Abstract
Dietary restriction (DR) is defined as a moderate reduction in food intake while avoiding malnutrition. The beneficial effects of DR are being increasingly acknowledged in aging and in a series of age-related neurodegenerative disorders, for example, Parkinson’s disease (PD). To date, the pathogenesis of PD remains elusive and there is no cure for it in spite of intensive research over decades. In this review, we summarize the current knowledge on the efficacy of DR on PD, focusing on the underlying mechanisms involving general metabolism, neuroendocrinolgy, neuroinflammation, gut microbiome, and so on. We anticipate that this review will provide future perspectives for PD prevention and treatment.
Keywords:
dietary restriction; Parkinson’s disease; gut microbiome; mechanism; patients; treatment; prevention 1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, with an incidence of 15/100,000/year and prevalence of 100–200/100,000 [1,2], imposing a growing socioeconomic burden globally. To date, PD is still incurable and its pathogenesis remains elusive [3,4]. Evidence from experimental studies reveals that mechanisms including protein misfolding and aggregation, neuroinflammation, mitochondrial dysfunction, and altered gut bacteria composition contribute to PD development [2,5,6,7,8,9]. In addition, PD is extremely heterogeneous regarding the age of onset, clinical manifestations, rate of progression, and therapeutic responsiveness [10,11]. As of now, a number of medication strategies have been widely applied to control the motor and non-motor symptoms of PD and improve the quality of life, among which levodopa remains the most effective [4,12,13,14]. Other common medications include dopamine agonists [15,16,17], monoamine oxidase B inhibitors [18,19,20], amantadine, and anticholinergic medication [4,12]. However, with long-term application of these drugs and as the disease progresses, adverse effects like gastrointestinal reactions, motor complications, psychotic disorders, and decreased efficacy emerge [12,13,14]. Moreover, these medications could neither effectively prevent the disease onset nor stop the disease progression. Recently, lifestyle interventions in the promotion of healthy brain aging and the prevention and treatment of central nervous system (CNS) diseases have risen into the spotlight [21,22], which could be promisingly complementary to the conventional PD pharmacotherapy.
Dietary restriction (DR), which involves a moderate reduction in food intake while avoiding malnutrition, has been proven to be effective in holding back aging and relieving age-related chronic diseases, including cancer, cardiovascular disease, diabetes, and neurodegenerative disorders [23]. The beneficial actions of DR involve metabolic, hormonal, and immunomodulatory mechanisms. DR could reduce obesity and visceral fat, thus preventing metabolic risk factors [24]. It increases insulin sensitivity, glucose tolerance, and ghrelin level [25,26]. It can also induce adipose tissue transcriptional reprogramming, involving ways to regulate mitochondrial bioenergy, anti-inflammatory response, and longevity [27]. Moreover, a potential role of DR in regulating the gut–brain axis has been well described in diseases of CNS and intestinal microbiota transplantation has been shown to be effective in Alzheimer’s disease (AD) and multiple sclerosis (MS) [28,29]. Here, we summarize the strategies of DR from clinical and laboratory studies, and review the current findings of DR in preventing and ameliorating PD, with an emphasis on the possible mechanisms.
2. Dietary Restriction
2.1. Methods of DR
DR strategies include long-term reductions in food intake or changes in the frequency and interval between meals [22,30,31]. Methods of DR vary in animal and clinical studies [32,33]; here, we describe the common dietary intervention strategies.
2.1.1. Calorie Restriction
Calorie restriction (CR) refers to a 10–50% reduction in daily food intake without changing the frequency of eating [22,34,35]. In humans, a two-year 25% CR has been reported to improve overall health in non-obese participants with few adverse effects [36]. In caenorhabditis elegans, the gene Sir-2.1 that mediates longevity [37,38] is widely expressed under CR induction [39].
2.1.2. Intermittent Fasting
Intermittent fasting (IF) refers to eating little or no food for a longer period of time (e.g., 16–48 h) and eating normally for the rest of the time [40]. In different studies, the specific protocols for achieving IF also vary.
- (a)
- Alternate day fasting (ADF)
In laboratory rodents, alternate day fasting is the most commonly used type of IF besides time-restricted feeding (TRF) [40]. It is also known as every-other-day fasting (EODF), which achieves a comparable effect to CR on reducing fasting blood glucose and insulin concentrations [41]. This dietary strategy has been confirmed by clinical trials for its safety and health promotion effects like weight loss and reducing the risk of cardiovascular diseases [41,42].
- (b)
- Time-restricted feeding
TRF is achieved by restricting the time window of food intake, with normal eating within it [34,43]. Interestingly, the mechanism of TRF to prolong lifespan and attenuate age-related cardiac decline is not equivalent to that of CR, which may be accomplished by enhancing the expression of circadian rhythm genes. The combined effect of the molecular circadian clock and the imposed feeding and fasting rhythms can improve the rhythm of gene expression under TRF and provide systemic metabolic benefits [43,44].
- (c)
- Periodic fasting
Periodic fasting refers to a type of arrhythmic IF that often involves 5 days without energy restriction and 2 days with less than 75% energy (intermittent calorie restriction (ICR)) [34,45]. Such a strategy has been proven to protect against diabetes, cancer, heart disease, and neurodegeneration in rodents; in humans, it is effective in reducing obesity, hypertension, asthma, and rheumatoid arthritis [46,47].
2.1.3. Fasting Mimicking Diet (FMD)
FMD is also known as periodic fasting or cycles of fasting, which is based on severe calorie restriction of 50% or more, low protein and sugar, with a relatively high fat content. It has been suggested to prolong lifespan and prevent cardiometabolic risk [48], as well as promote neural myelination, suppress autoimmunity [49], and induce cancer regression [50].
2.2. The Physiological Benefits of DR
From yeast and fruit flies to monkeys and humans, DR has shown positive effects of extending lifespan, promoting metabolism, preventing chronic diseases and neurodegeneration, and improving overall health [31,47,51]. Based on yeast models, researchers have revealed that the lifespan-extension effect of DR is related to mechanisms including preserving mitochondrial respiration [52], promoting Snf1 activation [53], regulating cell cycle [54], and so on. Moreover, DR could notably improve glucose metabolism and promote mobilization of fatty acids [55]. In addition, IF has been demonstrated to reduce blood pressure, increase heart rate variability, and reduce insulin resistance, as reviewed elsewhere by Dr. Mattson [40]. In AD transgenic mice, DR can alleviate cognitive deficits, amyloid lesions, and microglial reactivity [56]. In addition, prophylactic IF can reduce tissue damage and neurological deficits after ischemic stroke by inhibiting excitotoxicity, oxidative stress, and inflammation [57]. Moreover, DR also exerts positive effects on healthy obese people; after a month of 15–16 h daily fasting, the body weight, BMI, and fat content in healthy obese people were significantly reduced, accompanied with notably elevated gastrointestinal hormones that regulate satiety, including leptin, glucagon-like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY), and cholecystokinin (CCK), except for ghrelin. It is suggested that IF can be used as a strategy against obesity in the healthy population [58]. Analysis of data based on CALERIE phase 2 (CALERIE 2), the largest cohort study to date of sustained CR in non-obese healthy humans [59], showed that a two-year CR could produce parallel beneficial effects as in the animals receiving a similar intervention [60]. Besides improving mood, sleep, quality of life, and overall health [36], it also reduces inflammation and may prolong the health span by inhibiting the Pla2g7 gene [27], which encodes platelet activating factor acetyl hydrolase (PLA2G7), as well as inflammatory activators associated with a variety of diseases like cancer, neuropathy, and stroke [61]. Moreover, DR has been shown to exert immunomodulation by regulating multiple signaling pathways, for example, promoting the targeted regulation of rapamycin (mTOR) in mammals and the activation of AMP-activated protein kinase (AMPK) [62,63,64]. Inactivation of mTOR induces a metabolic transition from glycolysis to oxidative phosphorylation and counteracts the chronic activation of glycolysis in senescent T cells [65]. Activated AMPK improves immune function by promoting mitochondrial regeneration through the expression of nuclear respiratory factor (NRF)-1 and peroxisome proliferator-activated receptor (PPAR) g transcription factor [66,67]. However, it should be noted that chronic CR is not fully equivalent to intermittent energy restriction and may instead impair certain immune functions by inhibiting mTOR [67]; further, optimal dietary composition and fasting cycles remain to be defined.
2.3. Life-Extending Effect of DR and the Influencing Factors
DR can extend the lifespan of yeast by threefold, the lifespan of worms by two- to threefold, the lifespan of flies by twofold, and the lifespan of mice by 30–50% [51]. Despite the potential advantages of lifelong DR to slow aging and lengthen life span, it is hard to carry it out for a life-long period. DR started late in life may be more practical [68], but can dietary interventions that start at an older age have similar effects to those that start younger? DR started at 6 months of age did not increase the lifespan, but a reduction in mean lifespan of 15% was observed in A/J mice of 10 months old [69]. In another study, male C57BL/6 mice were fed either 40% DR or an ad libitum (AL) feeding scheme from 3 to 12 months of age and then were either switched to the other feeding regimen or kept in the same state for an extra 3 months. Both 9 and 12 months of DR regimens (long term), along with 3 months (short term, mid-life beginning) of DR, alleviated DNA damage and reduced the adipocyte size in the visceral fatty tissue with similar effectiveness, suggesting a metabolic retention of the DR phase [70].
In addition to the starting age of DR, the influence of gender should not be underestimated [71,72,73,74]. A 40% DR could significantly improve glucose tolerance [75,76], which has been proposed to play a role in the life-extending effects of DR in the male mice, and such a metabolic change could be maintained for two months after switching to a free diet [77], while in the female mice, such a transition not only resulted in a rapid loss of RNA expression patterns associated with DR, suggesting no or only weak memory of the previous DR regimen, but also significantly increased mortality in these mice after transition [78]. Moreover, the time spent carrying out DR is equally important [44,47,79]. In one study of intermittent TRF (iTRF), nighttime-biased fasting could extend the lifespan of Drosophila flies and enable them to show better flight ability and cardiac and flight muscle function, but fasting during the day, as opposed to what the circadian clock dictates, could abolish the benefits of iTRF. Long et al. suggested that such a phenomenon could be explained by enhanced autophagy at night [44]. In conclusion, the effect of DR on lifespan is affected by multiple factors, such as age, gender, and genotype, and the mechanism remains to be further explored.
2.4. Delay Aging and Age-Related Diseases
DR delays aging [80] and reduces the risk of age-related diseases like diabetes, cancer, cardiovascular disease, and neurodegenerative diseases in mounting studies based on laboratory animals and humans [42,51,81,82]. Aging is a key risk factor for neurodegenerative diseases [83,84,85] and DR can improve the adverse effects of aging on the brain [86,87]. Rumani Singh et al. found that 24-month-old Wistar strain male rats (equivalent to completing 70% of their lifespan) experiencing a 3-month ADF program showed improved age-related motor coordination, as well as learning and memory function, compared with the free-fed rats, despite that all of the indicators were inferior to the 3-month-old young control group. Meanwhile, fasting significantly reduced the protein carbonyl content in the cerebral cortex, hippocampus, and hypothalamus, which increased remarkably with aging, and it partially restored the decreased Syn and CaM expression in the hippocampal CA3 and DG areas, piriform cortex, and hypothalamus [88]. Moreover, DR could ameliorate late-life depression, which is increasingly acknowledged in elder people and is a key non-motor symptom of PD [89,90,91,92]. A community project for 90-year-olds in Italy showed a significant positive correlation between body fat and depression in older adults [93] and that CR can work as an antidepressant [94]. In a randomized controlled experiment, Rebecca et al. reported that, in overweight and obese women with polycystic ovary syndrome, DR improved depression assessed with CES-D scores [95]. Further, in rodent models, CR for just 10 days led to significant antidepressant responses [96]. In addition, community survey evaluations showed that myeloperoxidase levels were positively correlated with mortality in older individuals [97], while CR is able to attenuate the increased myeloperoxidase activity during aging [98].
Metabolically, DR can improve hyperglycemia, glucose tolerance, and insulin sensitivity in AL-fed aging mutant and normal mice, and reduce the consequences of aging-induced glycemic dysregulation, such as hepatic gluconeogenesis [99]. A recent randomized controlled trial in healthy middle-aged adults demonstrated that, after a 4-week ADF diet regimen, not only did cardiovascular indicators improve, but on fasting days, the pro-aging amino acid methionine was periodically depleted; additionally, after long-term ADF, levels of sICAM-1 (an age-related inflammatory marker), low-density lipoprotein, and the metabolic regulator triiodothyronine were all reduced. Such a fasting regimen showed no adverse effect even after six months [42], laying the basis for the safety and feasibility of ADF as an effective clinical intervention against aging and age-related disorders.
3. General Neuroprotective Effects of DR
3.1. Improve Cognitive and Motor Function
Aging causes changes in the structure, physiology, and metabolism of the brain, eventually leading to impaired cognitive and motor-neural abilities [100,101,102]. Fasting exerts positive effects on the brain via metabolic, cellular, and rhythmic mechanisms, directly or indirectly, which could enhance cognitive performance and protect against CNS disorders [100,103]. It is reported that 18-month-old rats on a 60% CR diet presented a higher survival rate, better locomotor activity, and a delayed age-related decline in cognition [104]. In addition, monkeys treated with MPTP that underwent 30% CR could accomplish more specified work compared with those with a normal diet, despite there being no statistical difference [105]. Another study on rats at the age of 24 months also demonstrated that even a late-onset short-term IF-DR regimen (EODF) could well relieve the age-associated decline in motor coordination and cognition performance [88]. Another clinical trial in 43 individuals with central obesity aged 35–75 years revealed that a four-week dietary intervention (either CR or IF) could improve cognition, but the intermittent energy restriction group showed a notable deterioration in recognition memory [106]. Fu et al. reported that different forms of CR exerted diverting effects on spatial recognition memory in developing male mice; that is, chronic CR showed no effect on the preference of mice for novelty in the Y-maze, but severe CR could impair spatial recognition memory, while acute CR could either improve or impair spatial recognition memory depending on the individual [107]. According to a randomized clinical study in 48 subjects under 6 months of CR, the energy deficit was not related to a consistent pattern of cognitive deficits [108]. These findings indicate that DR could affect cognitive and motor function differently depending on the intensity, duration, and time of initiation. However, it is plausible that mild to moderate DR started early in life is more likely to yield beneficial effects on the brain.
3.2. Promote Neurotrophic Factor Levels
Neurotrophic factors are critical mediators of neuronal plasticity by promoting axonal and dendritic development and remodeling [109,110]. They are involved in the generation of neurotransmitters as well as the formation of synapses [111]. A 6-month 30% CR regimen in monkeys has been proven to lead to three times higher glial-cell-line-derived neurotrophic factor (GDNF) levels in the right and left caudate nucleus (CN) compared with monkeys fed ad libitum [105]. Moreover, the DR regimen could increase the level of brain-derived neurotrophic factor (BDNF) dramatically in the hippocampus, cerebral cortex, and striatum, and the subsequent activation of BDNF signaling pathways plays a critical role in the neuroprotective effect of DR [112,113]. BDNF regulates the baseline level of neurogenesis in the dentate gyrus of adult mice and augments neurogenesis by DR [113]. Furthermore, formalized paraphrase in db/db mice, a model of insulin-resistant diabetes, CR, wheel running, or a combination of both increased the hippocampal BDNF level, which was accompanied by increases in dendritic spine density on the secondary and tertiary dendrites of dentate granule neurons. Moreover, in post-ischemic mouse models, a hypocaloric diet for 8 weeks could well confer neuroprotection and promote peri-infarct brain remodeling by upregulating BDNF [114].
3.3. Improve Neuronal Plasticity
Neuronal plasticity is a gradual process of the formation of neuronal structure and function during development and through learning. It involves trophic processes like neurogenesis and synaptogenesis as well as atrophic processes like degeneration of inactive neurons and neuronal contacts [115,116]. Despite there being much to be explored, there is mounting evidence that DR modulates brain structure and function throughout the lifespan [88,117]. In aging male rats, the expression of synaptic plasticity-related proteins, such as synaptophysin, calcineurin, and CaM kinase II, in the hippocampal CA3 and DG regions, piriform cortex, and hypothalamus are significantly reduced compared with young mice, but could be partially recovered after 3 months of ADF [88]. In addition, ADF significantly improved brain function and structure, as demonstrated by better learning and memory assessed with the Barnes maze and fear conditioning, a thicker CA1 pyramidal cell layer, greater expression of the dendritic protein drebrin, and lower oxidative stress, in the IF mice compared with mice fed ad libitum [118]. Besides, IF has been reported to effectively promote the regeneration of artificially crushed mouse sciatic nerve axons by promoting indole-3-propionic acid (IPA) production by the intestinal microbe Clostridium sporogenes, which can significantly improve epidermal innervation, thereby promoting sensory recovery [119]. A detailed description of the physiological and neuroprotective effect of DR is listed in Table 1.

Table 1.
The physiological benefits of different types of DR.
4. Effects of DR on PD and the Underlying Mechanisms
The key pathological features of PD include the formation of Lewy body [140] and the loss of dopaminergic neurons [2]. It is now established that genetic factors, α-synuclein misfolding and aggregation [141], neuroinflammation [5,142,143], oxidative stress [144,145], mitochondrial abnormalities [6], impaired protein clearance [146,147,148], and intestinal flora disorders and intestinal inflammation [149,150] all contribute to the onset and progression of PD.
4.1. DR and PD Animal Models
Despite that the pathogenesis of PD remains to be explored, several PD animal models have been well established: (1) The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model is the most commonly used PD animal model [151]. The subacute model is widely chosen because it could recapitulate PD progression, with the drawback of failing to elicit notable motor deficit. However, it could disrupt the blood–brain barrier (BBB) in the substantia nigra (SN) pars compacta (SNpc), activate astrocytes and microglial NLRP3 inflammasome in the striatum, upregulate α-synuclein expression, and induce Lewy-body-like pathology [151,152]. (2) The neurotoxin 6-hydroxydopamine (6-OHDA) intracerebral infusion is a classic PD model that causes massive destruction of nigrostriatal dopaminergic neurons [153]. Unlike the MPTP model, it is mainly used for the study of motor and biochemical dysfunction in PD. Although its manipulation carries a lower risk of toxicity, the effects of 6-OHDA in the brain appear to be heterogeneous and strongly influenced by the site of infusion [154]. (3) It is speculated that the α-synuclein pathology of PD originates in the gastrointestinal tract and spreads to the brain through the vagus nerve [155]; however, previous classical PD models fail to fully copy such a pathological process. It has recently been reported that, after injection of α-synuclein preformed fibers (PFFs) into the muscularis of the mouse duodenum and pylorus, pathological α-synuclein aggregates could be detected in the dorsal motor nucleus of the vagus (DMV) and locus coeruleus, which subsequently spread to the amygdala, dorsal raphe nucleus, and SN [156,157]. This model provides the most accurate characterization of α-synuclein gut-to-brain transmission to date and makes it possible to prevent PD by interfering with the production of pathological α-synuclein in the gut [158,159] (Figure 1). (4) A53T SNCA transgenic mice displayed a very similar expression pattern of human α-synuclein to endogenous mouse α-synuclein. They express truncated, oligomeric, and proteinase K-resistant phosphorylated forms of α-synuclein in areas specifically affected by PD and/or dementia with Lewy bodies, including the olfactory bulb, cerebral cortex, striatum, and SN [160].
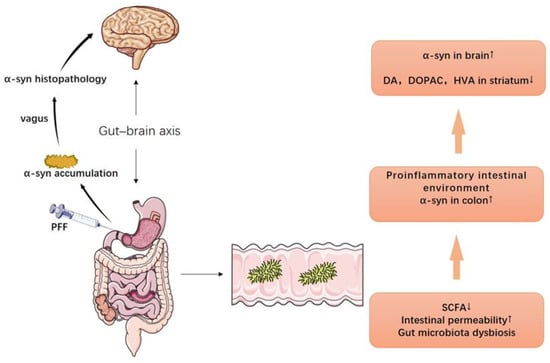
Figure 1.
The gut–brain axis and the PD model of PFF. Injection of α-synuclein preformed fibers (PFFs) into duodenum and gastric pylorus induces α-synuclein aggregates’ formation in the dorsal motor nucleus of the vagus (DMV) and locus coeruleus, which eventually travel to the brain via the vagus nerve (the left half). Decreased SCFAs in gut, increased intestinal permeability, and intestinal microbiota imbalance cause a proinflammatory intestinal environment and α-synuclein aggregation, which ultimately lead to an increase in α-synuclein and a decrease in dopamine and dopaminergic metabolites (DOPAC, HVA) in the brain (the right half).
With these animal models, the effects and the potential mechanisms of DR on PD have been increasingly studied [105,136,161]. In the MPTP-induced PD model, DR could decrease the neurotoxicity of MPTP on dopaminergic neurons by inducing stress proteins like heat-shock protein 70 and glucose regulate protein 78 [159]. Moreover, fasting for 3 days followed by feeding for 4 days, an FMD scheme, preserved motor function and attenuated MPTP-induced loss of SN dopaminergic neurons. BDNF, which promotes dopaminergic neuron survival, is elevated in PD mice after FMD, suggesting that BDNF is involved in FMD-mediated neuroprotection [132]. Additionally, adult male rhesus monkeys maintaining a 30% CR for 6 months and injected with MPTP into the right carotid artery showed significantly higher motor activity and higher levels of dopamine and dopamine metabolites in the striatal region compared with AL controls. Moreover, a notable increase in GDNF was also observed, suggesting that more than one form of neurotrophic factors mediate the anti-PD effect of CR [105]. In the yeast model, CR is performed by reducing the glucose concentration in the initial medium from 2% to 0.5% or 0.05%. Such a regimen yielded prolonged longevity and reduced α-synuclein-mediated toxicity, which were mediated by the maintenance of autophagy steady state [162]. SNCA mice expressing A53T mutant human α-synuclein under the control of the mouse Thy-1 promoter that causes familial PD have impaired autonomic control of their heart rate, which is exacerbated by a high-energy diet via a mechanism involving increased susceptibility of parasympathetic brainstem neurons to α-synuclein, and this could be partially restored by ADF [163,164]. Moreover, in normal rats of non-PD models, there is a considerable and continuous rise in α-synuclein mRNA expression in the cortex and hippocampus of rats with aging, while long-term DR could reverse the late age-related increase in α-synuclein expression [136]. More PD models based on different mechanisms are needed to fully elucidate the specific effect of DR on PD; for instance, whether DR can prevent PD progression in the PFF-induced PD model remains unknown.
4.2. DR and PD-Related Risk Factors
Regarding the potential risk factors for PD, a meta-analysis of seven observational cohort studies showed that people with diabetes had a 38% increased risk of developing PD [165]. It is speculated that diabetes may be an important confounding factor in PD pathogenesis [166]. Insulin signaling has been proven to cause neurodegeneration by affecting insulin dysregulation, amyloid aggregation, neuroinflammation, mitochondrial dysfunction, and altered synaptic plasticity [167]. In mice under 12 weeks of a high-fat diet (HFD), glucose intolerance promotes endogenous neurotoxin accumulation, which damages dopaminergic neurons in the brain and is associated with PD-like slowness and instability of gait [168]. Another well-studied risk factor of PD is obesity, which is associated with multiple pathological pathways, including metabolic abnormalities, altered hormonal signaling, and increased inflammation, which contribute to downstream neuropathology [169]. Gene-Jack Wang et al. reported that striatal dopamine D2 receptor availability is significantly lower in obese individuals than in the controls [170]. As a weight loss strategy, IF cannot only reduce body weight in the patients with type 2 diabetes (T2DM), but also lower glycated hemoglobin [171]. In db/db mice, an insulin-resistant diabetes model, the reduction in BDNF levels in the hippocampus and the density of dendritic spines on secondary and tertiary dendrites of DG granule neurons could be partially restored by CR, and in wild-type mice, CR showed an additive effect on the elevation of BDNF levels in the hippocampus [172]. Recently, Curie Kim et al. studied the effect of intermittent energy restriction (IER) and continuous energy restriction (CER) on cognition related to neurogenesis in the human hippocampus, and they found that both CER and IER improved adult-hippocampal neurogenesis-dependent cognition in adults with central obesity [106]. Moreover, increased β-cell death and impaired autophagic flux are present in the islets of obesity-induced diabetic mice, while IF could restore the islet autophagic flux and enhanced β-cell survival through the autophago-lysosomal pathway. Meanwhile, IF could also improve glucose tolerance and lower fasting blood glucose in these diabetic mice by enhancing glucose-stimulated insulin secretion and nuclear expression of the pancreatic regeneration marker NEUROG3 [129]. So far, no clinical guidelines have been published regarding the implementation of dietary interventions like IF in patients with diabetes [173], and more clinical data are needed to assess the safety and efficacy of such dietary regimens. As another risk factor for PD, the relationship between traumatic brain injury and neurodegenerative diseases is complex [174], but both IF and CR after traumatic brain injury have been proved to be neuroprotective by alleviating mitochondrial dysfunction, promoting hippocampal neurogenesis, inhibiting glial cell response, shaping nerve cell plasticity, and modulating autophagy and apoptosis [175,176].
4.3. Possible Mechanisms of DR on PD
4.3.1. Ameliorate Neuroinflammation
Neuroinflammation is the coordinated response of the central nervous system to noxious stimuli, for example, infection, traumatic brain injury, or other neurological disorders, and chronic neuroinflammation may take place if the predisposing factors persist [22,177,178]. Postmortem brain pathology of PD patients revealed inflammatory changes in microglia and elevated levels of proinflammatory mediators like IL1β, IL6, and TNFα in the striatum [5]. Microglial NLRP3 inflammasome activation induces dopaminergic neuron death and subsequent dyskinesia in PD, while dopamine can significantly reduce NLRP3-dependent inflammasome activation in mixed glial cells stimulated by NLRP3 agonists. It is indicated that the reduction in dopamine levels in PD may exacerbate neuroinflammation in the CNS by hindering the ability of dopamine to inhibit the inflammasome, thus forming a vicious circle [152].
Meanwhile, the NLRP3 inflammasome can assemble under stimulation by accumulated endogenous metabolites, such as fibrillar amyloid β and 25-hydroxycholesterol, leading to the activation of caspase-1 and subsequent secretion of IL-1β [152]. The former can truncate the normal multimeric α-synuclein into a form that is prone to aggregation, eventually leading to neuronal dysfunction and loss [179]. Chronic systemic expression of the latter is also able to exacerbate neurodegeneration and microglial activation in the SN. Furthermore, the downstream molecule NO of IL-1β action is partly responsible for the worsening of neurodegeneration observed in PD [180]. In mice, prolonged (48 h) fasting blunted NLRP3 inflammasome activation and reduced IL-1β levels [181]. This is consistent with previous observations in humans [182]. Mechanistically, the negative regulation of fasting-induced SIRT3 on NLRP3 is achieved through SIRT3-mediated mitochondrial SOD2 deacetylation, resulting in SOD2 activation. Therefore, the anti-inflammatory effects of the mimic fasting diet may be mediated in part through SIRT3-directed blunting of NLRP3 inflammasome assembly and activation [181]. Recently, peripheral circulating CD4+ and CD8+ T cells from PD patients were shown to produce cytokines in response to α-synuclein. Further, α-synuclein overexpression in the mouse midbrain results in upregulation of major histocompatibility complex II (MHCII) protein in CNS myeloid cells along with T cell infiltrating into the CNS; such an enhanced T-cell response primarily involves increased CD4+ T cells that produce the cytokine IFNγ, while knockout of CD4 T cells could attenuate α-synuclein induced neurodegeneration [183]. Francesca Cignarella et al. found that the proportion of CD4+ T cells in the draining lymph nodes of the IF group as well as the level of IL-17A and IFN-γ were lower than those of the free diet group in the multiple sclerosis (MS) mice, and thereby ameliorated symptoms in the EAE mice. In addition, IF improved T cell composition in the mouse gut lamina propria, thereby modulating systemic immunity [133]. Considering the gastrointestinal inflammation in PD [149], whether IF can play a similar role in PD is worth exploring.
4.3.2. Reduce Oxidative Stress
Reactive oxygen species (ROS) are the products of normal cellular metabolism and mediate a wide range of physiological processes, but at high concentrations, they can adversely modify biomolecules like proteins, lipids, and DNA [184,185]. When the antioxidant defense system in the cell fails to balance oxidants, the ratio of oxidants to antioxidants is disturbed and the oxidants dominate, thus forming oxidative stress [186]. Consequently, the defense mechanisms are unable to cope with ROS-induced damage [187]. DA metabolism, high levels of iron and calcium in SN, mitochondrial dysfunction, and neuroinflammation lead to increased oxidative stress and loss of dopaminergic neurons in the brains of PD patients [188,189]. While many antioxidants, such as creatine, vitamin E, coenzyme Q10, pioglitazone, melatonin, and deferoxamine, have been tested in clinical trials as potential PD treatments, none have been shown to improve neurodegeneration in PD patients [188,190]. Besides the dietary supplements, many studies have demonstrated the remarkable effect of DR in ameliorating oxidative stress.
Sirtuins (SIRTs 1–7) belong to the nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylases family and play important roles in the regulation of oxidative stress, of which SIRT1, SIRT3, and SIRT5 protect cells from activity oxygen damage and SIRT2, SIRT6, and SIRT7 regulate key oxidative stress genes and mechanisms [191]. There is increasing evidence showing that modulation of sirtuins’ activity by DR regimens can prevent neuronal loss and brain damage in PD models [192,193]. mTOR activation mediates neuronal apoptosis under oxidative stress; for example, the environmental toxin cadmium-induced ROS activates mTOR signaling, leading to neuronal cell death. Mechanistically, calcium/calmodulin-dependent protein kinase II (CaMKII) and calcium signaling pathways are involved in this process [194]. Meanwhile, IF can suppress mTOR levels [195], induce Sox2 and Ngn3 expression, and promote insulin production in pancreatic islets of human type 1 diabetic patients [196]. However, studies have also shown that mTOR activators can be used to prevent oxidative-stress-related neuronal cell death and protect dopaminergic neurons from oxidative stress in PD [194]. Different signaling pathways of DR on mTOR may be involved and more deeper research based on different PD models is necessary.
4.3.3. Preserve Mitochondrial Function and Reduce Mitochondrial Damage
Post-mortem studies in PD patients have shown lowered reactivity of the complex I of the respiratory chain in the SN as well as mitochondrial morphological and DNA losses [145]. Furthermore, mitochondrial failure has been identified in sporadic and hereditary types of PD, along with toxin-induced models [6]. DR can boost the heart mitochondrial respiratory rate while lowering cardiac ROS generation [197] and has a favorable effect on mitochondria by increasing mitochondrial biogenesis and efficiency [187]. Rumani Singh et al. discovered that the activity of the mitochondrial electron transport chain (ETC) decreased significantly in old (24-month-old) ad libitum (OAL) fed rats compared with their young (3-month-old) counterparts, but it could be reversed partially by the IF-DR regimen [88]. Meanwhile, chronic hypoxia-induced CR induces notable changes in myocardial mitochondrial metabolism and is responsible for better diffusion of ADP toward adenine nucleotide translocase, which may be mediated by enhanced permeability of the outer mitochondrial membrane [198].
4.3.4. Maintain Autophagy Homeostasis
Autophagy is a major, conserved cellular pathway through which cells transport cytoplasmic contents to lysosomes for degradation [199], which helps remove misfolded or aggregated proteins, as well as recycle damaged cellular components [200]. In PD, increased intracellular pathogenic α-synuclein can lead to Rab1a-related mislocalization of the autophagy protein Atg9, which inhibits autophagosome biogenesis [201]. In addition, the accumulation of α-synuclein aggregates can not only inhibit the in vivo maturation of autophagy and fusion with lysosomes, but also impair lysosome function, thereby reducing the ability to clear α-synuclein [199]. On the other hand, neuronal α-synuclein activates microglia, which engulf α-synuclein into autophagosomes for degradation through selective autophagy [202]. Injured microglial autophagy exacerbates pro-inflammatory responses by modulating the NLRP3 inflammasome and eventually exacerbates MPTP-induced neurodegeneration [146]. However, in the later stage of PD, overactive or dysfunctional autophagy can also lead to neuronal cell death [200]. Therefore, regulating autophagy homeostasis in PD is a promising target.
Dietary interventions like fasting and CR are the most potent non-genetic autophagy regulators that act in a variety of organs and tissues, including nerves, liver, kidney, and heart [200]. Autophagy is maintained at homeostatic levels following CR treatment in yeast cells expressing α-synuclein, and such autophagic homeostasis is able to reverse the decreased activity of the α-syuclein-promoted ubiquitin-proteasome system (UPS), another crucial α-synuclein degradation pathway [203]. In AD mice, Xigui Chen et al. showed that fasting increases the number, size, and signal strength of neuronal autophagosomes in the brain and, for the first time, they discovered a circadian rhythm of autophagy, that is, autophagy parameters increase during the day and decrease during the night [204]. In parallel, Matt Ulgherait et al. applied Drosophila to demonstrate that nighttime-specific fasting significantly prolongs lifespan, while only fasting during the day has no effect on lifespan [205]. In conclusion, DR in a specific time period may have the best effect on autophagy regulation and more research is needed to reveal the effect of DR on selective autophagy in microglia.
4.3.5. Regulate Gut Microbiota Composition and Richness
Recently, an increasing number of studies have highlighted the important role of the gut microbiota in neurological disorders, including AD [28], autism [206,207], MS [29,208], PD [132,149,209,210], and stroke [211,212]. The gut microbiota affects behavior, modulates the production of neurotransmitters in the gut and brain, and influences brain development and myelination patterns. Moreover, the dietary components are chemically transformed by the microbiota so that gut-derived metabolites spread to all organs, eventually affecting the brain [213]. The role of gastrointestinal inflammation in PD can be traced back to the well-known Braak hypothesis [155]: PD may originate in the gut as there may be a pathogen that can pass through the gastric epithelial layer to induce the misfolding and aggregation of α-synuclein in the submucosal plexus and ultimately project to the brain [155,214]. At the same time, multiple surveys have shown that patients with Helicobacter pylori infection and patients with inflammatory bowel disease have an increased risk of PD [215,216]. Recent studies have confirmed the hypothesis that a pro-inflammatory gut environment due to gut hyperpermeability and/or dysbiosi can induce or exacerbate PD, increase α-synuclein levels in the colon, and reduce DA and DA metabolites (DOPAC, HVA) in the striatum [217]. Mechanistically, short-chain fatty acids (SCFAs), one of the metabolites of gut microbes, can regulate gut permeability and blood–brain barrier (BBB) permeability by upregulating tight junction proteins [218]. In PD patients, there is a lack of correlation between SCFA-producing gut bacteria and fecal levels of SCFAs. PD patients show reduced fecal SCFAs, but increased plasma SCFAs, which may be due to increased intestinal permeability, which allows SCFAs to enter the systemic circulation, indicating a dysfunctional intestinal barrier [219]. Both human and animals under a CR or IF diet can develop a physically balanced composition of gut microbiota [133]. Zhou et al. have reported that TRF is effective in preserving motor function and attenuating dopaminergic neuron loss in MPTP-induced PD mice via promoting a favorable gut microbiota composition and metabolites [132]. Clinically, colon cleansing and a dietary intervention incorporating SCFAs have a beneficial impact not only on the gut microbiome, but also on the clinical course of PD [220]. In patients with metabolic syndrome, IF can induce profound changes in the gut microbial community, increase SCFA production, decrease circulating levels of lipopolysaccharides, and significantly reduce cardiovascular risk factors [122]. In addition, IF alters the gut microbiota composition of MPTP-induced PD mice, inhibiting the number of pro-inflammatory bacteria Clostridium in the gut and reducing the number of glial cells as well as the release of inflammatory factors such as IL-1β and TNF -α, thus exerting a neuroprotective effect, despite with no change in the SCFA level [132]. Even though many studies have demonstrated a close correlation between gut microbiota and PD, the mechanism remains unclear. Inflammation, metabolites, or specific probiotics may play a role (Figure 1 and Figure 2).
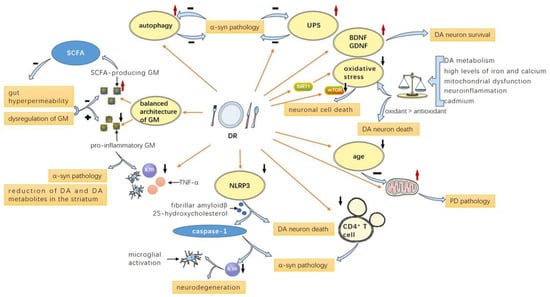
Figure 2.
Possible mechanisms of DR on PD: DR contributes to a balanced architecture of GM consisting of more SCFA-producing GM and less pro-inflammatory GM, as the former leads to less gut hyperpermeability and the latter leads to increased inflammatory factor levels, α-synuclein pathology, and reduction in DA and DA metabolites in the striatum; DR attenuates NLRP3 inflammasome and CD4+ T cells, leading to reduced α-syn pathology and caspase-1 and IL-1β levels, which could promote microglial activation and neurodegeneration; DR also delays aging and enhances mitochondrial function, thus ameliorating PD pathology; DR suppresses oxidative stress by modulating mTOR and SIRT1-related pathways and boosts the level of neurotrophic factors like BDNF and GDNF; DR enhances autophagy and UPS to facilitate α-synuclein degradation, thus ameliorating α-synuclein pathology. PD: Parkinson’s disease; SCFA: short-chain fatty acid; DR: dietary restriction; GM: gut microbiota; UPS: ubiquitin-proteasome system; DA: dopamine; BDNF: brain-derived neurotrophic factor; GDNF: glial-cell-line-derived neurotrophic factor; α-syn: α-synuclein.
5. Other Dietary Interventions on PD
5.1. Low Fat Diet
A high-fat diet (HFD) severely reduces striatal dopamine, SN microtubule-associated protein 2, manganese superoxide dismutase, and tyrosine hydroxylase levels in MPTP subacute mice, and significantly increases mortality of the MPTP acute mice [221]. Multiple studies have shown that a high fat intake can lead to excess production of circulating free fatty acids and systemic inflammation. Immune cells, free fatty acids, and circulating cytokines reach the hypothalamus and initiate local inflammation through several processes such as microglial proliferation [222,223,224,225]. In a randomized controlled trial, PD patients were randomly classified as low-fat diet (LFD) or ketogenic diet for 8 weeks, and both groups showed significantly reduced MDS-UPDRS scores and a notable improvement in both motor daily living experiences and non-motor symptoms like fatigue, sleepiness, and cognitive impairment [226]. In the mice under LFD combined with 40% CR starting at 24 months old, white matter microglia activation was reduced, as indicated by a number of phagocytic markers like Mac-2/Lgals3, Dectin-1/Clec7a, and CD16/CD32, which may explain the protective effects of CR during aging-related decline [227]. In addition, in 6-OHDA-induced PD rats, a high-fat diet induced insulin resistance and significantly increased DA consumption in the SN, while later conversion to LFD reversed such a phenomenon and improved proteins associated with mitochondrial function (e.g., AMPK and PGC-1α) and proteasome function (e.g., TCF11/Nrf1) [228].
5.2. Protein-Restricted Diet and Amino-Acid-Restricted Diet
There has been suggested a significant positive correlation between PD mortality and per capita total dietary protein consumption and meat consumption [229]. As early as 1987, low-protein dietary strategies have been clinically developed to reduce fluctuations in PD patients treated with levodopa [230,231,232]. This phenomenon is due to a significant increase in plasma concentrations of large amounts of neutral amino acids (LNAAs) caused by a conventional high-protein diet, which compete with levodopa for transportation across the BBB [233,234,235]. The results of the dietary pattern showed that severe motor fluctuations induced by daytime levodopa or carbidopa treatment were significantly alleviated by eliminating dietary proteins from breakfast and lunch, and the patient’s overall function and sensitivity to the drug improved significantly [236]. There were no adverse effects during the one-year follow-up period [236]. Another recent study also reported that patients with a high-protein diet had younger onset, greater motor fluctuations, and higher rates of PD family members [237]. We can infer that the protein-restricting diet may be more suitable for younger PD patients or familial PD patients. Furthermore, obesity, insulin resistance, and T2DM were associated with elevated levels of branched-chain amino acids (BCAAs) as risk factors for PD. Restricting BCAAs can improve metabolic health, induce weight loss, and increase insulin sensitivity and glycemic tolerance, as well as improve insulin resistance [238,239,240,241]. Reducing protein and amino acid content in the diet provides PD patients with an inexpensive and sustainable dietary intervention.
6. Perspectives
To sum up, we have reviewed the physiological benefits of DR, especially on PD, through various mechanisms including reducing PD-related risk factors, alleviating oxidative stress, improving mitochondrial function, maintaining autophagy homeostasis, and perfecting the gut microbiome, among others. The major concern of such a regimen is poor adherence in daily life and few guidelines have included DR for treating neurodegenerative diseases thus far. Further research is required to improve our understanding of the numerous DR protocols in PD and the underlying mechanisms. More clinical trials are necessary to assess whether DR could be an effective adjunctive therapeutic measure to the current medications.
Author Contributions
X.H., W.Y., and J.F. were responsible for conceiving, designing, and revising this review. Z.W. wrote the original manuscript. Y.C. completed the figures and tables. L.W. and H.Y. searched and collected the related references. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81901417), 345 talent project of Shengjing hospital, and Scientific Research Foundation of Educational Department of Liaoning Province (QN2019001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully thank members of Juan Feng’s lab for their intellectual input.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| dietary restriction | DR |
| Parkinson’s disease | PD |
| central nervous system | CNS |
| Alzheimer’s disease | AD |
| multiple sclerosis | MS |
| calorie restriction | CR |
| intermittent fasting | IF |
| alternate day fasting | ADF |
| time-restricted feeding | TRF |
| every-other-day fasting | EODF |
| intermittent calorie restriction | ICR |
| fasting mimicking diet | FMD |
| glucagon-like peptide-1 | GLP-1 |
| peptide tyrosine-tyrosine | PYY |
| cholecystokinin | CCK |
| platelet-activating factor acetyl hydrolase | PLA2G7 |
| AMP-activated protein kinase | AMPK |
| nuclear respiratory factor | NRF |
| peroxisome proliferator-activated receptor | PPAR |
| ad libitum | AL |
| intermittent TRF | iTRF |
| glial-cell-line-derived neurotrophic factor | GDNF |
| brain-derived neurotrophic factor | BDNF |
| indole-3-propionic acid | IPA |
| 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine | MPTP |
| substantia nigra | SN |
| substantia nigra pars compacta | SNpc |
| 6-hydroxydopamine | 6-OHDA |
| α-synuclein preformed fibers | PFF |
| dorsal motor nucleus of the vagus | DMV |
| high-fat diet | HFD |
| type 2 diabetes | T2DM |
| intermittent energy restriction | IER |
| continuous energy restriction | CER |
| multiple sclerosis | MS |
| reactive oxygen species | ROS |
| sirtuins | SIRTs |
| nicotinamide adenine dinucleotide | NAD |
| calcium/calmodulin-dependent protein kinase II | CaMKII |
| electron transport chain | ETC |
| old ad libitum | OAL |
| ubiquitin-proteasome system | UPS |
| short-chain fatty acids | SCFAs |
| blood–brain barrier | BBB |
| high-fat diet | HFD |
| low-fat diet | LFD |
| large amounts of neutral amino acids | LNAA |
| branched-chain amino acids | BCAAs |
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 687. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Singleton, A.B.; Farrer, M.J.; Bonifati, V. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov. Disord. 2013, 28, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Tambasco, N.; Romoli, M.; Calabresi, P. Levodopa in Parkinson’s Disease: Current Status and Future Developments. Curr. Neuropharmacol. 2018, 16, 1239–1252. [Google Scholar] [CrossRef]
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Parkinsons Dis. 2013, 3, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Alonso Canovas, A.; Luquin Piudo, R.; Garcia Ruiz-Espiga, P.; Burguera, J.A.; Campos Arillo, V.; Castro, A.; Linazasoro, G.; Lopez Del Val, J.; Vela, L.; Martinez Castrillo, J.C. Dopaminergic agonists in Parkinson’s disease. Neurologia 2014, 29, 230–241. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Rascol, O. Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs 2010, 24, 941–968. [Google Scholar] [CrossRef]
- Stocchi, F.; Torti, M.; Fossati, C. Advances in dopamine receptor agonists for the treatment of Parkinson’s disease. Expert Opin. Pharm. 2016, 17, 1889–1902. [Google Scholar] [CrossRef]
- Braga, C.A.; Follmer, C.; Palhano, F.L.; Khattar, E.; Freitas, M.S.; Romao, L.; Di Giovanni, S.; Lashuel, H.A.; Silva, J.L.; Foguel, D. The anti-Parkinsonian drug selegiline delays the nucleation phase of alpha-synuclein aggregation leading to the formation of nontoxic species. J. Mol. Biol. 2011, 405, 254–273. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Parkinsons Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Kakish, J.; Tavassoly, O.; Lee, J.S. Rasagiline, a suicide inhibitor of monoamine oxidases, binds reversibly to alpha-synuclein. ACS Chem. Neurosci. 2015, 6, 347–355. [Google Scholar] [CrossRef]
- Brocchi, A.; Rebelos, E.; Dardano, A.; Mantuano, M.; Daniele, G. Effects of Intermittent Fasting on Brain Metabolism. Nutrients 2022, 14, 1275. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Zanetti, M.; Klaus, K.A.; Semolic, A.; Johnson, M.L.; Nair, K.S. Higher unacylated ghrelin and insulin sensitivity following dietary restriction and weight loss in obese humans. Clin. Nutr. 2021, 40, 638–644. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age 2010, 32, 97–108. [Google Scholar] [CrossRef]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hao, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109884. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Dorling, J.L.; Martin, C.K.; Redman, L.M. Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res. Rev. 2020, 64, 101038. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Bauer, B.A.; Benarroch, E.E.; Savica, R. The Role of Intermittent Fasting in Parkinson’s Disease. Front. Neurol. 2021, 12, 682184. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Carmona-Gutierrez, D.; Mueller, M.I.; Madeo, F. The ups and downs of caloric restriction and fasting: From molecular effects to clinical application. EMBO Mol. Med. 2022, 14, e14418. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Horikawa, M.; Nomura, T.; Sakamoto, K. Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology 2010, 11, 31–43. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Li, L.; Wang, D. Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16. Biochem. Biophys. Res. Commun. 2010, 400, 613–618. [Google Scholar] [CrossRef]
- Bamps, S.; Wirtz, J.; Savory, F.R.; Lake, D.; Hope, I.A. The Caenorhabditis elegans sirtuin gene, sir-2.1, is widely expressed and induced upon caloric restriction. Mech. Ageing Dev. 2009, 130, 762–770. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Varady, K.A.; Hellerstein, M.K. Alternate-day fasting and chronic disease prevention: A review of human and animal trials. Am. J. Clin. Nutr. 2007, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef]
- Long, H.; Panda, S. Time-restricted feeding and circadian autophagy for long life. Nat. Rev. Endocrinol. 2022, 18, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Mishra, A.; Mirzaei, H.; Guidi, N.; Vinciguerra, M.; Mouton, A.; Linardic, M.; Rappa, F.; Barone, R.; Navarrete, G.; Wei, M.; et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat. Metab. 2021, 3, 1342–1356. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.Y.; Lee, S.K.; Lee, C.K. Caloric Restriction-Induced Extension of Chronological Lifespan Requires Intact Respiration in Budding Yeast. Mol. Cells 2017, 40, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.B.; Maqani, N.; Strickler, E.; Li, M.; Smith, J.S. Caloric Restriction Extends Yeast Chronological Life Span by Optimizing the Snf1 (AMPK) Signaling Pathway. Mol. Cell Biol. 2017, 37, e00562-16. [Google Scholar] [CrossRef]
- Leonov, A.; Feldman, R.; Piano, A.; Arlia-Ciommo, A.; Lutchman, V.; Ahmadi, M.; Elsaser, S.; Fakim, H.; Heshmati-Moghaddam, M.; Hussain, A.; et al. Caloric restriction extends yeast chronological lifespan via a mechanism linking cellular aging to cell cycle regulation, maintenance of a quiescent state, entry into a non-quiescent state and survival in the non-quiescent state. Oncotarget 2017, 8, 69328–69350. [Google Scholar] [CrossRef] [PubMed]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Gregosa, A.; Vinuesa, A.; Todero, M.F.; Pomilio, C.; Rossi, S.P.; Bentivegna, M.; Presa, J.; Wenker, S.; Saravia, F.; Beauquis, J. Periodic dietary restriction ameliorates amyloid pathology and cognitive impairment in PDAPP-J20 mice: Potential implication of glial autophagy. Neurobiol. Dis. 2019, 132, 104542. [Google Scholar] [CrossRef]
- Fann, D.Y.; Ng, G.Y.; Poh, L.; Arumugam, T.V. Positive effects of intermittent fasting in ischemic stroke. Exp. Gerontol. 2017, 89, 93–102. [Google Scholar] [CrossRef]
- Zouhal, H.; Bagheri, R.; Triki, R.; Saeidi, A.; Wong, A.; Hackney, A.C.; Laher, I.; Suzuki, K.; Ben Abderrahman, A. Effects of Ramadan Intermittent Fasting on Gut Hormones and Body Composition in Males with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5600. [Google Scholar] [CrossRef]
- Dorling, J.L.; van Vliet, S.; Huffman, K.M.; Kraus, W.E.; Bhapkar, M.; Pieper, C.F.; Stewart, T.; Das, S.K.; Racette, S.B.; Roberts, S.B.; et al. Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: Highlights from CALERIE phase 2. Nutr. Rev. 2021, 79, 98–113. [Google Scholar] [CrossRef]
- Rochon, J.; Bales, C.W.; Ravussin, E.; Redman, L.M.; Holloszy, J.O.; Racette, S.B.; Roberts, S.B.; Das, S.K.; Romashkan, S.; Galan, K.M.; et al. Design and conduct of the CALERIE study: Comprehensive assessment of the long-term effects of reducing intake of energy. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 97–108. [Google Scholar] [CrossRef]
- Rhoads, T.W.; Anderson, R.M. Caloric restriction has a new player. Science 2022, 375, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Lemus, M.B.; Stark, R.; Santos, V.V.; Thompson, A.; Rees, D.J.; Galic, S.; Elsworth, J.D.; Kemp, B.E.; Davies, J.S.; et al. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. 2016, 36, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Rallis, C. The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation. Genes 2020, 11, 1043. [Google Scholar] [CrossRef]
- Hu, Y.; Mai, W.; Chen, L.; Cao, K.; Zhang, B.; Zhang, Z.; Liu, Y.; Lou, H.; Duan, S.; Gao, Z. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 2020, 68, 1031–1045. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Longo, V.D.; Cortellino, S. Fasting, dietary restriction, and immunosenescence. J. Allergy Clin. Immunol. 2020, 146, 1002–1004. [Google Scholar] [CrossRef]
- Goto, S. Health span extension by later-life caloric or dietary restriction: A view based on rodent studies. Biogerontology 2006, 7, 135–138. [Google Scholar] [CrossRef]
- Goodrick, C.L.; Ingram, D.K.; Reynolds, M.A.; Freeman, J.R.; Cider, N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: Interaction of genotype and age. Mech. Ageing Dev. 1990, 55, 69–87. [Google Scholar] [CrossRef]
- Ishaq, A.; Dufour, D.; Cameron, K.; von Zglinicki, T.; Saretzki, G. Metabolic memory of dietary restriction ameliorates DNA damage and adipocyte size in mouse visceral adipose tissue. Exp. Gerontol. 2018, 113, 228–236. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Madrigal-Matute, J.; Scheibye-Knudsen, M.; Fang, E.; Aon, M.; Gonzalez-Reyes, J.A.; Cortassa, S.; Kaushik, S.; Gonzalez-Freire, M.; Patel, B.; et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016, 23, 1093–1112. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A.; Mitchell, J.R.; Mitchell, S.J. Sex differences in the response to dietary restriction in rodents. Curr. Opin. Physiol. 2018, 6, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Honjoh, S.; Ihara, A.; Kajiwara, Y.; Yamamoto, T.; Nishida, E. The Sexual Dimorphism of Dietary Restriction Responsiveness in Caenorhabditis elegans. Cell Rep. 2017, 21, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.I.; Cassidy, E.J.; Fricke, C.; Bonduriansky, R. The lifespan-reproduction trade-off under dietary restriction is sex-specific and context-dependent. Exp. Gerontol. 2013, 48, 539–548. [Google Scholar] [CrossRef]
- Cameron, K.M.; Miwa, S.; Walker, C.; von Zglinicki, T. Male mice retain a metabolic memory of improved glucose tolerance induced during adult onset, short-term dietary restriction. Longev. Healthspan 2012, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; De Tata, V.; Fierabracci, V.; Barbera, M.; Rossetti, R.; Masiello, P. Comparative study on the preventing effects of oral vanadyl sulfate and dietary restriction on the age-related glucose intolerance in rats. Aging Clin. Exp. Res. 2005, 17, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Matyi, S.; Jackson, J.; Garrett, K.; Deepa, S.S.; Unnikrishnan, A. The effect of different levels of dietary restriction on glucose homeostasis and metabolic memory. Geroscience 2018, 40, 139–149. [Google Scholar] [CrossRef]
- Hahn, O.; Drews, L.F.; Nguyen, A.; Tatsuta, T.; Gkioni, L.; Hendrich, O.; Zhang, Q.; Langer, T.; Pletcher, S.; Wakelam, M.J.O.; et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. 2019, 1, 1059–1073. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Chen, W.; Liu, Z. The neuroprotective effects of intermittent fasting on brain aging and neurodegenerative diseases via regulating mitochondrial function. Free Radic. Biol. Med. 2022, 182, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Lane, M.A.; Ingram, D.K. Dietary restriction in aging nonhuman primates. Interdiscip. Top. Gerontol. 2007, 35, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Pizza, V.; Agresta, A.; D’Acunto, C.W.; Festa, M.; Capasso, A. Neuroinflamm-aging and neurodegenerative diseases: An overview. CNS Neurol. Disord. Drug Targets 2011, 10, 621–634. [Google Scholar] [CrossRef]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- Murphy, T.; Dias, G.P.; Thuret, S. Effects of diet on brain plasticity in animal and human studies: Mind the gap. Neural. Plast. 2014, 2014, 563160. [Google Scholar] [CrossRef]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 2012, 34, 917–933. [Google Scholar] [CrossRef]
- Laudisio, A.; Antonelli Incalzi, R.; Gemma, A.; Giovannini, S.; Lo Monaco, M.R.; Vetrano, D.L.; Padua, L.; Bernabei, R.; Zuccala, G. Use of proton-pump inhibitors is associated with depression: A population-based study. Int. Psychogeriatr. 2018, 30, 153–159. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188. [Google Scholar] [CrossRef]
- Morimoto, S.S.; Kanellopoulos, D.; Manning, K.J.; Alexopoulos, G.S. Diagnosis and treatment of depression and cognitive impairment in late life. Ann. N. Y. Acad. Sci. 2015, 1345, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef]
- Giovannini, S.; Macchi, C.; Liperoti, R.; Laudisio, A.; Coraci, D.; Loreti, C.; Vannetti, F.; Onder, G.; Padua, L.; Mugello Study Working, G. Association of Body Fat with Health-Related Quality of Life and Depression in Nonagenarians: The Mugello Study. J. Am. Med. Dir. Assoc. 2019, 20, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Zhao, Y.; Zhang, X.; Li, B.; Cui, R. The Effects of Calorie Restriction in Depression and Potential Mechanisms. Curr. Neuropharmacol. 2015, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Buckley, J.D.; Lim, S.S.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 1812–1816. [Google Scholar] [CrossRef]
- Lutter, M.; Krishnan, V.; Russo, S.J.; Jung, S.; McClung, C.A.; Nestler, E.J. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 2008, 28, 3071–3075. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Leeuwenburgh, C.; Carter, C.; Marzetti, E.; Russo, A.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F. Myeloperoxidase levels and mortality in frail community-living elderly individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 369–376. [Google Scholar] [CrossRef]
- Son, T.G.; Zou, Y.; Yu, B.P.; Lee, J.; Chung, H.Y. Aging effect on myeloperoxidase in rat kidney and its modulation by calorie restriction. Free Radic. Res. 2005, 39, 283–289. [Google Scholar] [CrossRef]
- Arum, O.; Saleh, J.K.; Boparai, R.K.; Kopchick, J.J.; Khardori, R.K.; Bartke, A. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age 2014, 36, 9651. [Google Scholar] [CrossRef]
- Hadem, I.K.H.; Majaw, T.; Kharbuli, B.; Sharma, R. Beneficial effects of dietary restriction in aging brain. J. Chem. Neuroanat. 2019, 95, 123–133. [Google Scholar] [CrossRef]
- Sombric, C.J.; Torres-Oviedo, G. Cognitive and Motor Perseveration Are Associated in Older Adults. Front. Aging Neurosci. 2021, 13, 610359. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Frndak, S.; Drake, A.S.; Irwin, L.; Zivadinov, R.; Weinstock-Guttman, B.; Benedict, R.H. Differential effects of aging on motor and cognitive functioning in multiple sclerosis. Mult. Scler. 2017, 23, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Gudden, J.; Arias Vasquez, A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.Q.; Guan, J.T.; Xu, M.Y.; Xu, X.H.; Fu, Y.C. Behavioral study of calorie-restricted rats from early old age. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 2393–2395. [Google Scholar] [CrossRef]
- Maswood, N.; Young, J.; Tilmont, E.; Zhang, Z.; Gash, D.M.; Gerhardt, G.A.; Grondin, R.; Roth, G.S.; Mattison, J.; Lane, M.A.; et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 18171–18176. [Google Scholar] [CrossRef]
- Kim, C.; Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L.; Thuret, S. Energy Restriction Enhances Adult Hippocampal Neurogenesis-Associated Memory after Four Weeks in an Adult Human Population with Central Obesity; a Randomized Controlled Trial. Nutrients 2020, 12, 638. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.; Li, L.; Wang, Y.; Kong, X.; Wang, J. Food restriction affects Y-maze spatial recognition memory in developing mice. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2017, 60, 8–15. [Google Scholar] [CrossRef]
- Martin, C.K.; Anton, S.D.; Han, H.; York-Crowe, E.; Redman, L.M.; Ravussin, E.; Williamson, D.A. Examination of cognitive function during six months of calorie restriction: Results of a randomized controlled trial. Rejuvenation Res. 2007, 10, 179–190. [Google Scholar] [CrossRef]
- Xiao, N.; Le, Q.T. Neurotrophic Factors and Their Potential Applications in Tissue Regeneration. Arch. Immunol. Ther. Exp. 2016, 64, 89–99. [Google Scholar] [CrossRef]
- Gillespie, L.N. Regulation of axonal growth and guidance by the neurotrophin family of neurotrophic factors. Clin. Exp. Pharmacol. Physiol. 2003, 30, 724–733. [Google Scholar] [CrossRef]
- Di Benedetto, S.; Muller, L.; Wenger, E.; Duzel, S.; Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017, 75, 114–128. [Google Scholar] [CrossRef]
- Duan, W.; Guo, Z.; Mattson, M.P. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J. Neurochem. 2001, 76, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, T.S.; Sanchez-Mendoza, E.H.; Schultz Moreira, A.R.; Nascentes Melo, L.M.; Wang, C.; Sardari, M.; Hagemann, N.; Doeppner, T.R.; Kleinschnitz, C.; Hermann, D.M. Hypocaloric Diet Initiated Post-Ischemia Provides Long-Term Neuroprotection and Promotes Peri-Infarct Brain Remodeling by Regulating Metabolic and Survival-Promoting Proteins. Mol. Neurobiol. 2021, 58, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.; Caroni, P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat. Neurosci. 2016, 19, 1553–1562. [Google Scholar] [CrossRef]
- Katz, L.C.; Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 1996, 274, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zuo, Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS ONE 2013, 8, e66069. [Google Scholar] [CrossRef]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef]
- Castello, L.; Froio, T.; Maina, M.; Cavallini, G.; Biasi, F.; Leonarduzzi, G.; Donati, A.; Bergamini, E.; Poli, G.; Chiarpotto, E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic. Biol. Med. 2010, 48, 47–54. [Google Scholar] [CrossRef]
- Honjoh, S.; Yamamoto, T.; Uno, M.; Nishida, E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 2009, 457, 726–730. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Mindikoglu, A.L.; Abdulsada, M.M.; Jain, A.; Jalal, P.K.; Devaraj, S.; Wilhelm, Z.R.; Opekun, A.R.; Jung, S.Y. Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome. Sci. Rep. 2020, 10, 18341. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin. Nutr. 2018, 37, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Sundfor, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- Bruss, M.D.; Khambatta, C.F.; Ruby, M.A.; Aggarwal, I.; Hellerstein, M.K. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E108–E116. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Liu, H.; Javaheri, A.; Godar, R.J.; Murphy, J.; Ma, X.; Rohatgi, N.; Mahadevan, J.; Hyrc, K.; Saftig, P.; Marshall, C.; et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 2017, 13, 1952–1968. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Association between Time Restricted Feeding and Cognitive Status in Older Italian Adults. Nutrients 2021, 13, 191. [Google Scholar] [CrossRef]
- Baik, S.H.; Rajeev, V.; Fann, D.Y.; Jo, D.G.; Arumugam, T.V. Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav. 2020, 10, e01444. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Jia, X.B.; Sun, M.F.; Zhu, Y.L.; Qiao, C.M.; Zhang, B.P.; Zhao, L.P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Manzanero, S.; Erion, J.R.; Santro, T.; Steyn, F.J.; Chen, C.; Arumugam, T.V.; Stranahan, A.M. Intermittent fasting attenuates increases in neurogenesis after ischemia and reperfusion and improves recovery. J. Cereb. Blood Flow Metab. 2014, 34, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Coupe, B.; Leloup, C.; Asiedu, K.; Maillard, J.; Penicaud, L.; Horvath, T.L.; Bouret, S.G. Defective autophagy in Sf1 neurons perturbs the metabolic response to fasting and causes mitochondrial dysfunction. Mol. Metab. 2021, 47, 101186. [Google Scholar] [CrossRef] [PubMed]
- Mladenovic, A.; Perovic, M.; Tanic, N.; Petanceska, S.; Ruzdijic, S.; Kanazir, S. Dietary restriction modulates alpha-synuclein expression in the aging rat cortex and hippocampus. Synapse 2007, 61, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Herman, J.P.; Mattson, M.P. Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp. Neurol. 2000, 166, 435–441. [Google Scholar] [CrossRef]
- Rajabi, A.; Parinejad, N.; Ahmadi, K.; Khorramizadeh, M.R.; Raza, M. Anti-inflammatory effects of serum isolated from animals on intermittent feeding in C6 glioma cell line. Neurosci. Lett. 2011, 487, 32–35. [Google Scholar] [CrossRef]
- Rubovitch, V.; Pharayra, A.; Har-Even, M.; Dvir, O.; Mattson, M.P.; Pick, C.G. Dietary Energy Restriction Ameliorates Cognitive Impairment in a Mouse Model of Traumatic Brain Injury. J. Mol. Neurosci. MN 2019, 67, 613–621. [Google Scholar] [CrossRef]
- Du, X.Y.; Xie, X.X.; Liu, R.T. The Role of alpha-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, J.; Wang, P.; Liu, J.; Zhao, Y.; Jiang, F.; Lou, H. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain. Behav. Immun. 2021, 91, 324–338. [Google Scholar] [CrossRef]
- Tu, H.Y.; Yuan, B.S.; Hou, X.O.; Zhang, X.J.; Pei, C.S.; Ma, Y.T.; Yang, Y.P.; Fan, Y.; Qin, Z.H.; Liu, C.F.; et al. α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson’s disease. Aging Cell 2021, 20, e13522. [Google Scholar] [CrossRef]
- Tang, M.; Liu, T.; Jiang, P.; Dang, R. The interaction between autophagy and neuroinflammation in major depressive disorder: From pathophysiology to therapeutic implications. Pharm. Res. 2021, 168, 105586. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Lubomski, M.; Tan, A.H.; Lim, S.Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2020, 267, 2507–2523. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharm. Sin 2017, 38, 1317–1328. [Google Scholar] [CrossRef]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.T.; Martignoni, E. The 6-hydroxydopamine model: News from the past. Parkinsonism Relat. Disord. 2008, 14 (Suppl. 2), S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wood, H. New models show gut-brain transmission of Parkinson disease pathology. Nat. Rev. Neurol. 2019, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Yagi, H.; Uemura, M.T.; Hatanaka, Y.; Yamakado, H.; Takahashi, R. Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 2018, 13, 21. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Taguchi, T.; Ikuno, M.; Hondo, M.; Parajuli, L.K.; Taguchi, K.; Ueda, J.; Sawamura, M.; Okuda, S.; Nakanishi, E.; Hara, J.; et al. α-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: A prodromal Parkinson’s disease model. Brain 2020, 143, 249–265. [Google Scholar] [CrossRef]
- Duan, W.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999, 57, 195–206. [Google Scholar] [CrossRef]
- Guedes, A.; Ludovico, P.; Sampaio-Marques, B. Caloric restriction alleviates alpha-synuclein toxicity in aged yeast cells by controlling the opposite roles of Tor1 and Sir2 on autophagy. Mech. Ageing Dev. 2017, 161, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, K.J.; Rothman, S.M.; Ladenheim, B.; Wan, R.; Vranis, N.; Hutchison, E.; Okun, E.; Cadet, J.L.; Mattson, M.P. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant alpha-synuclein. Neurobiol. Aging 2013, 34, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.J.; McLean, J.R.; Kartunen, A.; Langston, J.W.; Isacson, O. alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol. Dis. 2012, 47, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Hsieh, T.F.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Chiang, J.H.; Li, T.C.; Lin, C.C. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine 2017, 96, e5921. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Han, K.; Kim, D.; Rhee, S.Y.; Jang, W.; Shin, D.W. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 2020, 35, 236–244. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; de Pablo-Fernandez, E.; Foltynie, T.; Noyce, A.J. The Association Between Type 2 Diabetes Mellitus and Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 775–789. [Google Scholar] [CrossRef]
- Wu, H.; Xie, B.; Ke, M.; Deng, Y. High-fat diet causes increased endogenous neurotoxins and phenotype of Parkinson’s disease in mice. Acta Biochim. Biophys. Sin. 2019, 51, 969–971. [Google Scholar] [CrossRef]
- Lee, E.B.; Mattson, M.P. The neuropathology of obesity: Insights from human disease. Acta Neuropathol. 2014, 127, 3–28. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Borgundvaag, E.; Mak, J.; Kramer, C.K. Metabolic Impact of Intermittent Fasting in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. J. Clin. Endocrinol. Metab. 2021, 106, 902–911. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Zhao, Z.; Dong, W.; Zhang, X.; Chen, X.; Ma, L. Short-term caloric restriction exerts neuroprotective effects following mild traumatic brain injury by promoting autophagy and inhibiting astrocyte activation. Behav. Brain Res. 2017, 331, 135–142. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Xu, S.; Li, C.; Li, M.; Cao, S.; Sun, Y.; Dai, H.; Guo, Y.; Chen, X.; et al. Scientific Evidences of Calorie Restriction and Intermittent Fasting for Neuroprotection in Traumatic Brain Injury Animal Models: A Review of the Literature. Nutrients 2022, 14, 1431. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Nuber, S.; Selkoe, D.J. Caspase-1 clipping causes complications for alpha-synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 9958–9960. [Google Scholar] [CrossRef]
- Pott Godoy, M.C.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef]
- Traba, J.; Geiger, S.S.; Kwarteng-Siaw, M.; Han, K.; Ra, O.H.; Siegel, R.M.; Gius, D.; Sack, M.N. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3-mediated activation of superoxide dismutase 2. J. Biol. Chem. 2017, 292, 12153–12164. [Google Scholar] [CrossRef]
- Traba, J.; Kwarteng-Siaw, M.; Okoli, T.C.; Li, J.; Huffstutler, R.D.; Bray, A.; Waclawiw, M.A.; Han, K.; Pelletier, M.; Sauve, A.A.; et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J. Clin. Investig. 2015, 125, 4592–4600. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Role of oxidant species in aging. Curr. Med. Chem. 2004, 11, 1105–1112. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Mladenovic Djordjevic, A.; Loncarevic-Vasiljkovic, N.; Gonos, E.S. Dietary Restriction and Oxidative Stress: Friends or Enemies? Antioxid. Redox Signal. 2021, 34, 421–438. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, C.M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Duarte-Jurado, A.P.; Gopar-Cuevas, Y.; Saucedo-Cardenas, O.; Loera-Arias, M.J.; Montes-de-Oca-Luna, R.; Garcia-Garcia, A.; Rodriguez-Rocha, H. Antioxidant Therapeutics in Parkinson’s Disease: Current Challenges and Opportunities. Antioxidants 2021, 10, 453. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Srivastava, S.; Haigis, M.C. Role of sirtuins and calorie restriction in neuroprotection: Implications in Alzheimer’s and Parkinson’s diseases. Curr. Pharm. Des. 2011, 17, 3418–3433. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H. Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J. Clin. Neurol. 2009, 5, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.P.; Chen, J.; Zhao, Y.; Chai, Z.; Hu, Y. mTOR Signaling in Parkinson’s Disease. Neuromolecular. Med. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Celebi-Birand, D.; Ardic, N.I.; Karoglu-Eravsar, E.T.; Sengul, G.F.; Kafaligonul, H.; Adams, M.M. Dietary and Pharmacological Interventions That Inhibit Mammalian Target of Rapamycin Activity Alter the Brain Expression Levels of Neurogenic and Glial Markers in an Age-and Treatment-Dependent Manner. Rejuvenation Res. 2020, 23, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e12. [Google Scholar] [CrossRef]
- Li, N.; Guenancia, C.; Rigal, E.; Hachet, O.; Chollet, P.; Desmoulins, L.; Leloup, C.; Rochette, L.; Vergely, C. Short-term moderate diet restriction in adulthood can reverse oxidative, cardiovascular and metabolic alterations induced by postnatal overfeeding in mice. Sci. Rep. 2016, 6, 30817. [Google Scholar] [CrossRef]
- Daneshrad, Z.; Novel-Chaté, V.; Birot, O.; Serrurier, B.; Sanchez, H.; Bigard, A.X.; Rossi, A. Diet restriction plays an important role in the alterations of heart mitochondrial function following exposure of young rats to chronic hypoxia. Pflügers Arch. 2001, 442, 12–18. [Google Scholar] [CrossRef]
- Djajadikerta, A.; Keshri, S.; Pavel, M.; Prestil, R.; Ryan, L.; Rubinsztein, D.C. Autophagy Induction as a Therapeutic Strategy for Neurodegenerative Diseases. J. Mol. Biol. 2020, 432, 2799–2821. [Google Scholar] [CrossRef]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef]
- Tenreiro, S.; Reimão-Pinto, M.M.; Antas, P.; Rino, J.; Wawrzycka, D.; Macedo, D.; Rosado-Ramos, R.; Amen, T.; Waiss, M.; Magalhães, F.; et al. Phosphorylation modulates clearance of alpha-synuclein inclusions in a yeast model of Parkinson’s disease. PLoS Genet. 2014, 10, e1004302. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Marques, B.; Pereira, H.; Santos, A.R.; Teixeira, A.; Ludovico, P. Caloric restriction rescues yeast cells from alpha-synuclein toxicity through autophagic control of proteostasis. Aging 2018, 10, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kondo, K.; Motoki, K.; Homma, H.; Okazawa, H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Sci. Rep. 2015, 5, 12115. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Midoun, A.M.; Park, S.J.; Gatto, J.A.; Tener, S.J.; Siewert, J.; Klickstein, N.; Canman, J.C.; Ja, W.W.; Shirasu-Hiza, M. Circadian autophagy drives iTRF-mediated longevity. Nature 2021, 598, 353–358. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029017. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A.; Schafer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Lai, F.; Jiang, R.; Xie, W.; Liu, X.; Tang, Y.; Xiao, H.; Gao, J.; Jia, Y.; Bai, Q. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1986–1999. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Nam, H.S. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Wang, J.H.; Lei, W.Y.; Chen, C.L.; Chang, C.Y.; Liou, L.S. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: A population-based retrospective cohort study. Parkinsonism Relat. Disord. 2018, 47, 26–31. [Google Scholar] [CrossRef]
- Lee, H.S.; Lobbestael, E.; Vermeire, S.; Sabino, J.; Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut 2021, 70, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Tran, S.M.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Hegelmaier, T.; Lebbing, M.; Duscha, A.; Tomaske, L.; Tönges, L.; Holm, J.B.; Bjørn Nielsen, H.; Gatermann, S.G.; Przuntek, H.; Haghikia, A. Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease. Cells 2020, 9, 376. [Google Scholar] [CrossRef]
- Morris, J.K.; Bomhoff, G.L.; Stanford, J.A.; Geiger, P.C. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1082–R1090. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef] [PubMed]
- Mendes, N.F.; Jara, C.P.; Zanesco, A.M.; de Araújo, E.P. Hypothalamic Microglial Heterogeneity and Signature under High Fat Diet-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 2256. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Raj, D.D.; Schaafsma, W.; van der Heijden, R.A.; Kooistra, S.M.; Reijne, A.C.; Zhang, X.; Moser, J.; Brouwer, N.; Heeringa, P.; et al. Low-Fat Diet with Caloric Restriction Reduces White Matter Microglia Activation During Aging. Front. Mol. Neurosci. 2018, 11, 65. [Google Scholar] [CrossRef]
- Ma, D.; Shuler, J.M.; Raider, K.D.; Rogers, R.S.; Wheatley, J.L.; Geiger, P.C.; Stanford, J.A. Effects of discontinuing a high-fat diet on mitochondrial proteins and 6-hydroxydopamine-induced dopamine depletion in rats. Brain Res. 2015, 1613, 49–58. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Pincus, J.H.; Karstaedt, P. An international comparison of dietary protein consumption and mortality from Parkinson’s disease. J. Neurol. 1992, 239, 236–237. [Google Scholar] [CrossRef]
- Pincus, J.H.; Barry, K.M. Dietary method for reducing fluctuations in Parkinson’s disease. Yale J. Biol. Med. 1987, 60, 133–137. [Google Scholar]
- Cereda, E.; Barichella, M.; Pedrolli, C.; Pezzoli, G. Low-protein and protein-redistribution diets for Parkinson’s disease patients with motor fluctuations: A systematic review. Mov. Disord. 2010, 25, 2021–2034. [Google Scholar] [CrossRef]
- Hirata, H.; Asanuma, M.; Kondo, Y.; Ogawa, N. Influence of protein-restricted diet on motor response fluctuations in Parkinson’s disease. Rinsho Shinkeigaku = Clin. Neurol. 1992, 32, 973–978. [Google Scholar]
- Pincus, J.H.; Barry, K.M. Plasma levels of amino acids correlate with motor fluctuations in parkinsonism. Arch. Neurol. 1987, 44, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.A.; Katzman, R. Synthetic amino acids and the nature of L-DOPA transport at the blood-brain barrier. J. Neurochem. 1975, 25, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Pincus, J.H.; Barry, K. Influence of dietary protein on motor fluctuations in Parkinson’s disease. Arch. Neurol. 1987, 44, 270–272. [Google Scholar] [CrossRef]
- Virmani, T.; Tazan, S.; Mazzoni, P.; Ford, B.; Greene, P.E. Motor fluctuations due to interaction between dietary protein and levodopa in Parkinson’s disease. J. Clin. Mov. Disord. 2016, 3, 8. [Google Scholar] [CrossRef]
- Anderson, J.G.; Hintze, K.; Marchant, E.D. Restricting branched-chain amino acids: An approach to improve metabolic health. J. Physiol. 2018, 596, 2469–2470. [Google Scholar] [CrossRef]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef]
- Hill, C.M.; Morrison, C.D. Dietary branched chain amino acids and metabolic health: When less is more. J. Physiol. 2018, 596, 555–556. [Google Scholar] [CrossRef]
- Asoudeh, F.; Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Dietary intake of branched-chain amino acids in relation to general and abdominal obesity. Eat Weight Disord. 2022, 20, 1303–1311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).