Astragalus Shiitake—A Novel Functional Food with High Polysaccharide Content and Anti-Proliferative Activity in a Colorectal Carcinoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Crude Polysaccharides
2.2.2. Characterization of the Polysaccharide-Rich Extracts
2.2.3. Anti-Proliferative Activities in HCT-116 Cells
2.2.4. Statistical Analysis
3. Results
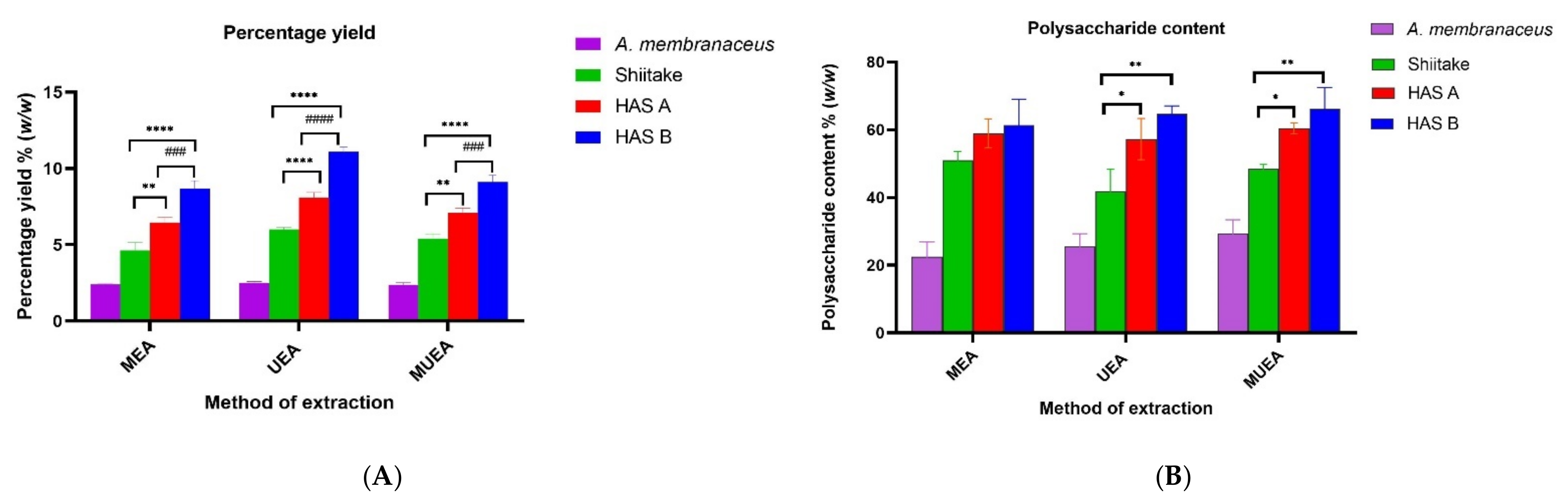
3.1. Effect of Different Extraction Methods on PRE Yield and Content of Total Polysaccharides
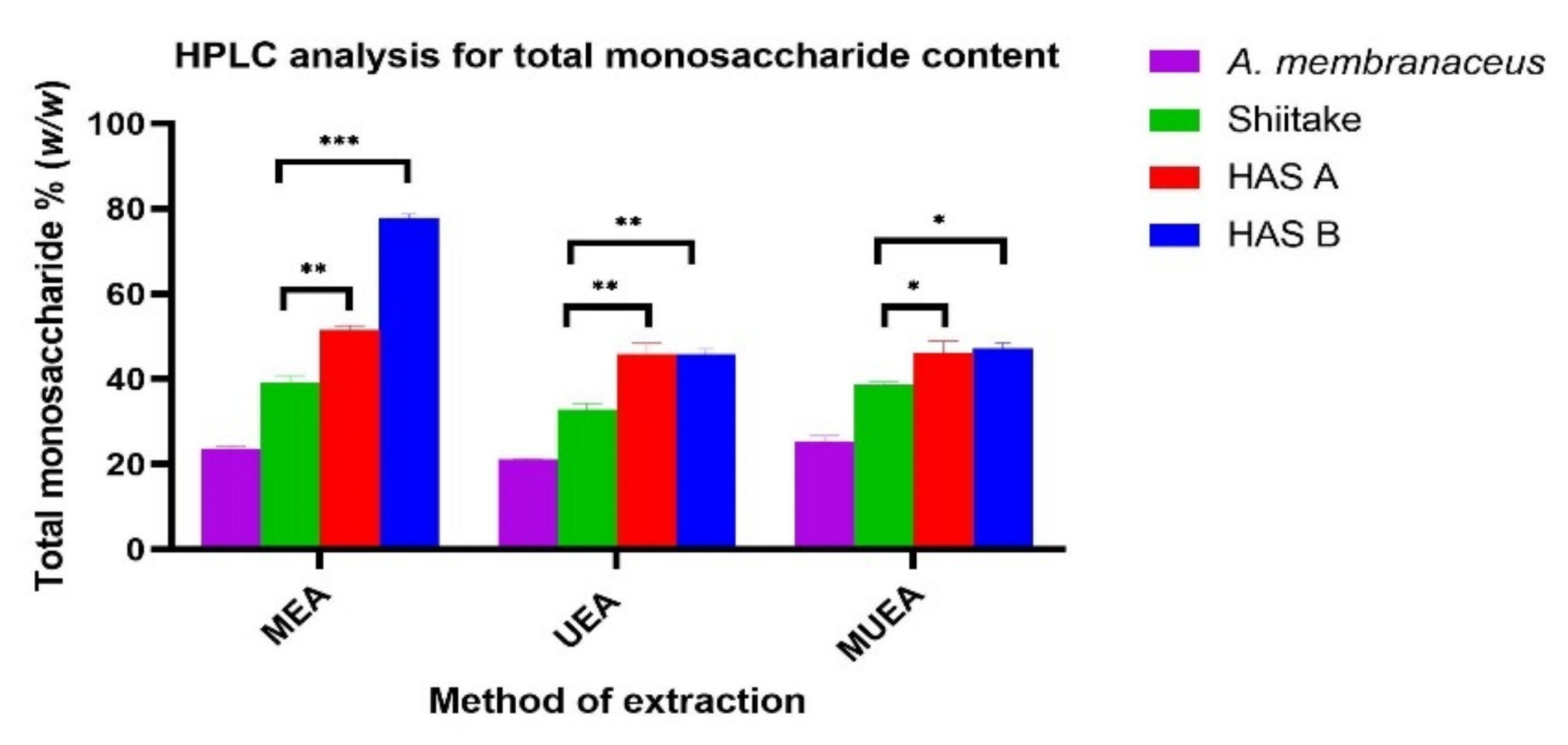
3.2. Effect of Different Extraction Methods on Monosaccharide Composition of Polysaccharide Extracts Analyzed by HPLC
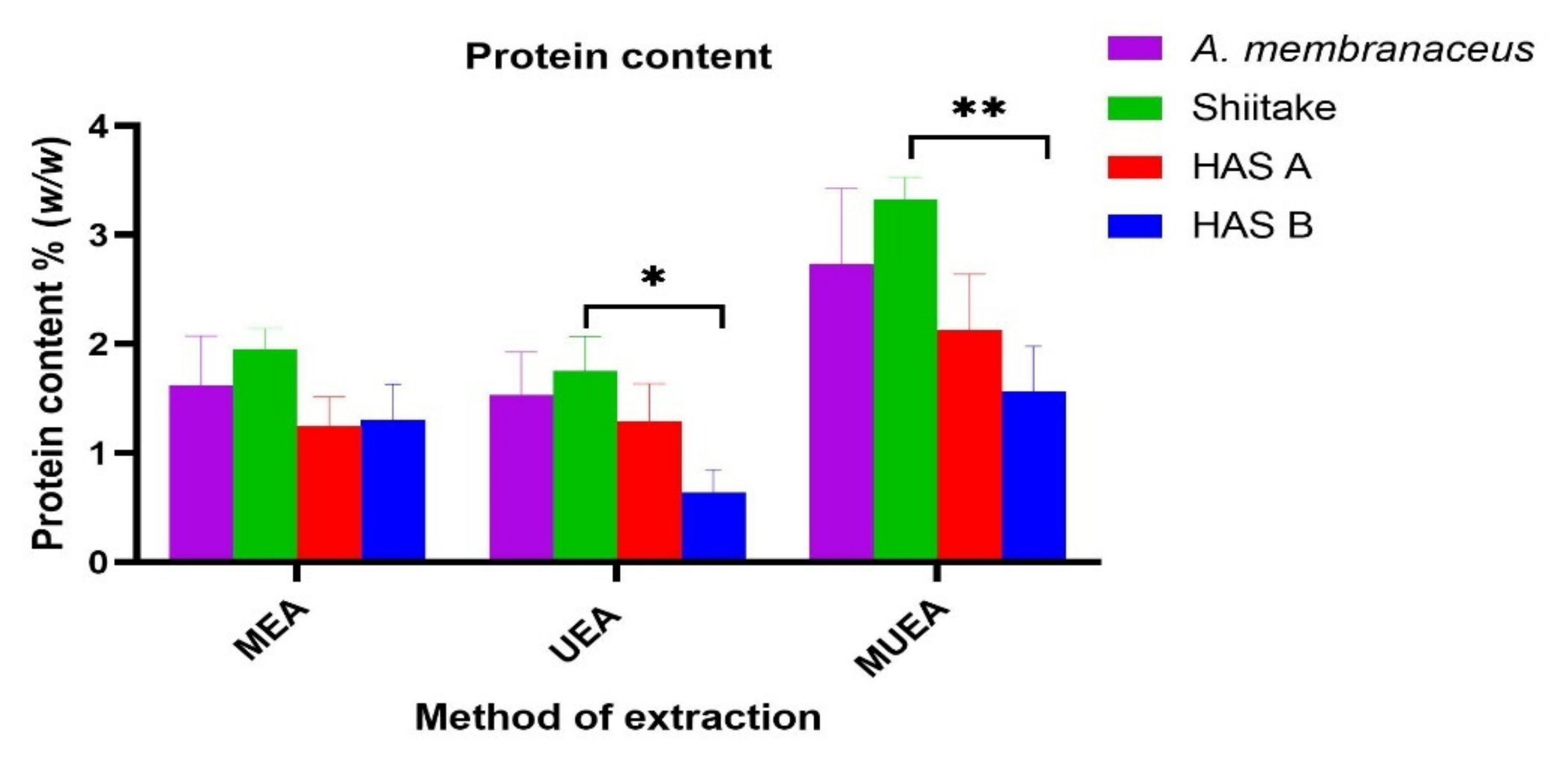
3.3. Effect of Different Extraction Methods on the Protein Content
3.4. Effect of Different Extraction Methods on Molecular Weight Profile of Polysaccharide-Rich Extracts
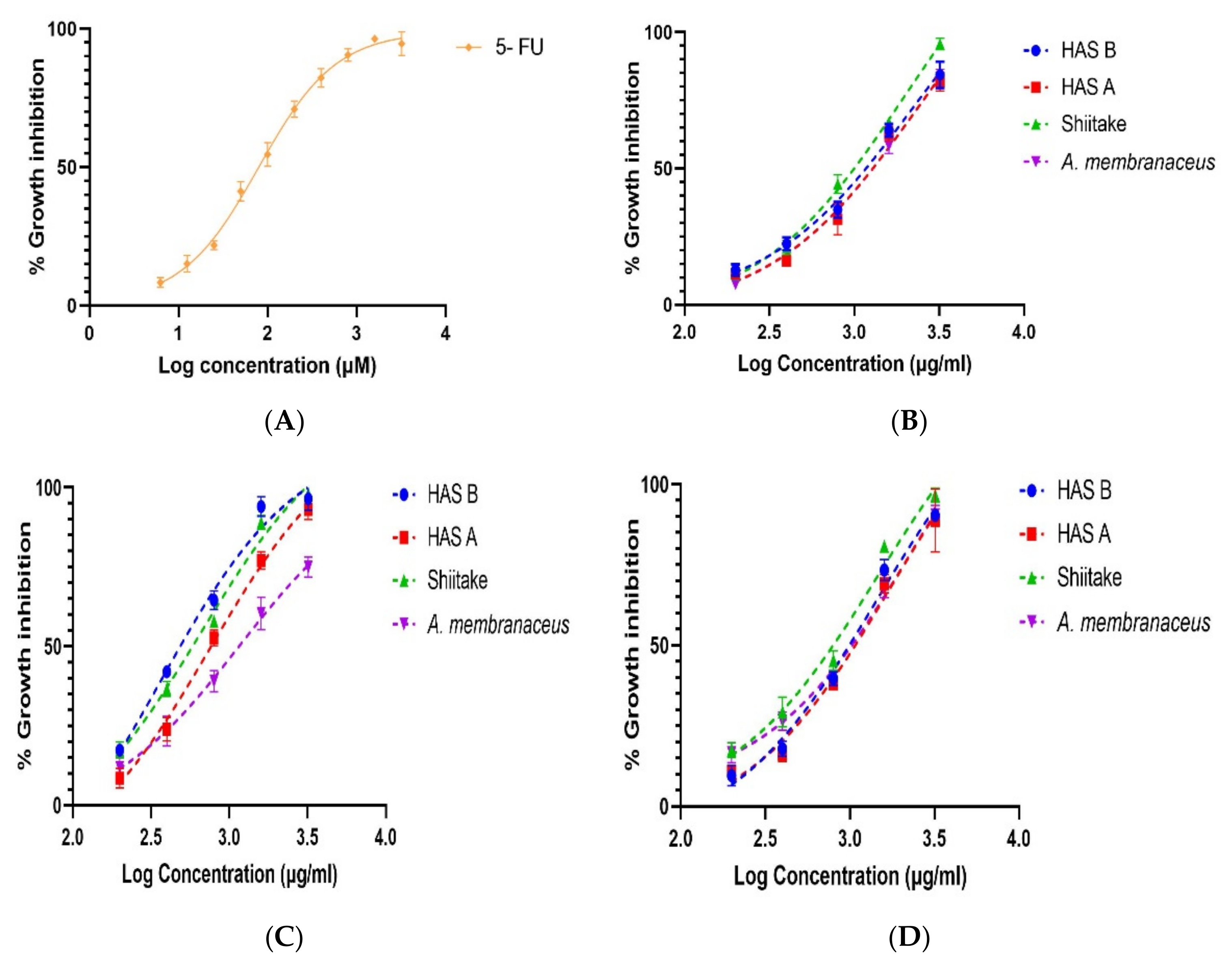
3.5. Effect of Different Extraction Methods on Anti-Proliferative Activity of PREs in HCT 116 Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hobbs, C. Medicinal value of Lentinus edodes (Berk.) Sing.(Agaricomycetideae). A literature review. Int. J. Med. Mushrooms 2000, 2, 1–16. [Google Scholar] [CrossRef]
- Miyaji, C.; Poersch, A.; Ribeiro, L.; Eira, A.; Colus, I. Shiitake (Lentinula edodes (Berkeley) Pegler) extracts as a modulator of micronuclei induced in HEp-2 cells. Toxicol. In Vitro 2006, 20, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Scola, G.; Scariot, F.J.; Dillon, A.J.; Moura, S.; Echeverrigaray, S.; Henriques, J.P.; Roesch-Ely, M. Extrinsic and intrinsic apoptotic responses induced by shiitake culinary-medicinal mushroom Lentinus edodes (Agaricomycetes) aqueous extract against a larynx carcinoma cell line. Int. J. Med. Mushrooms 2018, 20, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of α-and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, J.; Hu, H.; Li, Q.; Liu, Y.; Wang, K. Functional polysaccharide Lentinan suppresses human breast cancer growth via inducing autophagy and caspase-7-mediated apoptosis. J. Funct. Foods 2018, 45, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Han, Q.; Luo, Q.; Zhang, H.; Wang, Y. Construction of doxorubicin-conjugated lentinan nanoparticles for enhancing the cytotoxocity effects against breast cancer cells. Colloids Surf. Physicochem. Eng. Asp. 2019, 579, 123657. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Shu, Y.; Wang, H.; Zheng, Z.; Wang, J.; Wang, K. Induction of apoptosis in S180 tumour bearing mice by polysaccharide from Lentinus edodes via mitochondria apoptotic pathway. J. Funct. Foods 2015, 15, 151–159. [Google Scholar] [CrossRef]
- Yin, X.; Ying, J.; Li, L.; Zhang, H.; Wang, H. A meta-analysis of lentinan injection combined with chemotherapy in the treatment of nonsmall cell lung cancer. Indian J. Cancer 2015, 52, 29. [Google Scholar]
- Wang, X.-E.; Wang, Y.-H.; Zhou, Q.; Peng, M.; Zhang, J.; Chen, M.; Ma, L.-J.; Xie, G.-M. Immunomodulatory effect of lentinan on aberrant T subsets and cytokines profile in non-small cell lung cancer patients. Pathol. Oncol. Res. 2020, 26, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Wang, J.; Cheng, F.; Huang, X.; Cheng, Y.; Wang, K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016, 377, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, A.; Bonucci, M.; Pugliese, S.; D’ORTA, A.; Fioranelli, M. Polysaccharide from Lentinus edodes for integrative cancer treatment: Immunomodulatory effects on lymphocyte population. WCRJ 2016, 3, 1–7. [Google Scholar]
- Özçelik, E.; Pekşen, A. Hazelnut husk as a substrate for the cultivation of shiitake mushroom (Lentinula edodes). Bioresour. Technol. 2007, 98, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Diamantopoulou, P.A.; Philippoussis, A.N. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Budka, A.; Kalač, P.; Budzyńska, S.; Dawidowicz, L.; Hajduk, E.; Kozak, L.; Budzulak, J.; Sobieralski, K.; et al. The effect of different substrates on the growth of six cultivated mushroom species and composition of macro and trace elements in their fruiting bodies. Eur. Food Res. Technol. 2019, 245, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Kaleta, B.; Górski, A.; Zagożdżon, R.; Cieślak, M.; Kaźmierczak-Barańska, J.; Nawrot, B.; Klimaszewska, M.; Malinowska, E.; Górska, S.; Turło, J. Selenium-containing polysaccharides from Lentinula edodes—Biological activity. Carbohydr. Polym. 2019, 223, 115078. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Ke, Y.; Zeng, Y.-F.; Zhang, Y.-W.; Yu, H.-J. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017, 17, 115. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Hu, Y.; Wang, D.; Liu, J.; Zhang, J.; Zhao, X.; Liu, X.; Liu, C.; Yuan, J.; Ruan, S. Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohydr. Polym. 2012, 88, 68–74. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-J.; Yu, J.; Ji, H.-Y.; Zhang, H.-C.; Zhang, Y.; Liu, H.-P. Extraction of a novel cold-water-soluble polysaccharide from Astragalus membranaceus and its antitumor and immunological activities. Molecules 2018, 23, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Liang, Q.; Fenix, K.; Tamang, B.; Hauben, E.; Ma, L.; Zhang, W. Combining the Anticancer and Immunomodulatory Effects of Astragalus and Shiitake as an Integrated Therapeutic Approach. Nutrients 2021, 13, 2564. [Google Scholar] [CrossRef]
- Guo, S.; Ma, B.; Jiang, X.; Li, X.; Jia, Y. Astragalus polysaccharides inhibits tumorigenesis and lipid metabolism through miR-138-5p/SIRT1/SREBP1 pathway in prostate cancer. Front. Pharmacol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, S.; Xu, W.; Yu, B.; Wang, G.; Wang, H. Astragalus polysaccharide inhibits breast cancer cell migration and invasion by regulating epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020, 21, 1819–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Li, Q.; Mao, G.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Zhou, L.; Zhang, T.; Yang, J.; et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014, 101, 606–613. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Wang, J.; Wu, Z.-G.; Yang, J.-M.; Li, W.; Shen, L.-X. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2016, 93, 136–144. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Tang, X.; Fan, H.; Xie, X.; Wan, Q.; Wu, X.; Tang, J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohydr. Polym. 2016, 144, 263–270. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, X.; Huang, S.-Q.; Li, J.; Wang, X.; Tang, J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010, 46, 429–435. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Guo, M. Effects of Ultrasound Treatment on Extraction and Rheological Properties of Polysaccharides from Auricularia Cornea var. Li. Molecules 2019, 24, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Ramírez, A.; Smiderle, F.R.; Morales, D.; Iacomini, M.; Soler-Rivas, C. Strengths and weaknesses of the aniline-blue method used to test mushroom (1→ 3)-β-d-glucans obtained by microwave-assisted extractions. Carbohydr. Polym. 2019, 217, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzorqi, I.; Sudheer, S.; Lu, T.-J.; Manickam, S. Ultrasonically extracted β-d-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Yang, J.-M.; Liu, Y.-H.; Zhao, M.; Wang, J. Ultrasound assisted extraction of polysaccharides from Lentinus edodes and its anti-hepatitis B activity in vitro. Int. J. Biol. Macromol. 2018, 107, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Song, H.; Yang, Y.; Liu, Y.; Liu, Z.; Hu, H.; Zhang, Y. Optimization of microwave-assisted enzymatic extraction of polysaccharides from the fruit of Schisandra chinensis Baill. Int. J. Biol. Macromol. 2015, 76, 161–168. [Google Scholar] [CrossRef]
- Amigh, S.; Taghian Dinani, S. Combination of ultrasound-assisted aqueous enzymatic extraction and cooking pretreatment for date seed oil recovery. Heat Mass Transf. 2020, 56, 2345–2354. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Gao, H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 2018, 111, 446–454. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Wang, Q.; Wang, H.; Mei, Q. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005, 21, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Lorbeer, A.J.; Lahnstein, J.; Fincher, G.B.; Su, P.; Zhang, W. Kinetics of conventional and microwave-assisted fucoidan extractions from the brown alga, Ecklonia radiata. J. Appl. Phycol. 2015, 27, 2079–2087. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–24. [Google Scholar]
- You, L.; Gao, Q.; Feng, M.; Yang, B.; Ren, J.; Gu, L.; Cui, C.; Zhao, M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ma, G. Hypolipidemic effect of the polysaccharide from Pholiota nameko. Nutrition 2010, 26, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.A.; Hassan, A.I.; Mahmoud, M.G.; Asker, M.S. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complementary Altern. Med. 2016, 16, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Chen, G.; Chen, D.; Ye, H.; Zeng, X. The antidiabetic effect and potential mechanisms of natural polysaccharides based on the regulation of gut microbiota. J. Funct. Foods 2020, 75, 104222. [Google Scholar] [CrossRef]
- Cao, P.; Wu, S.; Wu, T.; Deng, Y.; Zhang, Q.; Wang, K.; Zhang, Y. The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohydr. Polym. 2020, 240, 116346. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, M.; Liu, S.; Yu, Q.; Chen, Y.; Xie, J. Ameliorative effect of Cyclocarya paliurus polysaccharides against carbon tetrachloride induced oxidative stress in liver and kidney of mice. Food Chem. Toxicol. 2020, 135, 111014. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Lai, X.; Xia, W.; Wei, J.; Ding, X. Therapeutic effect of Astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose-Response 2017, 15, 1559325816685182. [Google Scholar] [CrossRef] [Green Version]
- Chao, L.; Zhou, J.; Zhu, X.-X.; Ni, W.-P.; Wang, H.-D.; Wang, Q. Effect of Astragalus Polysaccharides on Proliferation and Cell Cycle of Human Gastric Carcinoma Cell Line MKN45. Chin. Arch. Tradit. Chin. Med. 2012, 30, 2474–2477. [Google Scholar]
- Lv, J.; Zhang, Y.; Tian, Z.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κB activation. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Chen, S.; Li, R.; Zhou, H.; Wu, H.; Song, H. Influences of extraction methods on physicochemical characteristics and activities of Astragalus cicer L. polysaccharides. Process Biochem. 2018, 73, 220–227. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, Y.; Xiong, Z. Effect of drying method on physicochemical properties and antioxidant activities of Hohenbuehelia serotina polysaccharides. Process Biochem. 2016, 51, 1100–1108. [Google Scholar] [CrossRef]
- Jeff, I.B.; Yuan, X.; Sun, L.; Kassim, R.M.; Foday, A.D.; Zhou, Y. Purification and in vitro anti-proliferative effect of novel neutral polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2013, 52, 99–106. [Google Scholar] [CrossRef]
- Unursaikhan, S.; Xu, X.; Zeng, F.; Zhang, L. Antitumor activities of O-sulfonated derivatives of (1→ 3)-α-D-glucan from different Lentinus edodes. Biosci. Biotechnol. Biochem. 2006, 70, 38–46. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between modification, conformation and functional activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Gu, J.; Ji, C.; Duan, Y.; Zhang, H. Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2019, 123, 1002–1011. [Google Scholar] [CrossRef]
- Hao, L.; Sheng, Z.; Lu, J.; Tao, R.; Jia, S. Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydr. Polym. 2016, 141, 54–59. [Google Scholar] [CrossRef]
- Lemmon, H.R.; Sham, J.; Chau, L.A.; Madrenas, J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: Characterization of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 2012, 142, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Method of Extraction | Extracts | Monosaccharides Concentration μg/mL ± S.D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rib | Rha | GlcAc | GalAc | Gluc | Gal | Xyl | Ara | Fuc | ||
| MEA | A. membranaceus | 60.0 ± 0.8 | 14.8 ± 0.2 | 43.6 ± 1.1 | 254.5 ± 7.1 | 61.1 ± 2 | 730.4 ± 15 | 303.6 ± 4.9 | 59.8 ± 1.1 | 209.8 ± 3 | 18.5 ± 0.3 |
| Shiitake | 194.5 ± 6.3 | 13.0 ± 1.5 | 9.6 ± 0.8 | 392.8 ± 37 | 22.2 ± 2.4 | 1352.7 ± 47 | 785.1 ± 28.3 | 22.4 ± 0.5 | 3.2 ± 0.7 | 109.2 ± 4.1 | |
| HAS-A | 158.6 ± 4.2 ## | 24.3 ± 1.8 | 11.8 ± 0.3 | 566.4 ± 23.9 # | 13.7 ± 9.6 | 2235.4 ± 70 ### | 688.9 ± 22 | 17.9 ± 3.5 | 5.7 ± 0.2 | 97.6 ± 2.7 | |
| HAS-B | 174.6 ± 0.8 * | 37.8 ± 3.6 | 11.1 ± 2.4 | 858.0 ± 6.6 *** | 21.4 ± 12.1 | 3581.7 ± 27 **** | 911.1 ± 2.2 | 17.1 ± 4.6 | 3.6 ± 0.3 | 156.0 ± 6 | |
| UEA | A. membranaceus | 67.9 ± 0.6 | 8.5 ± 0.4 | 53.4 ± 1.2 | 165.1 ± 1 | 49.5 ± 0.2 | 634.8 ± 2.5 | 374.9 ± 6.7 | 30.9 ± 0.1 | 192.9 ± 3.2 | 19.0 ± 0.2 |
| Shiitake | 171.3 ± 1.9 | 7.7 ± 0.6 | 8.9 ± 1.1 | 346.6 ± 0.9 | 25.1 ± 1.5 | 1131.7 ± 2.4 | 663.0 ± 36 | 24.4 ± 1.9 | 3.4 ± 0.3 | 90.8 ± 4.7 | |
| HAS-A | 160.3 ± 1.9 # | 9.5 ± 1.3 | 10.1 ± 1.4 | 487.3 ± 1.9 #### | 21.1 ± 3.9 | 1876.9 ± 2.6 #### | 736.6 ± 41.4 | 19.4 ± 0.7 | 4.7 ± 1.3 | 105.8 ± 6.2 | |
| HAS-B | 127.1 ± 1.5 *** | 10.0 ± 0.1 | 13.9 ± 13.1 | 551.1 ± 1.5 **** | 10.7 ± 1.2 | 2084.1 ± 1.5 **** | 557.1 ± 63 | 12.8 ± 1.2 | 5.0 ± 0.6 | 100.7 ± 12 | |
| MUEA | A. membranaceus | 53.7 ± 2.9 | 9.1 ± 1.6 | 44.8 ± 2.5 | 298.3 ± 25.9 | 45.1 ± 5 | 901.7 ± 37.7 | 313.1 ± 14.6 | 27.5 ± 0.48 | 169.0 ± 9.1 | 16.3 ± 0.5 |
| Shiitake | 193.4 ± 3.8 | 9.7 ± 2.5 | 9.2 ± 0.8 | 385.7 ± 3.8 | 25.8 ± 0.2 | 1365.3 ± 23.8 | 749.2 ± 15.1 | 21.5 ± 0.46 | 3.4 ± 1.3 | 103.5 ± 2 | |
| HAS-A | 149.2 ± 7.3 # | 40.7 ± 5.5 | 7.0 ± 0.9 | 501.4 ± 43 | 19.0 ± 8.7 | 1892.1 ± 97 # | 697.0 ± 35.5 | 17.5 ± 2.2 | 3.9 ± 2.2 | 101.9 ± 5.8 | |
| HAS-B | 94.8 ± 9.4 ** | 29.2 ± 5.3 | 10.4 ± 0.2 | 533.1 ± 40.8 * | 11.0 ± 2.5 | 2066.0 ± 61 * | 512.9 ± 45.3 | 10.2 ± 0.21 | 3.5 ± 0.8 | 89.8 ± 8 | |
| Extracts | Major Peaks for HAS-B | Major Peaks for HAS-A | Major Peaks for Shiitake | Major Peaks for Astragalus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rt, mins | % Concen-tration | Mw, kDa | Rt, mins | % Concen-tration | Mw, kDa | Rt, mins | % Concen-tration | Mw, kDa | Rt, mins | % Concen-tration | Mw, kDa | |
| MEA | 12.885 | 12.901 *** | 813.967 | 12.64 | 0.031 | 1092.567 | 16.514 | 80.829 | 10.399 | 15.055 | 1.953 | 60.021 |
| 16.015 | 33.56 | 18.94 | 16.693 | 72.565 | 8.387 | 17.605 | 10.873 | 2.804 | 16.665 | 56.546 | 8.674 | |
| 17.037 | 22.164 | 5.547 | 17.604 | 12.339 | 2.807 | 18.307 | 6.821 | 1.206 | 17.82 | 26.399 | 2.165 | |
| 17.655 | 10.848 | 2.64 | 18.315 | 7.68 | 1.195 | 18.318 | 10.229 | 1.19 | ||||
| 18.316 | 10.973 | 1.193 | ||||||||||
| UEA | 12.866 | 12.983 ** | 832.763 | 12.734 | 0.194 | 975.885 | 16.35 | 20.508 | 12.72 | 14.629 | 4.399 | 100.135 |
| 16.028 | 25.044 | 18.646 | 17.687 | 78.288 | 2.541 | 17.17 | 42.988 | 4.73 | 17.088 | 29.413 | 5.218 | |
| 17.719 | 36.966 | 2.445 | 18.326 | 16.625 | 1.179 | 17.5 | 22.04 | 3.19 | 17.706 | 39.937 | 2.483 | |
| 18.344 | 15.217 | 1.154 | 18.41 | 12.25 | 1.05 | 18.333 | 11.824 | 1.169 | ||||
| MUEA | 12.876 | 20.234 **** | 822.817 | 12.787 | 4.685 ## | 915.679 | 11.375 | 75.276 | 10.5 | 15.304 | 1.029 | 44.501 |
| 15.953 | 53.484 | 20.404 | 16.503 | 70.536 | 10.537 | 16.506 | 12.763 | 2.698 | 16.733 | 57.15 | 7.993 | |
| 17.638 | 8.581 | 2.695 | 17.604 | 9.554 | 2.807 | 17.637 | 10.199 | 1.213 | 17.671 | 25.882 | 2.59 | |
| 18.305 | 6.126 | 1.209 | 18.29 | 4.454 | 1.231 | 18.302 | 18.311 | 12.152 | 1.2 | |||
| Extracts | IC50 (mg/mL) ± S.D. (n = 3) | |||
|---|---|---|---|---|
| HAS-B | HAS-A | Shiitake | A. membranaceus | |
| MUEA | 1.444 ± 0.244 | 1.78 ± 0.443 | 1.491 ± 0.379 | 2.417 ± 0.298 |
| MEA | 2.224 ± 0.206 | 2.395 ± 0.52 | 1.975 ± 0.3 | 2.346 ± 0.181 |
| UEA | 0.367 ± 0.044 * | 0.76 ± 0.122 ## | 0.659 ± 0.059 | 1.181 ± 0.145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamang, B.; Liang, Q.; Balakrishnan, B.; Peng, S.; Zhang, W. Astragalus Shiitake—A Novel Functional Food with High Polysaccharide Content and Anti-Proliferative Activity in a Colorectal Carcinoma Cell Line. Nutrients 2022, 14, 2333. https://doi.org/10.3390/nu14112333
Tamang B, Liang Q, Balakrishnan B, Peng S, Zhang W. Astragalus Shiitake—A Novel Functional Food with High Polysaccharide Content and Anti-Proliferative Activity in a Colorectal Carcinoma Cell Line. Nutrients. 2022; 14(11):2333. https://doi.org/10.3390/nu14112333
Chicago/Turabian StyleTamang, Bunu, Qi Liang, Biju Balakrishnan, Su Peng, and Wei Zhang. 2022. "Astragalus Shiitake—A Novel Functional Food with High Polysaccharide Content and Anti-Proliferative Activity in a Colorectal Carcinoma Cell Line" Nutrients 14, no. 11: 2333. https://doi.org/10.3390/nu14112333
APA StyleTamang, B., Liang, Q., Balakrishnan, B., Peng, S., & Zhang, W. (2022). Astragalus Shiitake—A Novel Functional Food with High Polysaccharide Content and Anti-Proliferative Activity in a Colorectal Carcinoma Cell Line. Nutrients, 14(11), 2333. https://doi.org/10.3390/nu14112333






