Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Design
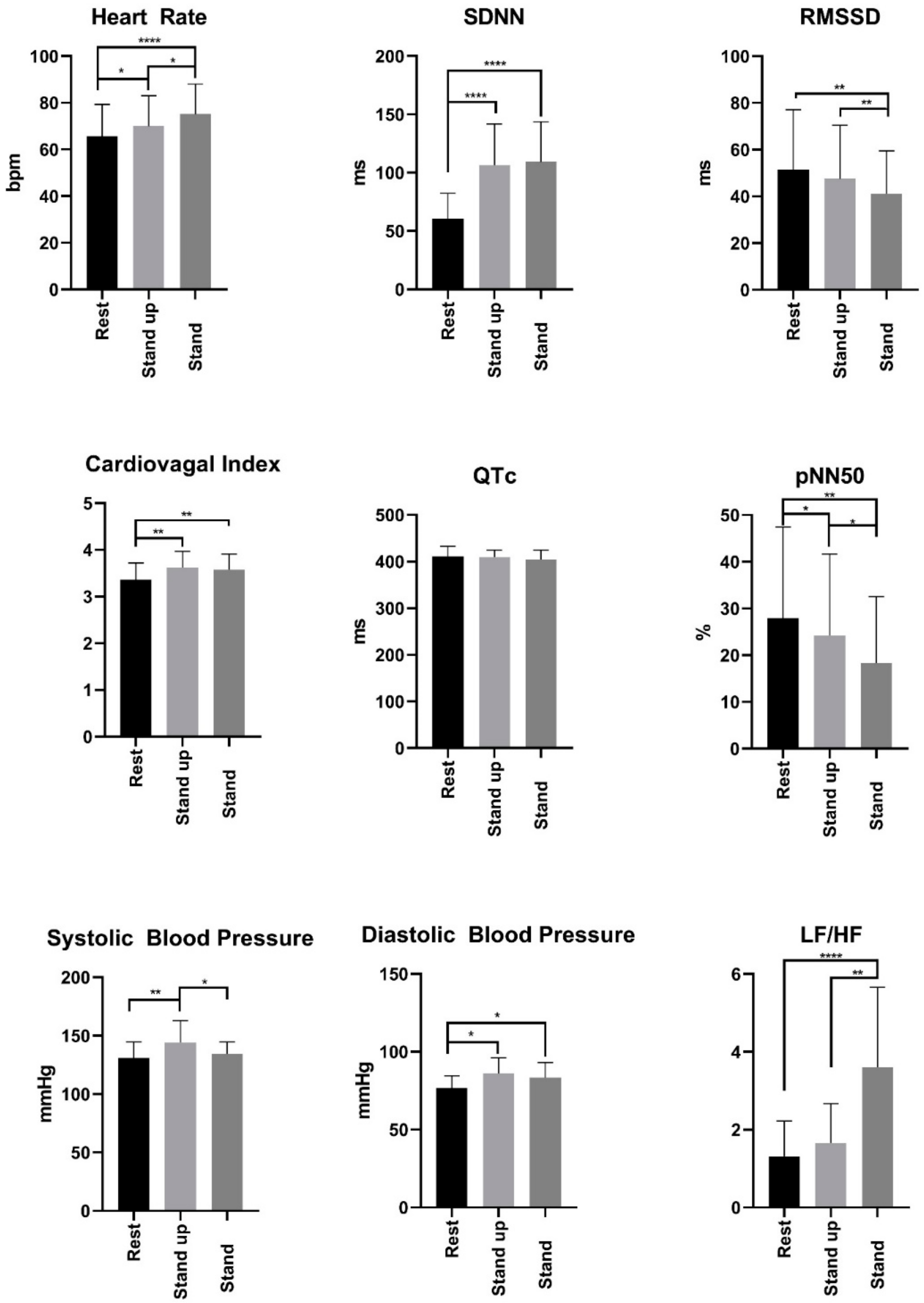
2.2.1. Screening Visit
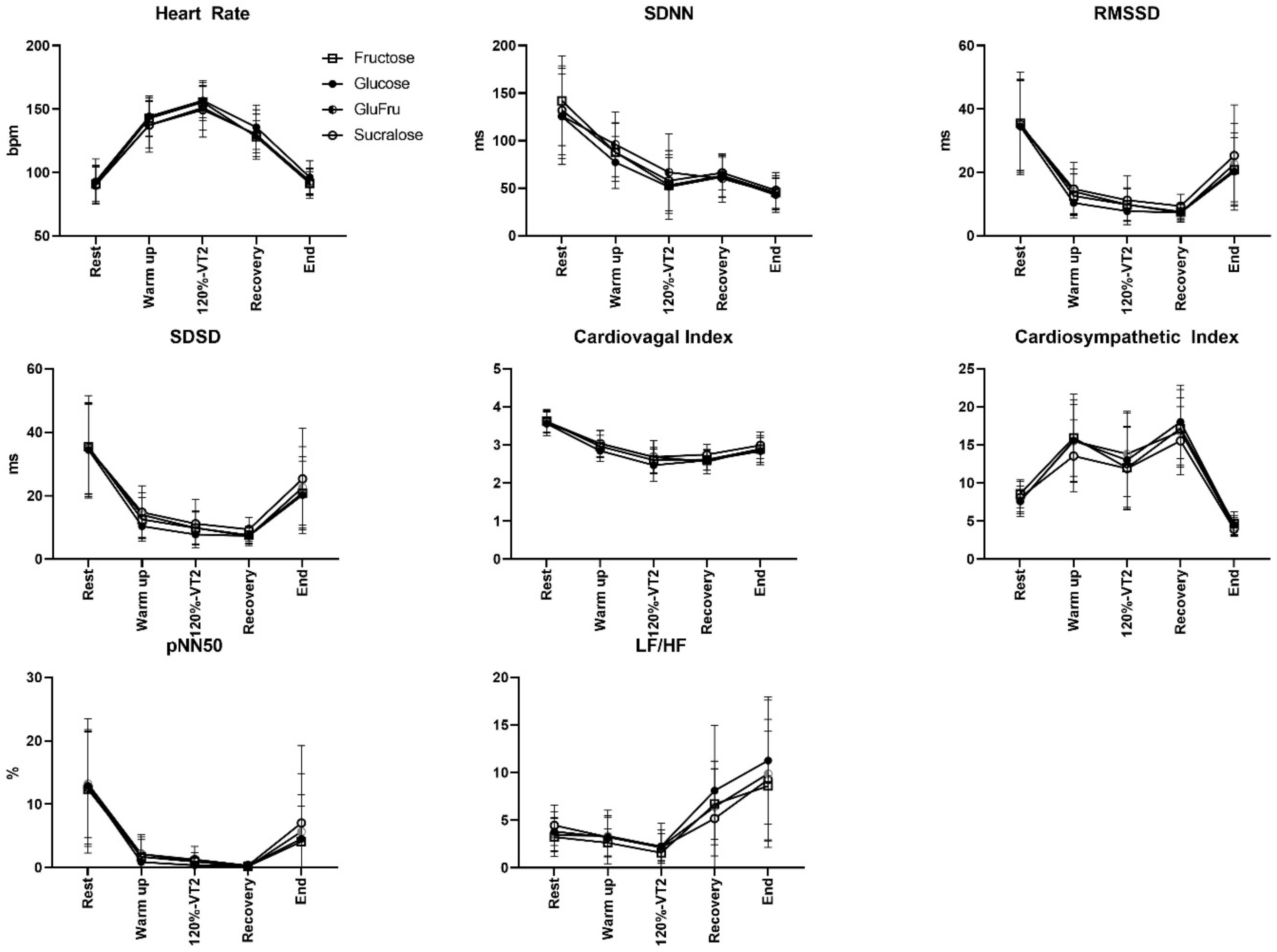
2.2.2. Trial Visits
2.3. HRV Measurement
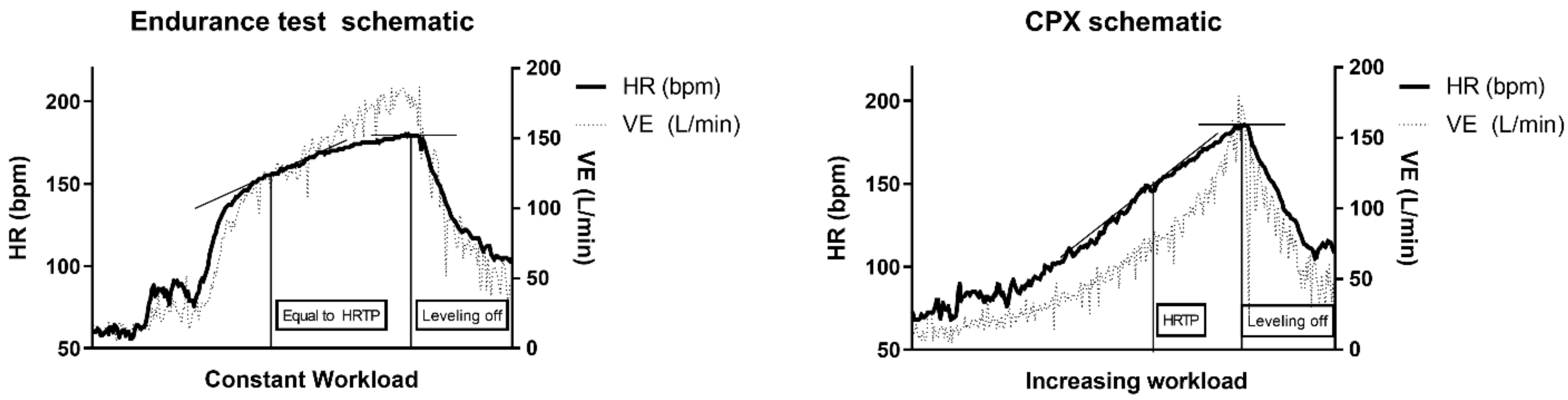
2.4. Heart Rate Curves
2.5. ECG Analysis
2.6. Statistics
3. Results
3.1. Anthropometry
3.2. Heart Rate Turn Point (HRTP) Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and Prevention of Cardiovascular Disease and Diabetes Mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Azzolina, D.; Folino, F.; Valentini, R.; Bendinelli, C.; Gafare, C.E.; Cainelli, E.; Vedovelli, L.; Iliceto, S.; Gregori, D.; et al. Dietary and Lifestyle Patterns Are Associated with Heart Rate Variability. J. Clin. Med. 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Adabag, S.; Whitsel, E.A.; Alonso, A.; Chen, L.Y. Low Heart Rate Variability in a 2-Minute Electrocardiogram Recording Is Associated with an Increased Risk of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities Study. PLoS ONE 2016, 11, e0161648. [Google Scholar] [CrossRef] [PubMed]
- Perpiñan, G.; Severeyn, E.; Wong, S.; Altuve, M. Cardiac Autonomic Modulation in Response to a Glucose Stimulus. Med. Biol. Eng. Comput. 2019, 57, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence Diagram of Physiological and Environmental Factors Affecting Heart Rate Variability: An Extended Literature Overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef]
- Plourde, B.; Sarrazin, J.-F.; Nault, I.; Poirier, P. Sudden Cardiac Death and Obesity. Expert Rev. Cardiovasc. 2014, 12, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.L.; Yadav, P.K.; Yadav, L.K.; Agrawal, K.; Sah, S.K.; Islam, M.N. Association between Obesity and Heart Rate Variability Indices: An Intuition toward Cardiac Autonomic Alteration—A Risk of CVD. Diabetes Metab. Syndr. Obes. 2017, 10, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Karpyak, V.M.; Romanowicz, M.; Schmidt, J.E.; Lewis, K.A.; Bostwick, J.M. Characteristics of Heart Rate Variability in Alcohol-Dependent Subjects and Nondependent Chronic Alcohol Users. Alcohol. Clin. Exp. Res. 2014, 38, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, A.; Prabhu, S.; Ling, L.-H.; Kalman, J.M.; Kistler, P.M. Alcohol and Atrial Fibrillation: A Sobering Review. J. Am. Coll. Cardiol. 2016, 68, 2567–2576. [Google Scholar] [CrossRef]
- Mandilaras, G.; Li, P.; Dalla-Pozza, R.; Haas, N.A.; Oberhoffer, F.S. Energy Drinks and Their Acute Effects on Heart Rhythm and Electrocardiographic Time Intervals in Healthy Children and Teenagers: A Randomized Trial. Cells 2022, 11, 498. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Heart Rate Variability in Risk Stratification of Cardiac Patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J. Interaction between Heart Rate and Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: https://Randomizer.Org/about/ (accessed on 27 September 2021).
- Eckstein, M.L.; Brockfeld, A.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Wachsmuth, N.; Zunner, B.; Zimmermann, P.; Obermayer-Pietsch, B.; Moser, O. Acute Metabolic Responses to Glucose and Fructose Supplementation in Healthy Individuals: A Double-Blind Randomized Crossover Placebo-Controlled Trial. Nutrients 2021, 13, 4095. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.L.; Brockfeld, A.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Wachsmuth, N.B.; Zunner, B.E.M.; Zimmermann, P.; Erlmann, M.; Obermayer-Pietsch, B.; et al. Acute Changes in Heart Rate Variability to Glucose and Fructose Supplementation in Healthy Individuals: A Double-Blind Randomized Crossover Placebo-Controlled Trial. Biology 2022, 11, 338. [Google Scholar] [CrossRef]
- Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381.
- Stys, A.; Stys, T. Current Clinical Applications of Heart Rate Variability. Clin. Cardiol. 1998, 21, 719–724. [Google Scholar] [CrossRef]
- Hofmann, P.; Pokan, R.; von Duvillard, S.P.; Seibert, F.J.; Zweiker, R.O.; Schmid, P.E. Heart Rate Performance Curve during Incremental Cycle Ergometer Exercise in Healthy Young Male Subjects. Med. Amp. Sci. Sports Amp. Exerc. 1997, 29, 762–768. [Google Scholar] [CrossRef]
- Samesima, N.; Azevedo, L.; Matos, L.; Echenique, L.; Negrao, C.; Pastore, C. Comparison of Electrocardiographic Criteria for Identifying Left Ventricular Hypertrophy in Athletes from Different Sports Modalities. Clinics 2017, 72, 343–350. [Google Scholar] [CrossRef]
- Pentikäinen, H.; Toivo, K.; Kokko, S.; Alanko, L.; Heinonen, O.J.; Korpelainen, R.; Selänne, H.; Vasankari, T.; Kujala, U.M.; Villberg, J.; et al. Resting Electrocardiogram and Blood Pressure in Young Endurance and Nonendurance Athletes and Nonathletes. J. Athl. Train. 2021, 56, 484–490. [Google Scholar] [CrossRef]
- Cirincione, B.; Sager, P.T.; Mager, D.E. Influence of Meals and Glycemic Changes on QT Interval Dynamics. J. Clin. Pharm. 2017, 57, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Birnbaumer, P.; Traninger, H.; Borenich, A.; Falgenhauer, M.; Modre-Osprian, R.; Harpf, H.; Hofmann, P. Heart Rate Performance Curve Is Dependent on Age, Sex, and Performance. Front. Public Health 2020, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.; Pokan, R. Value of the Application of the Heart Rate Performance Curve in Sports. Int. J. Sports Physiol. Perform. 2010, 5, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Conconi, F.; Ferrari, M.; Ziglio, P.G.; Droghetti, P.; Codeca, L. Determination of the Anaerobic Threshold by a Noninvasive Field Test in Runners. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1982, 52, 869–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, P.; Wonisch, M.; Pokan, R.; Schwaberger, G.; Smekal, G.; von Duvillard, S.P. Β1-Adrenoceptor Mediated Origin of the Heart Rate Performance Curve Deflection. Med. Sci. Sports Exerc. 2005, 37, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
| Participant | HR (bpm) | PQ (ms) | QRS (ms) | QT (ms) | QTc (ms) | Sokolow (mm) | Lewis (mm) | Cornell (mm) | Electrical Axis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | 136 | 84 | 406 | 464 | 9.4 | −5.6 | 6 | Vertical |
| 2 | 92 | 126 | 80 | 362 | 448 | 17.1 | −6 | 3.2 | Vertical |
| 3 | 60 | 154 | 90 | 422 | 422 | 27.3 | −4.8 | 4 | Vertical |
| 4 | 67 | 124 | 72 | 412 | 434 | 22.6 | −12 | 0.4 | Vertical |
| 5 | 48 | 176 | 104 | 458 | 409 | 17,4 | −6 | 8.6 | Vertical |
| 6 | 100 | 174 | 84 | 326 | 420 | 8.8 | 2 | 9.3 | Left |
| 7 | 72 | 158 | 106 | 400 | 439 | 25 | −1.8 | 9 | Vertical |
| 8 | 77 | 154 | 70 | 374 | 424 | 22 | 10.5 | 6.1 | Indifferent |
| 9 | 73 | 120 | 74 | 382 | 422 | 24 | 2 | 5 | Indifferent |
| 10 | 53 | 124 | 106 | 450 | 424 | 24.5 | −6.6 | 12.4 | Vertical |
| 11 | 57 | 162 | 68 | 444 | 434 | 26.4 | −10.2 | 1.4 | Vertical |
| 12 | 60 | 144 | 110 | 426 | 427 | 45.2 | −19.6 | 1.4 | Vertical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, M.L.; Zimmermann, P.; Erlmann, M.P.; Wachsmuth, N.B.; Haupt, S.; Zimmer, R.T.; Schierbauer, J.; Herz, D.; Aberer, F.; Sourij, H.; et al. Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial. Nutrients 2022, 14, 3257. https://doi.org/10.3390/nu14163257
Eckstein ML, Zimmermann P, Erlmann MP, Wachsmuth NB, Haupt S, Zimmer RT, Schierbauer J, Herz D, Aberer F, Sourij H, et al. Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial. Nutrients. 2022; 14(16):3257. https://doi.org/10.3390/nu14163257
Chicago/Turabian StyleEckstein, Max L., Paul Zimmermann, Maximilian P. Erlmann, Nadine B. Wachsmuth, Sandra Haupt, Rebecca T. Zimmer, Janis Schierbauer, Daniel Herz, Felix Aberer, Harald Sourij, and et al. 2022. "Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial" Nutrients 14, no. 16: 3257. https://doi.org/10.3390/nu14163257
APA StyleEckstein, M. L., Zimmermann, P., Erlmann, M. P., Wachsmuth, N. B., Haupt, S., Zimmer, R. T., Schierbauer, J., Herz, D., Aberer, F., Sourij, H., Obermayer-Pietsch, B., & Moser, O. (2022). Glucose and Fructose Supplementation and Their Acute Effects on Electrocardiographic Time Intervals during Anaerobic Cycling Exercise in Healthy Individuals: A Secondary Outcome Analysis of a Double-Blind Randomized Crossover-Controlled Trial. Nutrients, 14(16), 3257. https://doi.org/10.3390/nu14163257









