Suboptimal Plasma Vitamin C Is Associated with Lower Bone Mineral Density in Young and Early Middle-Aged Men: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Parameters, Definitions, and Cut-Offs
2.3. Assessment of Bone Mineral Density and Plasma/Serum Nutrient Status
2.3.1. Determination of Bone Mineral Density and the Rate of Osteoporotic Fracture
2.3.2. Blood Collection and Determination of Plasma Vitamin C Concentrations
2.3.3. Blood Collection and Determination of Serum 25(OH)D Concentrations
2.3.4. Blood Collection and Determination of Serum Vitamin B12 and Folic Acid Concentrations
2.4. Statistical Analysis
3. Results
3.1. Demographic/Anthropometric Characteristics and Laboratory and Radiology Results of the Study Population
3.2. Distribution of Suboptimal Circulating Nutrients in the Four Selected Nutrients
3.3. Primary Outcomes
3.3.1. Comparison of Age, BMI, and Nutrients’ Levels with Tertiles of Lumbar Spine BMD in Men and Women
3.3.2. The Correlations of the Lumbar Spine BMD Status with Age, BMI, and Nutrient Levels by Analysis of Logistic Regressions
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef]
- Teng, G.G.; Curtis, J.R.; Saag, K.G. Mortality and osteoporotic fractures: Is the link causal, and is it modifiable? Clin. Exp. Rheumatol. 2008, 26, S125–S137. [Google Scholar] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Noel, S.E.; Dawson-Hughes, B.; Tucker, K.L. Sufficient Plasma Vitamin C Is Related to Greater Bone Mineral Density among Postmenopausal Women from the Boston Puerto Rican Health Study. J. Nutr. 2021, 151, 3764–3772. [Google Scholar] [CrossRef]
- Krall, E.A.; Dawson-Hughes, B. Heritable and life-style determinants of bone mineral density. J. Bone Miner. Res. 1993, 8, 1–9. [Google Scholar] [CrossRef]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [CrossRef]
- Mohan, S.; Kapoor, A.; Singgih, A.; Zhang, Z.; Taylor, T.; Yu, H.; Chadwick, R.B.; Chung, Y.S.; Donahue, L.R.; Rosen, C.; et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J. Bone Miner. Res. 2005, 20, 1597–1610. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.J.; Yoo, H.S.; Song, D.H.; Chung, K.H.; Lee, K.J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/beta-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Beezhold, B.L.; Mostow, B.; Swan, P.D. Plasma vitamin C is inversely related to body mass index and waist circumference but not to plasma adiponectin in nonsmoking adults. J. Nutr. 2007, 137, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Schectman, G.; Byrd, J.C.; Gruchow, H.W. The influence of smoking on vitamin C status in adults. Am. J. Public Health 1989, 79, 158–162. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef]
- Dehghan, M.; Akhtar-Danesh, N.; McMillan, C.R.; Thabane, L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J. 2007, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef]
- Simon, J.A.; Hudes, E.S. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am. J. Epidemiol. 2001, 154, 427–433. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Associations of serum vitamins levels with bone mineral density in the different race-ethnicities US adults. BMC Musculoskelet. Disord. 2021, 22, 137. [Google Scholar] [CrossRef]
- Vaes, B.L.; Lute, C.; Blom, H.J.; Bravenboer, N.; de Vries, T.J.; Everts, V.; Dhonukshe-Rutten, R.A.; Muller, M.; de Groot, L.C.; Steegenga, W.T. Vitamin B(12) deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif. Tissue Int. 2009, 84, 413–422. [Google Scholar] [CrossRef]
- Herrmann, M.; Schmidt, J.; Umanskaya, N.; Colaianni, G.; Al Marrawi, F.; Widmann, T.; Zallone, A.; Wildemann, B.; Herrmann, W. Stimulation of osteoclast activity by low B-vitamin concentrations. Bone 2007, 41, 584–591. [Google Scholar] [CrossRef]
- Hsieh, R.L.; Huang, Y.L.; Chen, W.J.; Chen, H.H.; Shiue, H.S.; Lin, Y.C.; Hsueh, Y.M. Associations between Plasma Folate and Vitamin B12, Blood Lead, and Bone Mineral Density among Adults and Elderly Who Received a Health Examination. Nutrients 2022, 14, 911. [Google Scholar] [CrossRef]
- Kopiczko, A.; Lopuszanska-Dawid, M.; Gryko, K. Bone mineral density in young adults: The influence of vitamin D status, biochemical indicators, physical activity and body composition. Arch. Osteoporos. 2020, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Boot, A.M.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. The relation between 25-hydroxyvitamin D with peak bone mineral density and body composition in healthy young adults. J. Pediatr. Endocrinol. Metab. 2011, 24, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Uenishi, K.; Ohta, H.; Shiraki, M. Multiple vitamin deficiencies additively increase the risk of incident fractures in Japanese postmenopausal women. Osteoporos. Int. 2019, 30, 593–599. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grubler, M.R.; Verheyen, N. The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int. J. Endocrinol. 2017, 2017, 7454376. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2016. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef]
- Haseltine, K.N.; Chukir, T.; Smith, P.J.; Jacob, J.T.; Bilezikian, J.P.; Farooki, A. Bone Mineral Density: Clinical Relevance and Quantitative Assessment. J. Nucl. Med. 2021, 62, 446–454. [Google Scholar] [CrossRef]
- Shin, Y.W.; Park, K.I.; Moon, J.; Lee, S.T.; Chu, K.; Lee, S.K.; Roh, J.K.; Jung, K.H. Association of Bone Mineral Density With the Risk of Intracranial Aneurysm. JAMA Neurol. 2018, 75, 179–186. [Google Scholar] [CrossRef]
- Lloyd, J.T.; Alley, D.E.; Hawkes, W.G.; Hochberg, M.C.; Waldstein, S.R.; Orwig, D.L. Body mass index is positively associated with bone mineral density in US older adults. Arch. Osteoporos. 2014, 9, 175. [Google Scholar] [CrossRef]
- Salamat, M.R.; Salamat, A.H.; Abedi, I.; Janghorbani, M. Relationship between Weight, Body Mass Index, and Bone Mineral Density in Men Referred for Dual-Energy X-Ray Absorptiometry Scan in Isfahan, Iran. J. Osteoporos. 2013, 2013, 205963. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, C.Y.; Feng, P.H.; Chu, C.C.; So, E.C.; Hu, M.L. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin. J. Pain 2009, 25, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lin, Y.T.; Wang, L.K.; Hung, K.C.; Lan, K.M.; Ho, C.H.; Chang, C.Y. Hypovitaminosis Din Postherpetic Neuralgia-High Prevalence and Inverse Association with Pain: A Retrospective Study. Nutrients 2019, 11, 2787. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Lin, Y.T.; Chen, K.H.; Wang, L.K.; Chen, J.Y.; Chang, Y.J.; Wu, S.C.; Chiang, M.H.; Sun, C.K. The Effect of Perioperative Vitamin C on Postoperative Analgesic Consumption: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3109. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, K.C.; Lin, Y.T.; Chang, Y.J.; Wu, Z.F.; Ho, C.H.; Chen, J.Y. Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients 2020, 12, 2719. [Google Scholar] [CrossRef]
- Chen, J.Y.; Cheng, T.J.; Chang, C.Y.; Lan, K.M.; Weng, S.F.; Sheu, M.J.; Tseng, S.F.; Hu, M.L. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: A population-based cohort study. Int. J. Epidemiol. 2013, 42, 1873–1881. [Google Scholar] [CrossRef]
- Wang, L.K.; Lin, Y.T.; Hung, K.C.; Chang, C.Y.; Wu, Z.F.; Hu, M.L.; Chen, J.Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients 2020, 12, 2384. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, L.K.; Hung, K.C.; Chang, C.Y.; Wu, L.C.; Ho, C.H.; Chen, J.Y. Prevalence and Predictors of Insufficient Plasma Vitamin C in a Subtropical Region and Its Associations with Risk Factors of Cardiovascular Diseases: A Retrospective Cross-Sectional Study. Nutrients 2022, 14, 1108. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Bloomgarden, Z.T.; Zimmet, P.Z. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: Response to the International Expert Committee. Diabetes Care 2009, 32, e159. [Google Scholar] [CrossRef]
- Lorenzo, C.; Wagenknecht, L.E.; Hanley, A.J.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2104–2109. [Google Scholar] [CrossRef]
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421–445. [Google Scholar] [CrossRef]
- Sestak, I.; Singh, S.; Cuzick, J.; Blake, G.M.; Patel, R.; Gossiel, F.; Coleman, R.; Dowsett, M.; Forbes, J.F.; Howell, A.; et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: An international, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2014, 15, 1460–1468. [Google Scholar] [CrossRef]
- Goh, S.S.L.; Lai, P.S.M.; Tan, A.T.B.; Ponnampalavanar, S. Reduced bone mineral density in human immunodeficiency virus-infected individuals: A meta-analysis of its prevalence and risk factors: Supplementary presentation. Osteoporos. Int. 2018, 29, 1683. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.B.; Xie, X.B.; Peng, L.K.; Liu, L.; Song, L.; Dai, H.L. Current Status of Research on Osteoporosis after Solid Organ Transplantation: Pathogenesis and Management. Biomed. Res. Int. 2015, 2015, 413169. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Kim, D.J. Bone Diseases in Patients with Chronic Liver Disease. Int. J. Mol. Sci. 2019, 20, 4270. [Google Scholar] [CrossRef]
- Bover, J.; Bailone, L.; Lopez-Baez, V.; Benito, S.; Ciceri, P.; Galassi, A.; Cozzolino, M. Osteoporosis, bone mineral density and CKD-MBD: Treatment considerations. J. Nephrol. 2017, 30, 677–687. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Doria, C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020, 9, 1034. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Miller, P.D. Skeletal effects of primary hyperparathyroidism: Bone mineral density and fracture risk. J. Clin. Densitom. 2013, 16, 28–32. [Google Scholar] [CrossRef]
- Mazziotti, G.; Frara, S.; Giustina, A. Pituitary Diseases and Bone. Endocr. Rev. 2018, 39, 440–488. [Google Scholar] [CrossRef]
- Rochira, V. Late-onset Hypogonadism: Bone health. Andrology 2020, 8, 1539–1550. [Google Scholar] [CrossRef]
- Pereira, R.M.; Carvalho, J.F.; Canalis, E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics 2010, 65, 1197–1205. [Google Scholar] [CrossRef]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.A.; Viken, J.; Jensen, L.T.; Andersen, N.B. Bone mineral density in adult patients treated with various antiepileptic drugs. Seizure 2012, 21, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Crews, M.P.; Howes, O.D. Is antipsychotic treatment linked to low bone mineral density and osteoporosis? A review of the evidence and the clinical implications. Hum. Psychopharmacol. 2012, 27, 15–23. [Google Scholar] [CrossRef]
- Finck, H.; Hart, A.R.; Jennings, A.; Welch, A.A. Is there a role for vitamin C in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr. Res. Rev. 2014, 27, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Franssen, T.; Stijnen, M.; Hamers, F.; Schneider, F. Age differences in demographic, social and health-related factors associated with loneliness across the adult life span (19–65 years): A cross-sectional study in the Netherlands. BMC Public Health 2020, 20, 1118. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- German Nutrition, S. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J.O. Lifestyle Behaviours and Plasma Vitamin C and beta-Carotene Levels from the ELAN Population (Liege, Belgium). J. Nutr. Metab. 2011, 2011, 494370. [Google Scholar] [CrossRef]
- Allen, L.H. Folate and vitamin B12 status in the Americas. Nutr. Rev. 2004, 62, S29–S33. [Google Scholar] [CrossRef]
- Semeere, A.S.; Nakanjako, D.; Ddungu, H.; Kambugu, A.; Manabe, Y.C.; Colebunders, R. Sub-optimal vitamin B-12 levels among ART-naive HIV-positive individuals in an urban cohort in Uganda. PLoS ONE 2012, 7, e40072. [Google Scholar] [CrossRef]
- Oner, N.; Vatansever, U.; Karasalihoglu, S.; Ekuklu, G.; Celtik, C.; Biner, B. The prevalence of folic acid deficiency among adolescent girls living in Edirne, Turkey. J. Adolesc. Health 2006, 38, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Preveraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Sawicki, P.; Talalaj, M.; Zycinska, K.; Zgliczynski, W.S.; Wierzba, W. Comparison of the Characteristics of Back Pain in Women with Postmenopausal Osteoporosis with and without Vertebral Compression Fracture: A Retrospective Study at a Single Osteoporosis Center in Poland. Med. Sci. Monit. 2021, 27, e929853. [Google Scholar] [CrossRef]
- Robitaille, L.; Hoffer, L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2016, 15, 40. [Google Scholar] [CrossRef]
- Koivula, M.K.; Matinlassi, N.; Laitinen, P.; Risteli, J. Four automated 25-OH total vitamin D immunoassays and commercial liquid chromatography tandem-mass spectrometry in Finnish population. Clin. Lab. 2013, 59, 397–405. [Google Scholar] [CrossRef]
- Komaromy-Hiller, G.; Nuttall, K.L.; Ashwood, E.R. Effect of storage on serum vitamin B12 and folate stability. Ann. Clin. Lab. Sci. 1997, 27, 249–253. [Google Scholar]
- Berger, C.; Langsetmo, L.; Joseph, L.; Hanley, D.A.; Davison, K.S.; Josse, R.; Kreiger, N.; Tenenhouse, A.; Goltzman, D.; Canadian Multicentre Osteoporosis Study Research Group. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ 2008, 178, 1660–1668. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Chen, J.H.; Chiou, J.M.; Tsai, K.S.; Lee, Y.Y.; Tsao, C.K.; Chen, Y.C. Association of Renal Function and Menopausal Status with Bone Mineral Density in Middle-aged Women. Sci. Rep. 2015, 5, 14956. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, A.; Polgreen, L.E.; Hurley, D.L.; Gross, M.D.; Sidney, S.; Jacobs, D.R., Jr. A cross-sectional association between bone mineral density and parathyroid hormone and other biomarkers in community-dwelling young adults: The CARDIA study. J. Clin. Endocrinol. Metab. 2013, 98, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Bala, Y.; Doublier, A.; Farlay, D.; Ste-Marie, L.G.; Meunier, P.J.; Delmas, P.D. The role of mineralization and organic matrix in the microhardness of bone tissue from controls and osteoporotic patients. Bone 2008, 43, 532–538. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Li, X.; Agrawal, C.M. Age-related changes in the collagen network and toughness of bone. Bone 2002, 31, 1–7. [Google Scholar] [CrossRef]
- Currey, J.D. The many adaptations of bone. J. Biomech. 2003, 36, 1487–1495. [Google Scholar] [CrossRef]
- Chan, D.; Lamande, S.R.; Cole, W.G.; Bateman, J.F. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem. J. 1990, 269, 175–181. [Google Scholar] [CrossRef]
- Ongphiphadhanakul, B.; Rajatanavin, R.; Chanprasertyothin, S.; Piaseu, N.; Chailurkit, L. Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. Clin. Endocrinol. 1998, 49, 803–809. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Boonen, S.; Ederveen, A.G.; de Coster, R.; Van Herck, E.; Moermans, K.; Vandenput, L.; Verstuyf, A.; Bouillon, R. Skeletal effects of estrogen deficiency as indduced by an aromatase inhibitor in an aged male rat model. Bone 2000, 27, 611–617. [Google Scholar] [CrossRef]
- Sahni, S.; Hannan, M.T.; Gagnon, D.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. High vitamin C intake is associated with lower 4-year bone loss in elderly men. J. Nutr. 2008, 138, 1931–1938. [Google Scholar] [CrossRef]
- Sahni, S.; Hannan, M.T.; Gagnon, D.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture--a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos. Int. 2009, 20, 1853–1861. [Google Scholar] [CrossRef]
- Morton, D.J.; Barrett-Connor, E.L.; Schneider, D.L. Vitamin C supplement use and bone mineral density in postmenopausal women. J. Bone Miner. Res. 2001, 16, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mattia, C.; Coluzzi, F.; Celidonio, L.; Vellucci, R. Bone pain mechanism in osteoporosis: A narrative review. Clin. Cases Miner. Bone Metab. 2016, 13, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Qiao, N.; Jacques, P.F.; Selhub, J.; Cupples, L.A.; Kiel, D.P. Low plasma vitamin B12 is associated with lower BMD: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2005, 20, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nashimoto, M.; Matsuyama, S.; Yamamoto, M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: A cross sectional study. Nutrition 2001, 17, 921–925. [Google Scholar] [CrossRef]
- Manickam, B.; Washington, T.; Villagrana, N.E.; Benjamin, A.; Kukreja, S.; Barengolts, E. Determinants of circulating 25-hydroxyvitamin D and bone mineral density in young physicians. Endocr. Pract. 2012, 18, 219–226. [Google Scholar] [CrossRef]
- Wei, Q.S.; Chen, Z.Q.; Tan, X.; Su, H.R.; Chen, X.X.; He, W.; Deng, W.M. Relation of Age, Sex and Bone Mineral Density to Serum 25-Hydroxyvitamin D Levels in Chinese Women and Men. Orthop. Surg. 2015, 7, 343–349. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, T.C.; Chen, P.Y.; Hsieh, L.Y.; Yeh, S.L. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: A case-control study. Asia Pac. J. Clin. Nutr. 2010, 19, 393–401. [Google Scholar]
- Han, X.; Ding, S.; Lu, J.; Li, Y. Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine 2022, 44, 101299. [Google Scholar] [CrossRef]
- Bierhals, I.O.; Dos Santos Vaz, J.; Bielemann, R.M.; de Mola, C.L.; Barros, F.C.; Goncalves, H.; Wehrmeister, F.C.; Assuncao, M.C.F. Associations between body mass index, body composition and bone density in young adults: Findings from a southern Brazilian cohort. BMC Musculoskelet. Disord. 2019, 20, 322. [Google Scholar] [CrossRef]
- Li, Y. Association between obesity and bone mineral density in middle-aged adults. J. Orthop. Surg. Res. 2022, 17, 268. [Google Scholar] [CrossRef]
- Cherukuri, L.; Kinninger, A.; Birudaraju, D.; Lakshmanan, S.; Li, D.; Flores, F.; Mao, S.S.; Budoff, M.J. Effect of body mass index on bone mineral density is age-specific. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.K.; Beck, B.R. The BPAQ: A bone-specific physical activity assessment instrument. Osteoporos. Int. 2008, 19, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
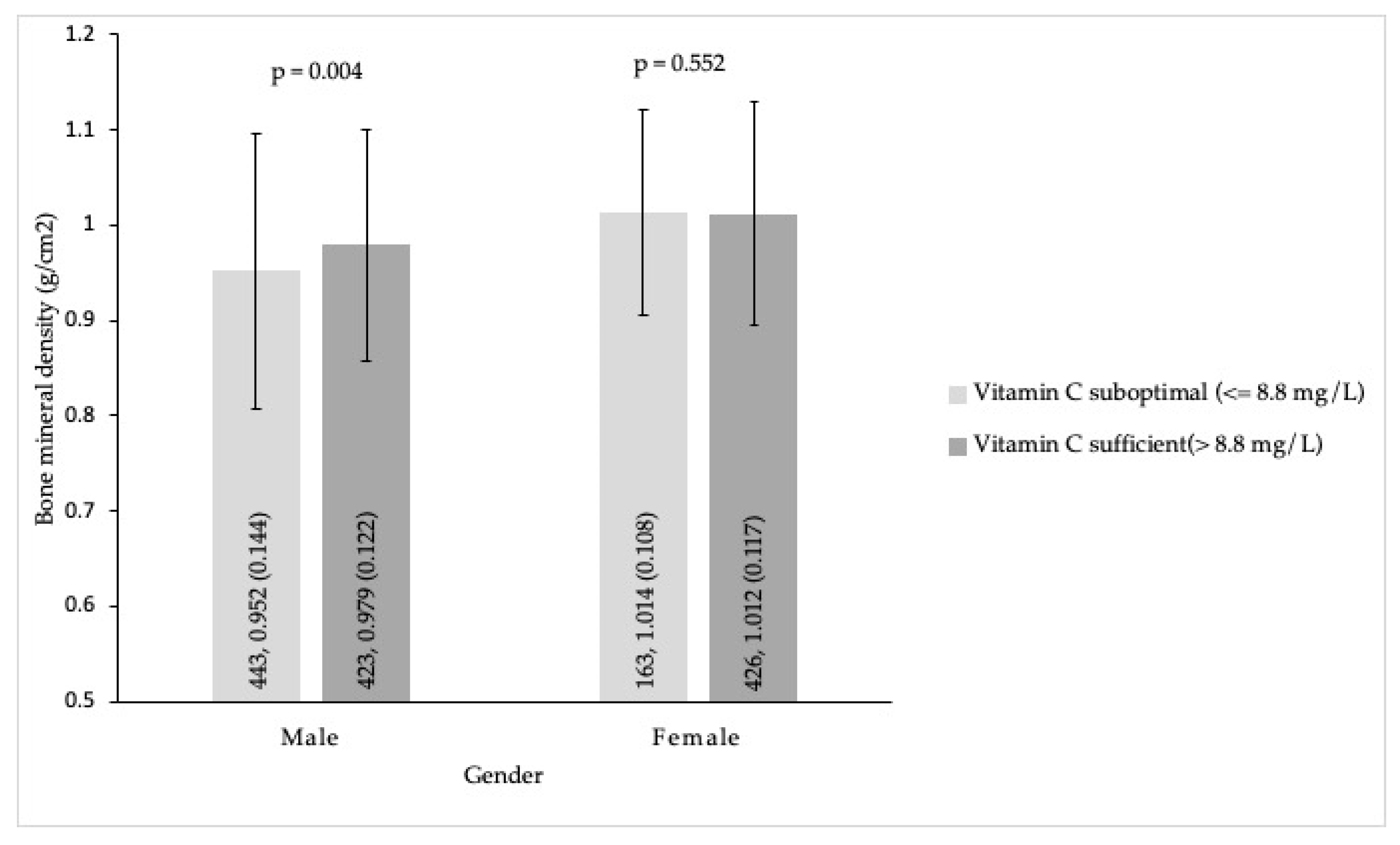
| Variables | Male (n = 866) | Female (n = 589) | p |
|---|---|---|---|
| Age groups (years), median (IQR) | 43 (6) | 43 (6) | 0.097 |
| 20–35, n (%) | 92 (10.6%) | 69 (11.7%) | 0.515 |
| 36–49, n (%) | 774 (89.4%) | 520 (88.3%) | |
| Body height (kg), median (IQR) | 172.1 (7.2) | 159.6 (7.6) | <0.001 |
| Body weight (cm), median (IQR) | 74.5 (14.5) | 55.1 (11.0) | <0.001 |
| Body mass index (kg/m2), median (IQR) | 25.1 (4.2) | 21.5 (3.9) | <0.001 |
| High BMI (≥23 kg/m2) (overweight/obesity), n (%) | 671 (77.5%) | 186 (31.6%) | <0.001 |
| Low BMI (<23 kg/m2) (underweight/normal weight), n (%) | 195 (22.5%) | 403 (68.4%) | |
| Seasons | |||
| Winter/Spring, n (%) | 385 (44.5%) | 301 (51.1%) | 0.013 |
| Summer/Autumn, n (%) | 481 (55.5%) | 288 (48.9%) | |
| Alcohol consumption | 4 (0.4%) | 2 (0.3%) | 0.983 |
| Osteoporotic fracture, n (%) | 0 | 0 | - |
| Bone mineral density at the lumbar spine (g/cm2), median (IQR) | 0.953 (0.173) | 1.011 (0.151) | <0.001 |
| Plasma vitamin C (mg/L), median (IQR) | 8.8 (4.2) | 10.4 (3.8) | <0.001 |
| Sufficient (>8.8 mg/L), n (%) | 423 (48.8%) | 426 (72.3%) | <0.001 |
| Suboptimal (≤8.8 mg/L), n (%) | 443 (51.2%) | 163 (27.7%) | |
| Insufficient (6.1–8.8 mg/L), n | 678 | 558 | |
| Deficient (≤6.0 mg/L), n | 188 | 31 | |
| Serum 25(OH)D (ng/mL), median (IQR) | 26.8 (10.6) | 23.2 (9.2) | <0.001 |
| Sufficient (≥30 ng/mL), n (%) | 290 (33.5%) | 108 (18.3%) | <0.001 |
| Suboptimal (<30 ng/mL), n (%) | 576 (66.5%) | 481 (81.7%) | |
| Insufficient (20–30 ng/mL), n | 710 | 405 | |
| Deficient (<20 ng/mL), n | 156 | 184 | |
| Serum vitamin B12 (pg/mL), median (IQR) | 509 (274) | 621 (303) | <0.001 |
| Sufficient (>300 pg/mL), n (%) | 803 (92.7%) | 569 (96.6%) | 0.002 |
| Suboptimal (≤300 pg/mL), n (%) | 63 (7.3%) | 20 (3.4%) | |
| Insufficient (200–300 pg/mL), n | 858 | 587 | |
| Deficient (<200 pg/mL), n | 8 | 2 | |
| Serum folic acid (ng/mL), median (IQR) | 7.7 (5.1) | 10.4 (5.1) | <0.001 |
| Sufficient (>6 ng/mL), n (%) | 619 (71.5%) | 528 (89.6%) | <0.001 |
| Suboptimal (≤6 ng/mL), n (%) | 247 (28.5%) | 61 (10.4%) | |
| Insufficient (3–6 ng/mL), n | 860 | 588 | |
| Deficient (<3 ng/mL), n | 6 | 1 |
| Subjects with Suboptimal Circulating Nutrients | None | 1 Nutrient | 2 Nutrients | 3–4 Nutrients | p | Mean (SD) | p |
|---|---|---|---|---|---|---|---|
| Total (n = 1455) | 192 (13.2%) | 663 (45.6%) | 427 (29.3%) | 173 (11.9%) | 1.4 (0.9) | ||
| Gender, n (%) | |||||||
| Men (n = 866), n (%) | 117 (13.5%) | 330 (38.1%) | 275 (31.8%) | 144 (16.6%) | <0.001 | 1.5 (1.0) | <0.001 |
| Women (n = 589), n (%) | 75 (12.7%) | 333 (56.5%) | 152 (25.8%) | 29 (4.9%) | 1.2 (0.7) | ||
| Age groups | |||||||
| 20–35 (n = 161), n (%) | 10 (6.2%) | 67 (41.6%) | 46 (28.6%) | 38 (23.6%) | <0.001 | 1.7 (0.9) | <0.001 |
| 36–49 (n = 1294), n (%) | 182 (14.1%) | 596 (46.1%) | 381 (29.4%) | 135 (10.4%) | 1.4 (0.9) | ||
| Lumbar spine BMD | |||||||
| The lowest BMD (n = 483), n (%) | 53 (11.0%) | 223 (46.2%) | 144 (29.8%) | 63 (13.0%) | 0.290 | 1.5 (0.9) | 0.093 |
| The middle and highest (n = 972), n (%) | 141 (14.5%) | 436 (44.9%) | 282 (29.0%) | 113 (11.6%) | 1.4 (0.9) |
| (a) | ||||
| Bone Mineral Density of Lumbar Spine in Men | ||||
| Variables (n = 866) | Lowest (n = 286) | Middle (n = 288) | Highest (n = 292) | p |
| Age groups (years) | 0.271 | |||
| 20–35, n (%) | 37 (12.9%) | 29 (10.1%) | 26 (8.9%) | |
| 36–49, n (%) | 249 (87.1%) | 259 (89.9%) | 266 (91.1%) | |
| Body mass index (kg/m2) | 0.009 | |||
| High BMI (≥23 kg/m2) (overweight/obesity), n (%) | 214 (74.8%) | 213 (74.0%) | 244 (83.6%) | |
| Low BMI (<23 kg/m2) (underweight/normal weight), n (%) | 72 (25.2%) | 75 (26.0%) | 48 (16.4%) | |
| Vitamin C (mg/L) | <0.001 | |||
| Sufficient (>8.8 mg/L), n (%) | 112 (39.2%) | 162 (56.3%) | 149 (51.0%) | |
| Suboptimal (≤8.8 mg/L), n (%) | 174 (60.8%) | 126 (43.8%) | 143 (49.0%) | |
| Serum 25(OH)D (ng/mL) | 0.806 | |||
| Sufficient (≥30 ng/mL), n (%) | 100 (35.0%) | 95 (33.0%) | 95 (32.5%) | |
| Suboptimal (<30 ng/mL), n (%) | 186 (65.0%) | 193 (67.0%) | 197 (67.5%) | |
| Vitamin B12 (pg/mL) | 0.114 | |||
| Sufficient (>300 pg/mL), n (%) | 261 (91.3%) | 263 (91.3%) | 278 (95.2%) | |
| Suboptimal (≤300 pg/dL), n (%) | 25 (8.7%) | 25 (8.7%) | 14 (4.8%) | |
| Folic acid (ng/mL) | 0.426 | |||
| Sufficient (>6 ng/mL), n (%) | 201 (70.3%) | 214 (74.3%) | 204 (69.9%) | |
| Suboptimal (≤6.0 ng/mL), n (%) | 85 (29.7%) | 74 (25.7%) | 88 (30.1%) | |
| Alcohol consumption | - | |||
| − | 284 (99.3%) | 288 (100.0%) | 290 (99.3%) | |
| + | 2 (0.7%) | 0 (0%) | 2 (0.7%) | |
| (b) | ||||
| Bone Mineral Density of Lumbar Spine in Women | ||||
| Variables (n = 589) | Lowest (n = 197) | Middle (n = 197) | Highest (n = 195) | p |
| Age groups (years) | 0.724 | |||
| 20–35, n (%) | 22 (11.2%) | 26 (13.2%) | 21 (10.8%) | |
| 36–49, n (%) | 175 (88.8%) | 171 (86.8%) | 174 (89.2%) | |
| Body mass index (kg/m2) | <0.001 | |||
| High BMI (≥23 kg/m2) (overweight/obesity), n (%) | 35 (17.8%) | 65 (33.0%) | 85 (43.6%) | |
| Low BMI (<23 kg/m2) (underweight/normal weight), n (%) | 161 (81.7%) | 132 (67.0%) | 110 (56.4%) | |
| Vitamin C (mg/L) | 0.520 | |||
| Sufficient (>8.8 mg/L), n (%) | 148 (75.1%) | 138 (70.1%) | 140 (71.8%) | |
| Suboptimal (≤8.8 mg/L), n (%) | 49 (24.9%) | 59 (29.9%) | 55 (28.2%) | |
| Serum 25(OH)D (ng/mL) | 0.763 | |||
| Sufficient (≥30 ng/mL), n (%) | 39 (19.8%) | 36 (18.3%) | 33 (16.9%) | |
| Suboptimal (<30 ng/mL), n (%) | 158 (80.2%) | 161(81.7%) | 162 (83.1%) | |
| Vitamin B12 (pg/mL) | 0.317 | |||
| Sufficient (>300 pg/mL), n (%) | 190 (96.4%) | 187 (94.9%) | 191 (97.9%) | |
| Suboptimal (≤300 pg/dL), n (%) | 7 (3.6%) | 10 (5.1%) | 4 (2.1%) | |
| Folic acid (ng/mL) | 0.271 | |||
| Sufficient (>6 ng/mL), n (%) | 175 (88.8%) | 182 (92.4%) | 171 (87.7%) | |
| Suboptimal (≤6.0 ng/mL), n (%) | 22 (11.2%) | 15 (7.6%) | 24 (12.3%) | |
| Alcohol consumption | - | |||
| − | 196 (99.5%) | 196 (99.5%) | 195 (100%) | |
| + | 1 (0.5%) | 1 (0.5%) | 0 (0%) | |
| (a) | ||||||
| Men | Lowest BMD (n = 286) | Highest BMD (n = 292) | Crude OR (95% CI) | p | Adjusted OR (95% CI) | p |
| Age groups (years) | ||||||
| 20–35, n (%) | 37 (58.73) | 26 (41.27) | 1.0 | 1.0 | ||
| 36–49, n (%) | 249 (48.35) | 266 (51.65) | 0.66 (0.39–1.12) | 0.122 | 0.65 (0.38–1.14) | 0.132 |
| Body mass index (kg/m2) | ||||||
| High BMI (≥23 kg/m2) (overweight/obesity), n (%) | 211 (46.68) | 241 (53.32) | 1.0 | 1.0 | ||
| Low BMI (<23 kg/m2) (underweight/normal weight), n (%) | 75 (59.52) | 51 (40.48) | 1.68 (1.13–2.51) | 0.011 | 1.68 (1.12–2.53) | 0.012 |
| Vitamin C (mg/L) | ||||||
| Sufficient (>8.8 mg/L), n (%) | 112 (42.91) | 149 (57.09) | 1.0 | 1.0 | ||
| Suboptimal (≤8.8 mg/L), n (%) | 174 (54.89) | 143 (45.11) | 1.62 (1.16–2.25) | 0.004 | 1.64 (1.16–2.31) | 0.004 |
| Serum 25 (OH)D (ng/mL) | ||||||
| Sufficient (≥30 ng/mL), n (%) | 100 (51.28) | 95 (48.72) | 1.0 | 1.0 | ||
| Suboptimal (<30 ng/mL), n (%) | 186 (48.56) | 197 (51.44) | 0.90 (0.64–1.27) | 0.537 | 0.91 (0.64–1.30) | 0.611 |
| Vitamin B12 (pg/mL) | ||||||
| Sufficient (>300 pg/mL), n (%) | 261 (48.42) | 278 (51.58) | 1.0 | 1.0 | ||
| Suboptimal (≤300 pg/dL), n (%) | 25 (64.10) | 14 (35.90) | 1.90 (0.97–3.74) | 0.062 | 2.05 (1.02–4.12) | 0.043 |
| Folic acid (ng/mL) | ||||||
| Sufficient (>6 ng/mL), n (%) | 201 (49.63) | 204 (50.37) | 1.0 | 1.0 | ||
| Suboptimal (≤6.0 ng/mL), n (%) | 85 (49.13) | 88 (50.87) | 0.98 (0.69–1.40) | 0.912 | 0.78 (0.53–1.14) | 0.204 |
| (b) | ||||||
| Women | Lowest BMD (n = 197) | Highest BMD (n = 195) | Crude OR (95% CI) | p | Adjusted OR (95% CI) | p |
| Age groups (years) | ||||||
| 20–35, n (%) | 22 (51.16) | 21 (48.84) | 1.0 | 1.0 | ||
| 36–49, n (%) | 175 (50.14) | 174 (49.86) | 0.96 (0.51–1.81) | 0.899 | 1.17 (0.61–2.27) | 0.634 |
| Body mass index (kg/m2) | ||||||
| High BMI (≥23 kg/m2) (overweight/obesity), n (%) | 35 (29.91) | 82 (70.09) | 1.0 | 1.0 | ||
| Low BMI (<23 kg/m2) (underweight/normal weight), n (%) | 162 (58.91) | 113 (41.09) | 3.36 (2.11–5.34) | <0.001 | 3.42 (2.13–5.50) | <0.001 |
| Vitamin C (mg/L) | ||||||
| Sufficient (>8.8 mg/L), n (%) | 148 (51.39) | 140 (48.61) | 1.0 | 1.0 | ||
| Suboptimal (≤8.8 mg/L), n (%) | 49 (47.12) | 55 (52.88) | 0.82 (0.54–1.32) | 0.456 | 0.87 (0.54–1.39) | 0.552 |
| Serum 25 (OH)D (ng/mL) | ||||||
| Sufficient (≥30 ng/mL), n (%) | 33 (45.83) | 1.0 | 1.0 | |||
| Suboptimal (<30 ng/mL), n (%) | 158 (49.38) | 162 (50.63) | 0.83 (0.49–1.38) | 0.464 | 1.01 (0.59–1.73) | 0.959 |
| Vitamin B12 (pg/mL) | ||||||
| Sufficient (>300 pg/mL), n (%) | 190 (49.87) | 191(50.13) | 1.0 | 1.0 | ||
| Suboptimal (≤300 pg/dL), n (%) | 7 (63.64) | 4 (36.36) | 1.76 (0.51–6.11) | 0.374 | 2.01 (0.55–7.39) | 0.295 |
| Folic acid (ng/mL) | ||||||
| Sufficient (>6 ng/mL), n (%) | 175 (50.58) | 171 (49.42) | 1.0 | 1.0 | ||
| Suboptimal (≤6.0 ng/mL), n (%) | 22 (47.83) | 24 (52.17) | 0.90 (0.48–1.66) | 0.726 | 0.99 (0.51–1.92) | 0.966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, K.-M.; Wang, L.-K.; Lin, Y.-T.; Hung, K.-C.; Wu, L.-C.; Ho, C.-H.; Chang, C.-Y.; Chen, J.-Y. Suboptimal Plasma Vitamin C Is Associated with Lower Bone Mineral Density in Young and Early Middle-Aged Men: A Retrospective Cross-Sectional Study. Nutrients 2022, 14, 3556. https://doi.org/10.3390/nu14173556
Lan K-M, Wang L-K, Lin Y-T, Hung K-C, Wu L-C, Ho C-H, Chang C-Y, Chen J-Y. Suboptimal Plasma Vitamin C Is Associated with Lower Bone Mineral Density in Young and Early Middle-Aged Men: A Retrospective Cross-Sectional Study. Nutrients. 2022; 14(17):3556. https://doi.org/10.3390/nu14173556
Chicago/Turabian StyleLan, Kuo-Mao, Li-Kai Wang, Yao-Tsung Lin, Kuo-Chuan Hung, Li-Ching Wu, Chung-Han Ho, Chia-Yu Chang, and Jen-Yin Chen. 2022. "Suboptimal Plasma Vitamin C Is Associated with Lower Bone Mineral Density in Young and Early Middle-Aged Men: A Retrospective Cross-Sectional Study" Nutrients 14, no. 17: 3556. https://doi.org/10.3390/nu14173556
APA StyleLan, K.-M., Wang, L.-K., Lin, Y.-T., Hung, K.-C., Wu, L.-C., Ho, C.-H., Chang, C.-Y., & Chen, J.-Y. (2022). Suboptimal Plasma Vitamin C Is Associated with Lower Bone Mineral Density in Young and Early Middle-Aged Men: A Retrospective Cross-Sectional Study. Nutrients, 14(17), 3556. https://doi.org/10.3390/nu14173556







