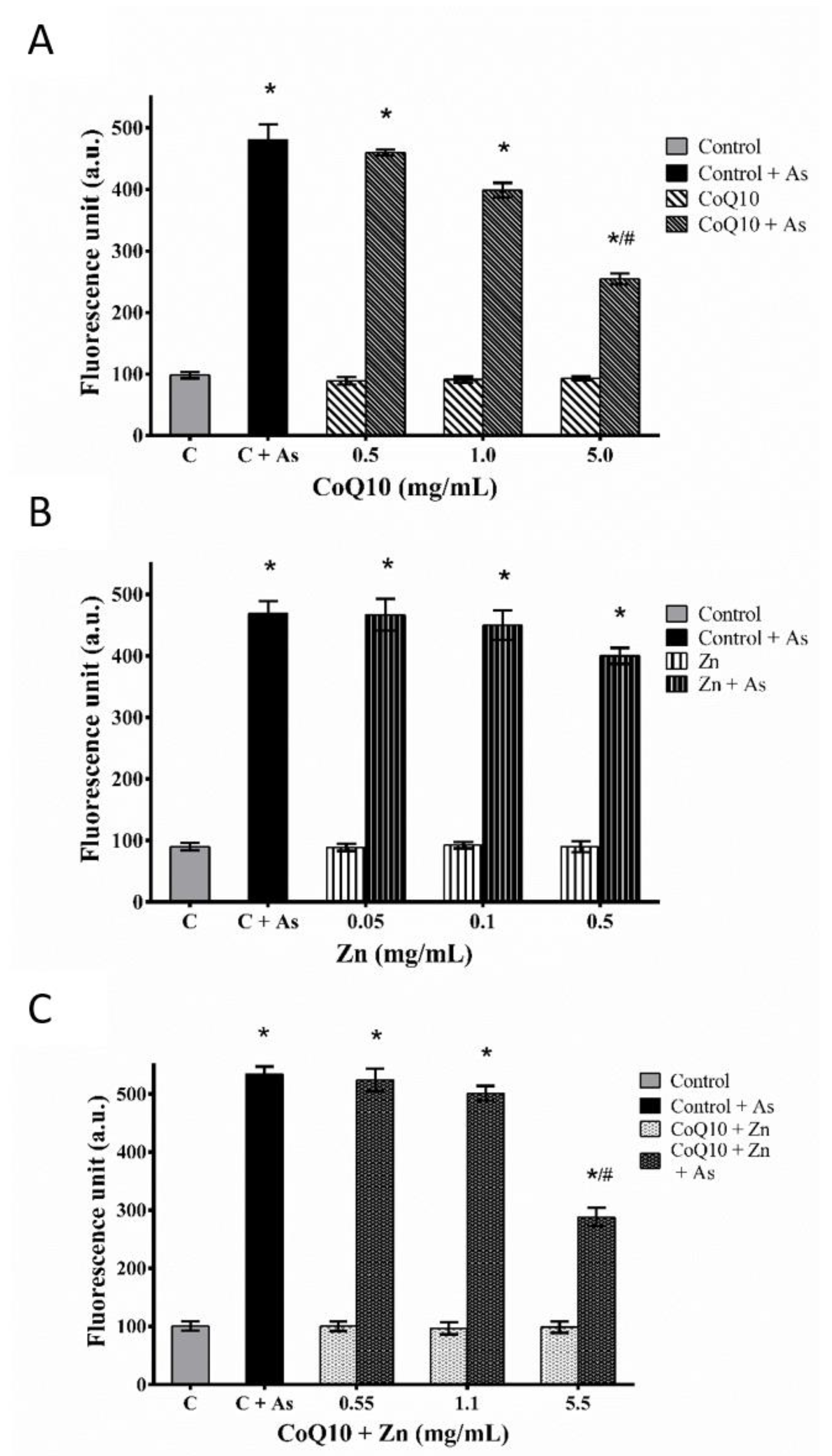
3.4. Determination of Intracellular ROS
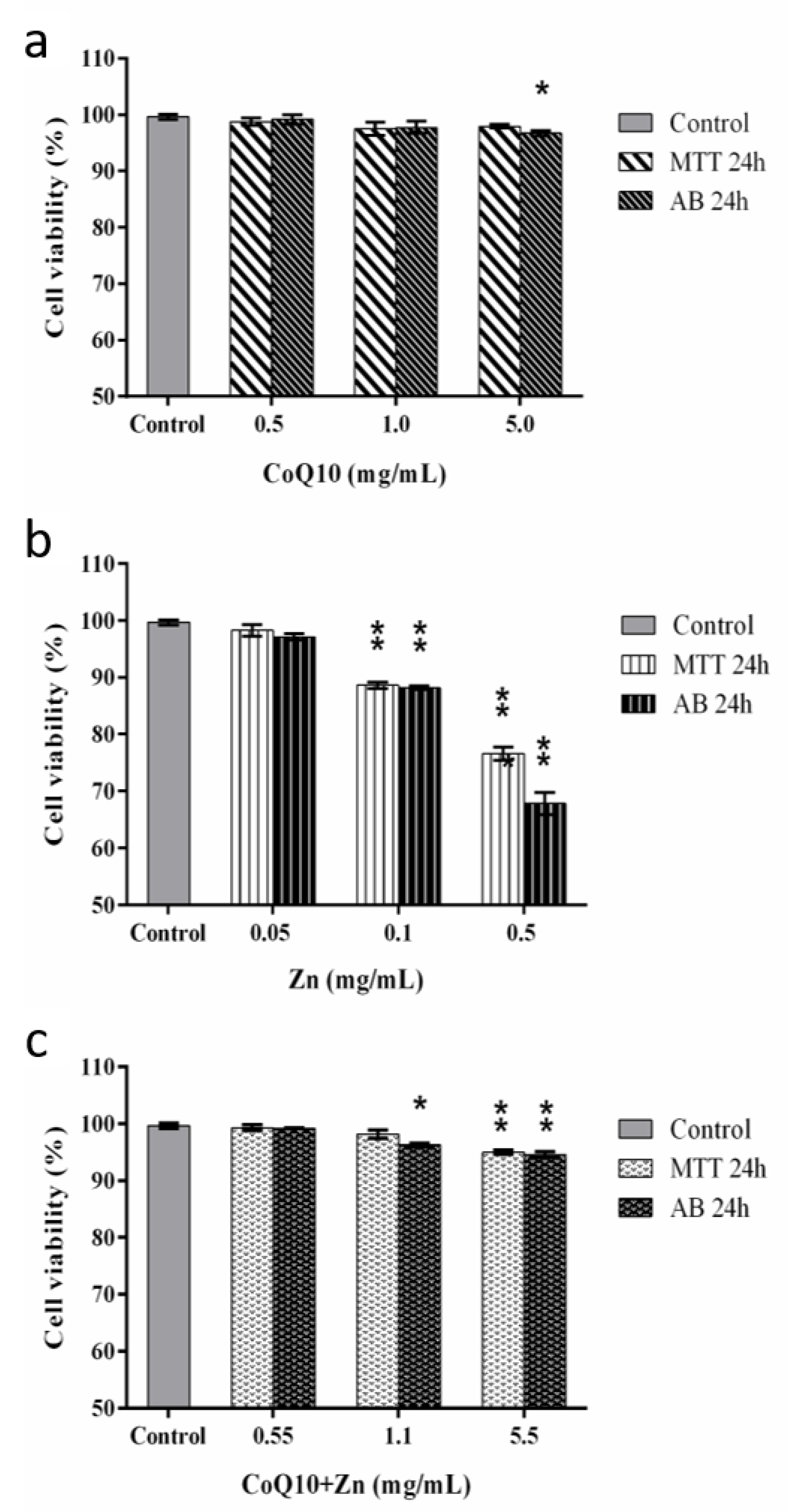
3.5. Effects of Treatments on CHO-K1 Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.L.; Terzidaki, A.A. A meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases. Biochem. J. 2017, 474, 2713–2731. [Google Scholar] [CrossRef]
- Stamp, L.K.; Khalilova, I.; Tarr, J.M.; Senthilmohan, R.; Turner, R.; Haigh, R.C.; Winyard, P.G.; Kettle, A.J. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 2012, 51, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.Z.; Gheita, T.A.; Kenawy, A.S.; Fahim, A.T.; El-sorougy, I.M.; Abdou, M.S. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: Relationship to disease manifestations and activity. Int. J. Rheum. Dis. 2011, 14, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogarasi, E.; Croitoru, M.D.; Fülöp, I.; Muntean, D.L. Is the Oxidative Stress Really a Disease? Acta Med. Marisiensis 2016, 62, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Yasukazu, Y.; Mieko, H.; Yoko, H.; Etsuo, N. Evaluation of the dietary effects of coenzyme Q in vivo by the oxidative stress marker, hydroxyoctadecadienoic acid and its stereoisomer ratio. Biochim. Biophys. Acta 2006, 1760, 1558–1568. [Google Scholar]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Dozor, A.J. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann. N. Y. Acad. Sci. 2010, 1203, 133–137. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Soria, E.A.; Quiroga, P.L.; Albrecht, C.; SRamos Elizagaray, I.; Cantero, J.J.; Bongiovanni, G.A. Development of an antioxidant phytoextract of Lantana grisebachii with lymphoprotective activity against in vitro arsenic toxicity. Adv. Pharmacol. Sci. 2014, 2014, 416761. [Google Scholar]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Ramos Elizagaray, S.I.; Quiroga, P.L.; Pérez, R.D.; Pérez, C.A.; Bongiovanni, G.A.; Soria, E.A. Effect of the aqueous extract of Lantana grisebachii Stuck. against bioaccumulated arsenic-induced oxidative and lipid dysfunction in rat splenocyt. J. Diet. Suppl. 2018, 16, 401–407. [Google Scholar] [CrossRef]
- De Barcelos, I.P.D.; Haas, R.H. CoQ10 and Aging. Biology 2019, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell. Mater. 2015, 30, 89–103. [Google Scholar] [CrossRef]
- Kim, H.N.; Jeon, D.G.; Lim, Y.; Jang, I.-S. The effects of coenzyme Q10 supplement on blood lipid indices and hepatic antioxidant defense system in SD rats fed a high cholesterol diet. Lab. Anim. Res. 2019, 35, 13. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.F.; Burón, I.; Espinosa, J.C.; Castilla, J.; Villalba, J.M.; Torres, J.M. Coenzyme Q and proteinllipid oxidation in a BSE-infected transgenic mouse model. Free Radic. Biol. Med. 2007, 42, 1723–1729. [Google Scholar] [CrossRef]
- Ayer, A.; Macdonald, P.; Stocker, R. CoQ10 Function and Role in Heart Failure and Ischemic Heart Disease. Annu. Rev. Nutr. 2015, 35, 175–213. [Google Scholar] [CrossRef]
- Díaz-Casado, M.E.; Quiles, J.L.; Barriocanal-Casado, E.; Gonzáles-García, P.; Battino, M.; López, L.C. The Paradox of Coenzyme Q10 in Aging. Nutrients 2019, 11, 2221. [Google Scholar] [CrossRef] [Green Version]
- Al-Megrin, A.W.; Soliman, D.; Kassab, R.D.; Metwally, D.M.; Moneim, A.E.; ElKhadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against Lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, A.O.; Fahad, A.; Moneim, A.E.; Metwally, D.M.; El-Khadragy, M.F.; Kassab, R.B. The Neuroprotective role of coenzyme Q10 against Lead acetate-induced neurotoxicity is mediated by antioxidant, anti-inflammatory and antiapoptotic activities. Int. J. Environ. Res. Public Health 2019, 16, 2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Yi, X.; da Silveira, E.; Sá, R.C.; Zhang, Y.; Liu, K.; Xiao, F.; Zhong, C. CoQ10 deficiency may indicate mitochondrial dysfunction in Cr (VI) toxicity. Int. J. Mol. Sci. 2017, 18, 816. [Google Scholar] [CrossRef] [Green Version]
- Marashi, S.M.; Majidi, M.; Sadeghian, M.; Jafarzadeh, M.; Mohammadi, S.; NasriNasrabadi, Z. Protective role of coenzyme Q10 as a means of alleviating the toxicity of aluminum phosphide: An evidence-based review. Tzu Chi Med. J. 2015, 27, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolova, N.; Victor, V.M. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Maret, W.; Vallee, B.L. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc. Natl. Acad. Sci. USA 2001, 98, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70. [Google Scholar] [CrossRef] [Green Version]
- Therrien, B. Coordination chemistry of 2,4,6-tri (pyridyl)-1,3,5-triazine ligands. J. Organomet. Chem. 2011, 696, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Und Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Soria, E.A.; Goleniowski, M.E.; Cantero, J.J. Bongiovanni, G.A. Antioxidant activity of different extracts of Argentinean medicinal plants against arsenic-induced toxicity in renal cells. Hum. Exp. Toxicol. 2008, 27, 341–346. [Google Scholar] [CrossRef]
- Soria, E.A.; Pérez, R.D.; Queralt, I.; Pérez, C.A.; Bongiovanni, G.A. Immunotoxicological effects of arsenic bioaccumulation on spatial metallomics and cellular enzyme response in the spleen of male Wistar rats after oral intake. Toxicol. Lett. 2017, 266, 65–73. [Google Scholar] [CrossRef]
- Soria, E.A.; Eynard, A.R.; Bongiovanni, G.A. Cytoprotective effects of silymarin on epithelial cells against arsenic-induced apoptosis in contrast with quercetin cytotoxicity. Life Sci. 2010, 87, 309–315. [Google Scholar] [CrossRef]
- Mardirosian, M.N.; Lascano, C.I.; Bongiovanni, G.A.; Venturino, A. Chronic toxicity of arsenic during Rhinella arenarum embryonic and larval development: Potential biomarkers of oxidative stress and antioxidant response. Environ. Toxicol. Chem. 2017, 36, 1614–1621. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Luz, J.R.D.; Nascimento, T.E.S.; Morais, L.V.F.; Cruz, A.K.M.; Rezende, A.A.; Brandão-Neto, J.; Ururahy, M.A.G.; Luchessi, A.D.; López, J.A.; Rocha, H.A.O.; et al. Thrombin Inhibition: Preliminary Assessment of the Anticoagulant Potential of Turnera subulata (Passifloraceae). J. Med. Food 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Brito, A.S.; Cavalcante, R.S.; Cavalheiro, R.P.; Palhares, L.C.G.F.; Nobre, L.T.D.B.; Andrade, G.P.V.; Nader, H.B.; Lima, M.A.; Chavante, S.F. Anti-IIa activity and antitumor properties of a hybrid heparin/heparan sulfate-like compound from Litopenaeus vannamei shrimp. Int. J. Biol. Macromol. 2018, 118, 1470–1478. [Google Scholar] [CrossRef]
- Binu, P.; Priya, N.; Abhilash, S.; Vineetha, R.C.; Nair, R.H. Studies on curative efficacy of monoterpene eugenol on anti- leukemic drug arsenic trioxide induced cardiotoxicity. Biomed. Pharmacother. 2017, 91, 559–566. [Google Scholar] [CrossRef]
- Pace, C.; Dagda, R.; Angermann, J. Antioxidants Protect against Arsenic Induced Mitochondrial Cardio-Toxicity. Toxics 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Fang, H.; Huang, Z.; Shang, W.; Hou, T.; Cheng, A.; Cheng, H. Imaging ROS signaling in cells and animals. J. Mol. Med. 2013, 91, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neergheen, V.; Chalasani, A.; Wainwright, L.; Yubero, D.; Montero, R.; Artuch, R.; Hargreaves, I. Coenzyme Q10 in the Treatment of Mitochondrial Disease. J. Inborn Errors Metab. 2017, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Clegg, M.S.; Hanna, L.A.; Niles, B.J.; Momma, T.Y.; Keen, C.L. Zinc deficiency-induced cell death. IUBMB Life 2005, 57, 661–669. [Google Scholar] [CrossRef]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef]
- Ohgami, N.; Yamanoshita, O.; Thang, N.D.; Yajima, I.; Nakano, C.; Wenting, W.; Ohnuma, S.; Kato, M. Carcinogenic risk of chromium, copper and arsenic in CCA-treated wood. Environ. Pollut. 2015, 206, 456–460. [Google Scholar] [CrossRef]
- Roy, P.; Saha, A. Metabolism and toxicity of arsenic: A human carcinogen. Curr. Sci. 2002, 82, 38–45. [Google Scholar]
- WHO (World Health Organization). Exposure to Arsenic: A Major Public Health Concern. Available online: http://www.who.int/ipcs/features/arsenic.pdf (accessed on 5 January 2021).
- Kumar, A.; Sharma, B. Consequences of heavy metals pollution in environment and bioremediation practices. In Recent Advances in Environmental Management; Bharagava, R.N., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 247–273. [Google Scholar]
- Gupta, V.K.; Kumar, A.; Yadav, S.H.; Pandey, R.; Sharma, B. Acetylcholinesterase as a biomarker of arsenic induced cardiotoxicity in mammals. Forensic Sci. Int. 2017, 5, 142–149. [Google Scholar]
- Kumar, A.; Singh, N.; Pandey, R.; Gupta, V.K.; Sharma, B. Biochemical and molecular targets of heavy metals and their actions. In Biomedical Applications of Metals; Rai, M., Ingle, A.P., Medici, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 297–319. [Google Scholar]
- Matos, R.C.; Oliveira, H.; Fonseca, H.M.A.C.; Morais, S.; Sharmaf, B.; Santos, C.; Pereira, M.L. Comparative Cr, As and CCA induced Cytostaticity in mice kidney: A contribution to assess CCA toxicity. Environ. Toxicol. Pharmacol. 2020, 73, 103297. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Fukao, Y.; Ferjani, L.; Tomioka, R.; Nagasaki, N.; Kurata, R.; Nishimori, Y.; Fujiwara, M.; Maeshima, M. iTRAQ Analysis Reveals Mechanisms of Growth Defects Due to Excess Zinc in Arabidopsis. Plant Physiol. 2011, 155, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, Z.; Poorakbar, L. Zinc toxicity on antioxidative response in (Zea mays L.) at two different pH. J. Stress Physiol. Biochem. 2013, 9, 66–73. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, N.; Stenson, M.; Lawson, J.; Abeykoon, J.; Patnaik, M.; Wu, X.; Witzig, T. Drugs with anti-oxidant properties can interfere with cell viability measurements by assays that rely on the reducing property of viable cells. Pathobiol. Focus 2017, 97, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicology 2004, 18, 703–710. [Google Scholar] [CrossRef]
- Ling, S.; Xie, H.; Yang, F.; Shan, Q.; Dai, H.; Zhuo, J.; Wei, X.; Song, P.; Zhou, L.; Xu, X.; et al. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: Roles of p38 MAPK, ERK3, and mTORC1. J. Hematol. Oncol. 2017, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Malhotra, A.; Nair, P.; Garg, M.; Dhawan, D.K. Protective role of zinc in ameliorating arsenic-induced oxidative stress and histological changes in rat liver. J. Environ. Pathol. Toxicol. Oncol. 2010, 29, 91–100. [Google Scholar] [CrossRef]
- Ruz, M.; Carrasco, F.; Rojas, P.; Codoceo, J.; Inostroza, J.; Basfi-fer, K.; Valencia, A.; Vásquez, K.; Galgani, J.; Pérez, A.; et al. Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr. Bull. 2013, 34, 215–221. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidante. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.V.e.; Gallia, M.C.; Luz, J.R.D.d.; Rezende, A.A.d.; Bongiovanni, G.A.; Araujo-Silva, G.; Almeida, M.d.G. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients 2022, 14, 3265. https://doi.org/10.3390/nu14163265
Silva SVe, Gallia MC, Luz JRDd, Rezende AAd, Bongiovanni GA, Araujo-Silva G, Almeida MdG. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients. 2022; 14(16):3265. https://doi.org/10.3390/nu14163265
Chicago/Turabian StyleSilva, Saulo Victor e, María Celeste Gallia, Jefferson Romáryo Duarte da Luz, Adriana Augusto de Rezende, Guillermina Azucena Bongiovanni, Gabriel Araujo-Silva, and Maria das Graças Almeida. 2022. "Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent" Nutrients 14, no. 16: 3265. https://doi.org/10.3390/nu14163265
APA StyleSilva, S. V. e., Gallia, M. C., Luz, J. R. D. d., Rezende, A. A. d., Bongiovanni, G. A., Araujo-Silva, G., & Almeida, M. d. G. (2022). Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients, 14(16), 3265. https://doi.org/10.3390/nu14163265









