Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Setting
2.3. Eligibility
2.4. Recruitment
2.5. Randomisation and Blinding
2.6. Usual Dietetic Care and Control Group
2.7. Intervention Groups
2.8. Community Involvement
2.9. Data Collection
2.10. Primary Outcome
2.11. Secondary Outcomes
2.12. Sample Size Calculation
2.13. Data Analysis
3. Results
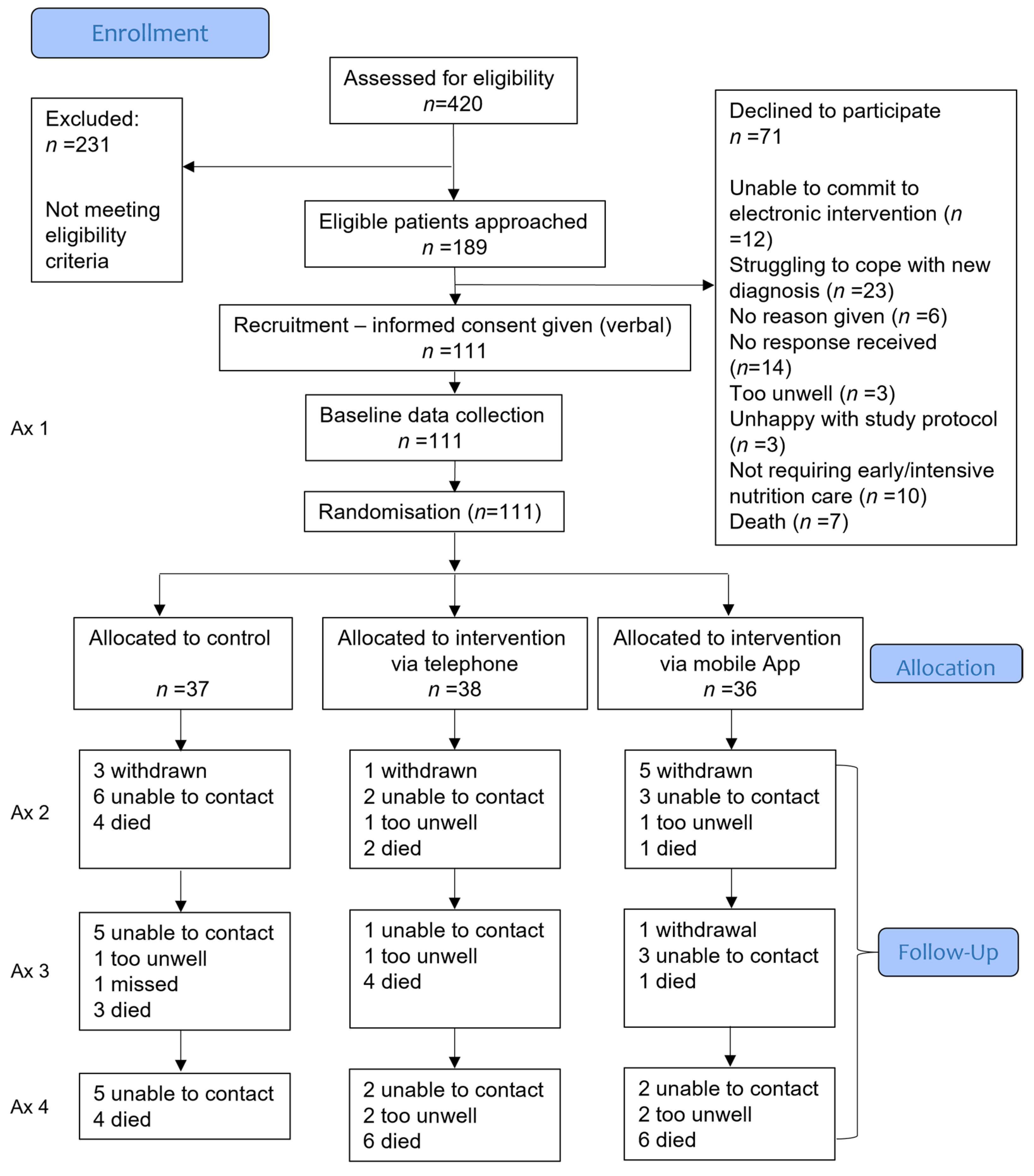
3.1. Participants
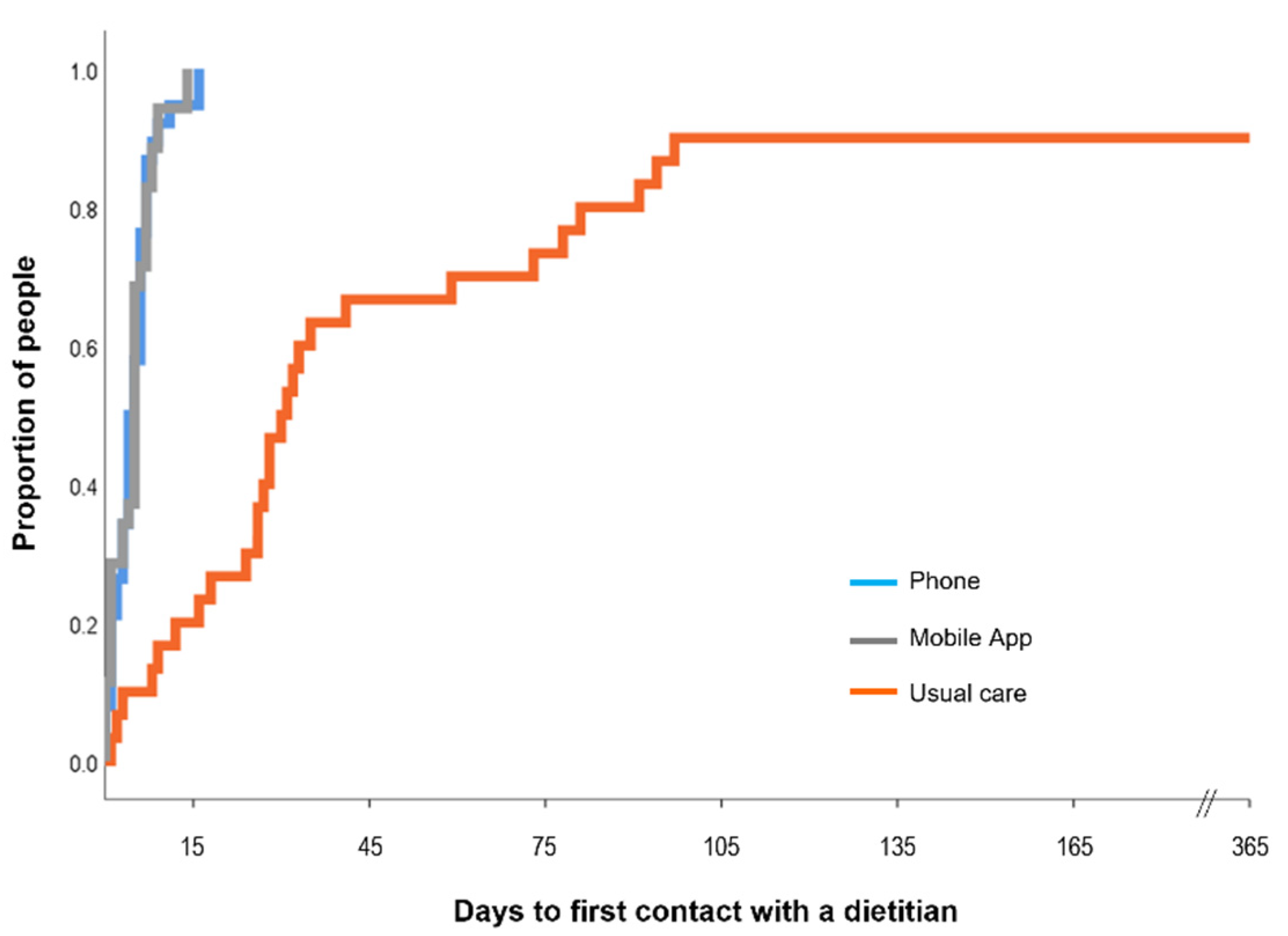
3.2. Dietetic Contact
3.3. Numbers Analysed and Missing Data
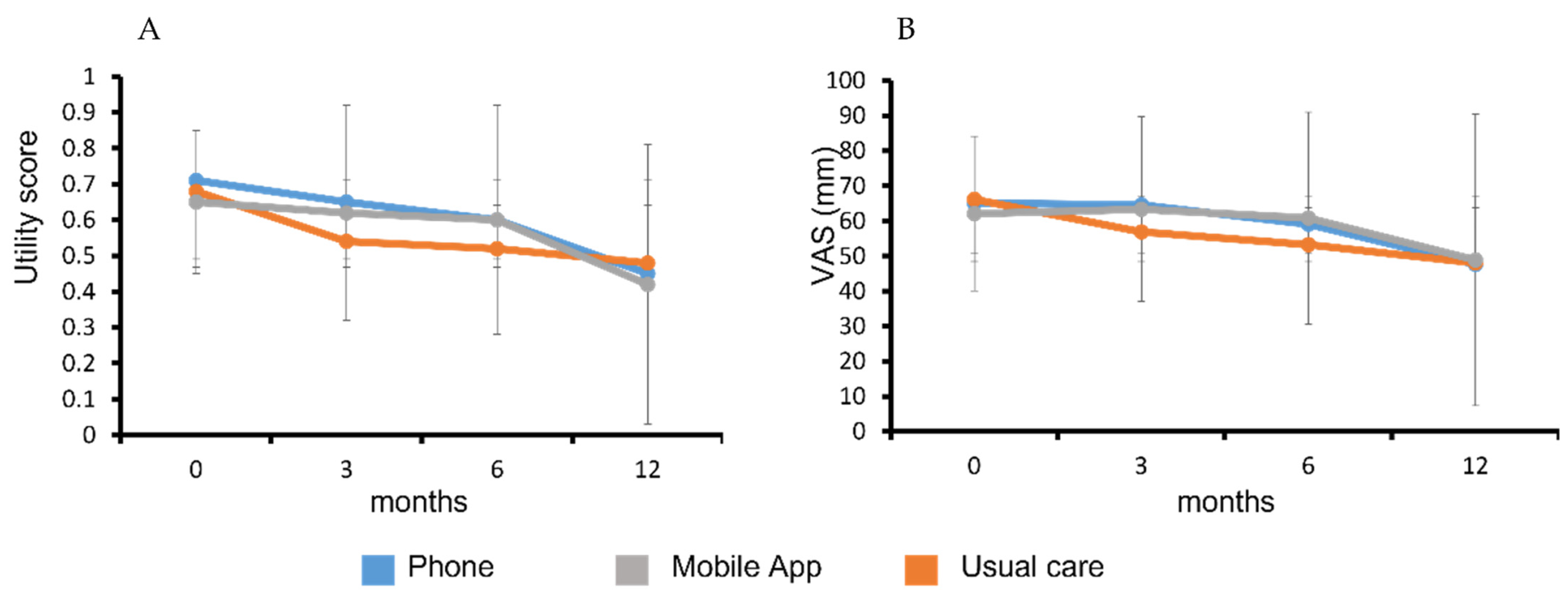
3.4. Primary Outcome—Quality-Adjusted Life Years (QALY)
3.5. Secondary Outcomes
4. Discussion
Implications for Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Q.; Yu, J.M.; Li, W.; Fu, Z.M.; Lin, Y.; Shi, Y.Y.; Hu, W.; Ba, Y.; Li, S.Y.; Li, Z.N.; et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2020, 28, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Silvers, M.A.; Savva, J.; Huggins, C.E.; Truby, H.; Haines, T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Supportive Care Cancer 2014, 22, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Lorton, C.M.; Griffin, O.; Higgins, K.; Roulston, F.; Stewart, G.; Gough, N.; Barnes, E.; Aktas, A.; Walsh, T.D. Late referral of cancer patients with malnutrition to dietitians: A prospective study of clinical practice. Supportive Care Cancer 2020, 28, 2351–2360. [Google Scholar] [CrossRef]
- Trujillo, E.B.; Claghorn, K.; Dixon, S.W.; Hill, E.B.; Braun, A.; Lipinski, E.; Platek, M.E.; Vergo, M.T.; Spees, C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019, 2019, 7462940. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, C.; McGough, C.; Norman, A.R.; Frost, G.S.; Cunningham, D.C.; Andreyev, H.J. Failure of dietetic referral in patients with gastrointestinal cancer and weight loss. Eur. J. Cancer 2006, 42, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Dunne, R.F. The Impact of Early Referrals to Dietitians for Patients with Esophagogastric Cancer. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer--a systematic review of the epidemiological literature. Nutr. J. 2012, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deftereos, I.; Kiss, N.; Isenring, E.; Carter, V.M.; Yeung, J.M. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Thordardottir, E.; West, C.P.; Jatoi, A. Does dietary counseling improve quality of life in cancer patients? A systematic review and meta-analysis. J. Supportive Oncol. 2008, 6, 234–237. [Google Scholar]
- Zhang, F.; Jin, Y.; Qiang, W. The effects of dietary advice on malnutrition in Cancer patients: A systematic review and meta-analysis. Supportive Care in Cancer 2020, 28, 1579–1585. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Oosterlaan, F.; van Huis, L.H.; Thielen, N.; Vondeling, A.; van den Bos, F. Nutritional status and interventions for patients with cancer—A systematic review. J. Geriatr. Oncol. 2021, 12, 6–21. [Google Scholar] [CrossRef]
- Spelten, E.R.; Hardman, R.N.; Pike, K.E.; Yuen, E.Y.N.; Wilson, C. Best practice in the implementation of telehealth-based supportive cancer care: Using research evidence and discipline-based guidance. Patient Educ. Couns. 2021, 104, 2682–2699. [Google Scholar] [CrossRef]
- Griffiths, F.; Lindenmeyer, A.; Powell, J.; Lowe, P.; Thorogood, M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J. Med. Internet Res. 2006, 8, e10. [Google Scholar] [CrossRef]
- Nguyen, O.T.; Alishahi Tabriz, A.; Huo, J.; Hanna, K.; Shea, C.M.; Turner, K. Impact of Asynchronous Electronic Communication–Based Visits on Clinical Outcomes and Health Care Delivery: Systematic Review. J. Med. Internet Res. 2021, 23, e27531. [Google Scholar] [CrossRef]
- Furness, K.; Sarkies, M.N.; Huggins, C.E.; Croagh, D.; Haines, T.P. Impact of the Method of Delivering Electronic Health Behavior Change Interventions in Survivors of Cancer on Engagement, Health Behaviors, and Health Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e16112. [Google Scholar] [CrossRef]
- Hanna, L.; Huggins, C.E.; Furness, K.; Silvers, M.A.; Savva, J.; Frawley, H.; Croagh, D.; Cashin, P.; Low, L.; Bauer, J.; et al. Effect of early and intensive nutrition care, delivered via telephone or mobile application, on quality of life in people with upper gastrointestinal cancer: Study protocol of a randomised controlled trial. BMC Cancer 2018, 18, 707. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Cancer Council Victoria and Department of Health Victoria. Optimal Care Pathway for People with Oesophagogastric Cancer, 2nd ed.; Cancer Council Victoria: Melbourne, VIC, Australia, 2021. [Google Scholar]
- Barnett, J.; Harricharan, M.; Fletcher, D.; Gilchrist, B.; Coughlan, J. myPace: An Integrative Health Platform for Supporting Weight Loss and Maintenance Behaviors. IEEE J. Biomed. Health Inform. 2015, 19, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, R.; Cronin, P.; Viney, R. A pilot discrete choice experiment to explore preferences for EQ-5D-5L health states. Appl. Health Econ. Health Policy 2013, 11, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Cankurtaran, E.S.; Ozalp, E.; Soygur, H.; Ozer, S.; Akbiyik, D.I.; Bottomley, A. Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. Eur. J. Cancer Care 2008, 17, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Michels, F.A.; Latorre Mdo, R.; Maciel Mdo, S. Validity, reliability and understanding of the EORTC-C30 and EORTC-BR23, quality of life questionnaires specific for breast cancer. Rev. Bras. Epidemiol. Braz. J. Epidemiol. 2013, 16, 352–363. [Google Scholar] [CrossRef]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Dong, Y.; Peng, C.Y. Principled missing data methods for researchers. SpringerPlus 2013, 2, 222. [Google Scholar] [CrossRef] [Green Version]
- Faria, R.; Gomes, M.; Epstein, D.; White, I.R. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014, 32, 1157–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ginkel, J.R.; Linting, M.; Rippe, R.C.A.; van der Voort, A. Rebutting Existing Misconceptions About Multiple Imputation as a Method for Handling Missing Data. J. Personal. Assess. 2020, 102, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenring, E.A.; Capra, S.; Bauer, J.D. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer 2004, 91, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Dang, A.K.; Duong, P.T.; Phan, H.B.T.; Pham, C.T.T.; Nguyen, A.T.L.; Le, H.T. Nutrition intervention is beneficial to the quality of life of patients with gastrointestinal cancer undergoing chemotherapy in Vietnam. Cancer Med. 2021, 10, 1668–1680. [Google Scholar] [CrossRef]
- Uster, A.; Ruefenacht, U.; Ruehlin, M.; Pless, M.; Siano, M.; Haefner, M.; Imoberdorf, R.; Ballmer, P.E. Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: A randomized controlled trial. Nutrition 2013, 29, 1342–1349. [Google Scholar] [CrossRef]
- Deftereos, I.; Yeung, J.M.C.; Arslan, J.; Carter, V.M.; Isenring, E.; Kiss, N.; Group oboTNPPS. Assessment of Nutritional Status and Nutrition Impact Symptoms in Patients Undergoing Resection for Upper Gastrointestinal Cancer: Results from the Multi-Centre NOURISH Point Prevalence Study. Nutrients 2021, 13, 3349. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Ole, N.; Dorthe, F.; Louise, K.; Astrid, K.; Roy, B.; Lars, K.; Richard, H.O. The e-health literacy framework: A conceptual framework for characterizing e-health users and their interaction with e-health systems. Knowl. Manag. E-Learn. 2015, 7, 522–540. [Google Scholar]
- Cooley, M.E.; Nayak, M.M.; Abrahm, J.L.; Braun, I.M.; Rabin, M.S.; Brzozowski, J.; Lathan, C.; Berry, D.L. Patient and caregiver perspectives on decision support for symptom and quality of life management during cancer treatment: Implications for eHealth. Psycho-Oncol. 2017, 26, 1105–1112. [Google Scholar] [CrossRef]
- Furness, K.; Huggins, C.E.; Truby, H.; Croagh, D.; Haines, T.P. Attitudes of Australian Patients Undergoing Treatment for Upper Gastrointestinal Cancers to Different Models of Nutrition Care Delivery: Qualitative Investigation. JMIR Form. Res. 2021, 5, e23979. [Google Scholar] [CrossRef]
- Steer, B.L.J. Cancer Malnutrition Point Prevalence Study 2018 Summary Report; VCMC, Ed.; Peter MacCallum Cancer Centre: Melbourne, VIC, Australia, 2020. [Google Scholar]
- de Oliveira Faria, S.; Simões Lima, G.A.; Lopes Carvalho, A.; Nader Marta, G.; Howell, D.; Eluf-Neto, J. Clinically significant changes in health-related quality of life in head and neck cancer patients following intensive nutritional care during radiotherapy. Eur. J. Oncol. Nurs. 2022, 56, 102065. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, H.; Hedeker, D. An imputation strategy for incomplete longitudinal ordinal data. Stat. Med. 2008, 27, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Carlin, J.B. Multiple imputation for missing data: Fully conditional specification versus multivariate normal imputation. Am. J. Epidemiol. 2010, 171, 624–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Control (n = 37) | Telephone (n = 38) | Mobile App (n = 36) | |
|---|---|---|---|
| Age—mean (sd) | 63.2 (9.9) | 67.5 (10.3) | 66.6 (9.7) |
| Gender—n (%) | |||
| Male | 23 (62) | 25 (66) | 26 (72) |
| Female | 14 (38) | 13 (34) | 10 (28) |
| Tumour location—n (%) | |||
| Oesophageal | 13 (35) | 16 (42) | 17 (47) |
| Gastric | 8 (22) | 4 (11) | 9 (25) |
| Pancreatic | 16 (43) | 18 (47) | 10 (28) |
| Clinical stage of cancer—n (%) | |||
| Resectable | 16 (43) | 15 (39) | 18 (44) |
| Borderline resectable | 2 (5) | 1 (3) | 3 (5) |
| Locally advanced | 12 (32) | 12 (32) | 9 (30) |
| Metastatic | 7 (19) | 10 (26) | 6 (21) |
| Height—mean (sd) | 168.9 (10.7) | 170.7 (8.9) | 171.6 (9.3) |
| Weight—mean (sd) | 75.0 (20.0) | 71.9 (12.7) | 76.4 (14.7) |
| EQ-5D-5L—median (IQR) | |||
| Mobility | 1 (1, 2) | 1 (1, 1) | 1 (1, 2) |
| Personal care | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| Usual activities | 1 (1, 3) | 1 (1, 1) | 1 (1, 3) |
| Pain or discomfort | 2 (1, 3) | 2 (1, 2) | 2 (1, 3) |
| Anxiety or Depression | 2 (1, 2) | 1.5 (1, 3) | 2 (1, 2) |
| EQ-5D-5L-utility score—mean (sd) | 0.68 (0.19) | 0.71 (0.23) | 0.65 (0.20) |
| EQ-5D-5L visual analogue scale mean (sd) | 66.16 (20.27) | 65.04 (22.9) | 62.08 (22.01) |
| First language—n (%) | |||
| English | 33 (89) | 33 (89) | 30 (86) |
| Familiarity with technology n “yes” (%) | |||
| Do you use email? | 33 (89%) | 29 (76) | 29 (81) |
| Do you have a smartphone? | 30 (81%) | 32 (84) | 30 (83) |
| Do you have a tablet device? | 16 (43%) | 25 (66)) | 21 (58) |
| Do you feel confident to communicate with your health professional using electronic messages from your smartphone or tablet device? | 33 (89%) | 31 (82) | 26 (72) |
| Do you regularly (at least once per day) use your smartphone or tablet device for purposes other than receiving or making phone calls? | 30 (81%) | 25 (66) | 26 (72) |
| PG-SGASF score—mean (sd) | 8.4 (6.5) | 8.5 (6.2) | 8.5 (6.5) |
| EORTC QLQ-C30 score—mean (sd) | |||
| Global health | 59.32 (25.72) | 63.41 (26.17) | 61.22 (24.60) |
| Physical functioning | 79.28 (22.21) | 81.23 (20.08) | 77.22 (25.67) |
| Role functioning | 63.51 (36.61) | 67.54 (34.43) | 65.28 (35.04) |
| Emotional functioning | 70.49 (21.02) | 72.15 (21.26) | 73.38 (25.34) |
| Cognitive functioning | 83.33 (21.52) | 85.09 (18.90) | 76.85 (23.66) |
| Social functioning | 72.52 (29.71) | 71.49 (30.49) | 74.07 (32.96) |
| Fatigue | 38.74 (25.48) | 35.38 (29.21) | 42.90 (31.44) |
| Nausea and vomiting | 10.81 (21.59) | 11.84 (20.47) | 11.11 (18.26) |
| Pain | 27.93 (27.51) | 25.44 (29.70) | 29.63 (33.36) |
| Dyspnoea | 8.10 (18.27) | 13.16 (23.94) | 12.96 (18.30) |
| Insomnia | 46.85 (36.39) | 28.95 (29.17) | 31.48 (29.76) |
| Appetite loss | 26.13 (30.60) | 35.09 (34.61) | 28.70 (35.77) |
| Constipation | 19.82 (29.89) | 17.54 (28.72) | 22.22 (31.87) |
| Diarrhoea | 10.81 (23.64) | 7.02 (22.13) | 4.63 (19.76) |
| Financial difficulties | 10.81 (22.30) | 15.79 (29.75) | 16.66 (40.19) |
| Control | Telephone | Mobile App | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 Months (n = 26) | 6 Months (n = 20) | 12 Months (n = 18) | 3 Months (n = 32) | 6 Months (n = 28) | 12 Months (n = 21) | 3 Months (n = 26) | 6 Months (n = 24) | 12 Months (n = 17) | |
| Dietitian contact prior to this follow-up—n “Yes” (%) | 23 (88) | 12 (60) | 11 (61) | 21 (66) | 15 (54) | 6 (29) | 16 (61) | 16 (67) | 8 (47) |
| Median number of contacts with dietitian (range) | 2.5 (0–13) | 2.5 (0–26) | 1 (0–15) | 2 (0–14) | 1.5 (0–23) | 0 (0–2) | 2 (0–7) | 2 (0–17) | 0 (0–5) |
| Control (n = 37) | Telephone (n = 38) | Mobile App (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 12 Months | 3 Months | 6 Months | 12 Months | 3 Months | 6 Months | 12 Months | |
| QALY—mean (sd) | - | - | 0.55 (0.28) | - | - | 0.57 (0.28) | - | - | 0.59 (0.23) |
| Mortality prior to this follow-up—n (cumulative %) | 4 (11%) | 3 (19%) | 4 (30%) | 2 (5%) | 4 (16%) | 6 (33%) | 1 (3%) | 1 (6%) | 6 (22%) |
| n (% relative to baseline) # | n = 30 (81%) | n = 28 (76%) | n = 30 (81%) | n = 33 (87%) | n = 34 (89%) | n = 34 (89%) | n = 28 (78%) | n = 29 (80%) | n = 28 (78%) |
| EQ-5D-5L utility score—mean (sd) | 0.54 (0.37) | 0.52 (0.35) | 0.48 (0.42) | 0.65 (0.29) | 0.60 (0.35) | 0.45 (0.41) | 0.62 (0.30) | 0.60 (0.32) | 0.42 (0.39) |
| EQ-5D-5L visual analogue scale—mean (sd) | 56.8 (29.9) | 53.2 (35.6) | 48.1 (41.5) | 64.5 (24.1) | 59.1 (32.8) | 47.6 (41.4) | 63.3 (26.4) | 60.8 (30.2) | 48.9 (41.6) |
| n (% relative to baseline) * | n = 26 (70%) | n = 20 (54%) | n = 18 (49%) | n = 32 (84%) | n = 28 (74%) | n = 21 (55%) | n = 26 (72%) | n = 25 (69%) | n = 17 (47%) |
| EQ-5D-5L—median (IQR) | |||||||||
| Mobility | 1.5 (1, 2) | 1 (1, 2) | 1 (1, 1) | 1 (1, 2.5) | 1 (1, 2.5) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 2 (1, 2) |
| Personal care | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 2) |
| Usual activities | 2 (1, 3) | 1 (1, 2) | 1 (1, 1) | 1.5 (1, 3) | 1.5 (1, 2) | 1 (1, 2) | 1.5 (1, 3) | 1 (1, 2) | 2 (1, 2) |
| Pain or discomfort | 2 (1, 2) | 2 (1, 2) | 1.5 (1, 2) | 2 (1, 3) | 1 (1, 3) | 2 (1, 2) | 1 (1, 2) | 2 (1, 2) | 1 (1, 2) |
| Anxiety or depression | 2 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Weight (kg)—mean (sd) | 75.6 (20.3) | 75.6 (17.5) | 73.2 (18.4) | 71.7 (11.8) | 70.2 (11.7) | 68.6 (13.3) | 71.7 (15.6) | 68.7 (14.1) | 68.5 (14.1) |
| PG-SGASF score—mean (sd) | 7.5 (5.0) | 4.6 (3.6) | 4.1 (4.1) | 7.8 (5.7) | 6.2 (5.1) | 4.3 (4.7) | 8.4 (6.1) a | 7.2 (4.0) | 4.9 (3.6) |
| EORTC QLQ-C30 score—mean (sd) | |||||||||
| Global health | 54.3 (25.1) | 69.8 (12.2) | 72.7 (15.9) | 66.4 (19.7) b | 68.0 (28.13) | 74.8 (23.8) | 62.3 (24.5) | 59.25 (21.10) | 73.5 (20.5) |
| Physical functioning | 70.8 (26.0) | 81.3 (14.1) | 86.7 (15.5) | 74.0 (18.5) | 75.95 (21.72) | 80.6 (20.0) | 73.8 (26.8) | 73.33 (17.32) | 82.7 (16.3) |
| Role functioning | 48.7 (31.9) | 71.7 (25.4) | 78.7 (25.4) | 62.0 (32.9) | 68.45 (32.18) | 75.4 (34.0) | 59.6 (35.3) | 54.67 (25.24) | 77.4 (16.7) |
| Emotional functioning | 72.8 (24.8) | 80.8 (16.7) | 85.6 (11.4) | 82.6 (18.7) | 80.65 (22.23) | 84.1 (15.1) | 76.3 (22.40) | 73.33 (22.31) | 83.8 (17.8) |
| Cognitive functioning | 72.4 (26.6) | 80.0 (17.6) | 82.4 (16.6) | 82.8 (19.0) | 83.93 (21.98) | 85.7 (20.6) | 78.7 (24.8) | 80 (20.41) | 82.4 (21.6) |
| Social functioning | 58.3 (41.7) | 80.0 (20.7) | 82.4 (27.7) | 71.5 (30.2) | 76.79 (25.0) | 84.1 (26.1) | 74.0 (32.3) | 66.6 (26.8) | 87.2 (21.7) |
| Fatigue | 54.7 (26.6) | 37.2 (22.0) | 25.3 (22.5) | 42.9 (23.5) | 39.28 (23.7) | 33.3 (24.3) | 45.7 (26.2) | 45.8 (26.3) | 34.0 (26.5) |
| Nausea and vomiting | 14.1 (16.1) | 8.3 (14.8) | 11.1 (21.4) | 7.8 (15.8) | 9.5 (12.4) | 7.1 (11.3) | 9.6 (14.8) | 6.0 (9.5) | 8.8 (19.6) |
| Pain | 29.5 (32.1) | 29.2 (24.7) | 22.2 (23.6) | 22.4 (30.1) | 25.0 (30.6) | 22.2 (22.0) | 20.5 (29.9) | 16.0 (21.2) | 18.6 (15.5) |
| Dyspnoea | 19.2 (30.1) | 11.7 (19.6) | 14.8 (23.5) | 21.9 (24.8) | 19.0 (24.7) | 23.8 (28.2) | 21.8 (23.0) | 18.7 (23.7) | 15.7 (23.9) |
| Insomnia | 35.9 (35.2) | 28.3 (29.2) | 25.9 (21.6) | 29.2 (37.6) | 22.6 (27.3) | 25.0 (30.3) | 34.6 (40.5) | 32.0 (31.1) | 31.4 (34.3) |
| Appetite loss | 38.5 (33.6) | 20 (27.4) | 14.8 (23.5) | 32.3 (28.7) | 23.8 (29.9) | 20.6 (26.8) | 35.9 (38.8) | 26.7 (25.5) | 25.5 (32.3) |
| Constipation | 16.7 (30.2) | 11.7 (24.8) | 9.3 (22.3) | 12.5 (22.0) | 17.9 (23.1) | 12.7 (19.6) | 19.2 (34.2) | 13.3 (25.6) | 7.8 (18.7) |
| Diarrhoea | 23.1 (32.3) | 18.3 (22.9) | 22.2 (30.2) | 19.3 (29.5) | 20.2 (27.7) | 19.0 (29.0) | 18.7 (27.5) | 22.7 (31.5) | 15.7 (24.0) |
| Financial difficulties | 21.8 (33.9) | 13.3 (29.4) | 13.0 (23.3) | 8.6 (19.2) | 9.5 (23.8) | 17.5 (34.3) | 19.3 (34.0) | 20.7 (33.8) | 19.6 (37.4) |
| Control vs. Telephone | Mobile App vs. Telephone | Mobile App vs. Control | |
|---|---|---|---|
| QALY—coef (95% CI), p-value † | 0.04 (0.43, 2.3), p = 0.998 | −0.02 (−0.13, 0.08), p = 0.712 | −0.08 (−0.18, 0.02), p = 0.135 |
| Survival—HR (95% CI), p-value * | 0.999 (−0.45, 2.39), p = 0.923 | 0.61 (0.27, 1.74), p = 0.434 | 0.52 (0.23, 1.50), p = 0.265 |
| EORTC QLQ-C30 score #,† | |||
| Global health | −4.02 (−10.4, 2.4), p = 0.22 | −6.00 (−12.70, 0.75), p = 0.082 | −0.67 (−7.62, 6.28), p = 0.850 |
| Physical functioning | −2.75 (−9.63, 4.12), p = 0.433 | −3.20 (−10.03, 3.63), p = 0.359 | −2.31 (−8.29, 3.67), p = 0.448 |
| Role functioning | −6.11 (−16.78, 4.56), p = 0.262 | −6.31 (−16.16, 3.54), p = 0.210 | −0.12 (−9.95, 9.71), p = 0.980 |
| Emotional functioning | −0.88 (−8.08, 6.33), p = 0.812 | −7.07 (−14.37, 0.22), p = 0.057 | 4.95 (−1.88, 11.78), p = 0.155 |
| Cognitive functioning | −7.36 (−14.15, −0.57), p = 0.034 | −1.60 (−8.57, 5.37), p = 0.652 | −6.43 (−13.90, 1.04), p = 0.092 |
| Social functioning | −5.38 (−16.73, 6.00), p = 0.353 | −3.01, (−12.30, 6.28), p = 0.525 | −4.93 (−16.54, 6.68), p = 0.405 |
| Fatigue | 3.08 (−5.77, 11.93), p = 0.496 | 3.28 (−5.63, 12.19), p = 0.471 | 1.47 (−7.63, 10.58), p = 0.751 |
| Nausea and vomiting | 0.02 (−5.68, 5.72), p = 0.994 | −1.94 (−6.91, 3.04), p = 0.445 | 3.17 (−2.16, 8.50), p = 0.244 |
| Pain | 1.22 (−9.04, 11.47), p = 0.816 | −5.87 (−15.60, 3.85), p = 0.237 | 11.63 (1.20, 22.06), p = 0.029 |
| Dyspnoea | −0.76 (−9.66, 8.13), p = 0.867 | 1.26 (−7.14, 9.65), p = 0.769 | 1.67 (−6.67, 10.02), p = 0.694 |
| Insomnia | −1.94 (−14.94, 11.06), p = 770 | 5.21 (−8.13, 18.56), p = 0.444 | −2.06 (−15.12, 11.00), p = 0.757 |
| Appetite loss | 0.65 (−9.71, 11.00), p = 0.902 | 3.49 (−6.74, 13.72), p = 0.504 | −2.01 (−12.71, 8.70), p = 0.713 |
| Constipation | −2.44 (−12.35, 7.35), p = 0.625 | 2.75 (−7.11, 12.62), p = 0.584 | −0.35 (−12.25, 11.56), p = 0.955 |
| Diarrhoea | 4.84 (−7.16, 16.83), p = 0.429 | −0.61 (−12.53, 11.31), p = 0.920 | 3.94 (−8.47, 16.35), p = 0.534 |
| Financial difficulties | 6.00 (−4.96, 16.97), p = 0.283 | 8.54 (−1.37, 18.46), p = 0.091 | −4.0 (−16.00, 8.00), p = 0.514 |
| PG-SGASF score * | −0.87 (−2.69, 0.94), p = 0.346 | 0.57 (−1.42, 2.55), p = 0.575 | −1.20 (−2.98, 0.58), p = 0.186 |
| Weight † | −2.43 (−5.11, 0.25), p = 0.075 | −2.56 (−4.89, −0.23), p = 0.031 | 0.92 (−1.65, 3.50), p = 0.481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huggins, C.E.; Hanna, L.; Furness, K.; Silvers, M.A.; Savva, J.; Frawley, H.; Croagh, D.; Cashin, P.; Low, L.; Bauer, J.; et al. Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial. Nutrients 2022, 14, 3234. https://doi.org/10.3390/nu14153234
Huggins CE, Hanna L, Furness K, Silvers MA, Savva J, Frawley H, Croagh D, Cashin P, Low L, Bauer J, et al. Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial. Nutrients. 2022; 14(15):3234. https://doi.org/10.3390/nu14153234
Chicago/Turabian StyleHuggins, Catherine E., Lauren Hanna, Kate Furness, Mary Anne Silvers, June Savva, Helena Frawley, Daniel Croagh, Paul Cashin, Liang Low, Judy Bauer, and et al. 2022. "Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial" Nutrients 14, no. 15: 3234. https://doi.org/10.3390/nu14153234
APA StyleHuggins, C. E., Hanna, L., Furness, K., Silvers, M. A., Savva, J., Frawley, H., Croagh, D., Cashin, P., Low, L., Bauer, J., Truby, H., & Haines, T. P. (2022). Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial. Nutrients, 14(15), 3234. https://doi.org/10.3390/nu14153234






