Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
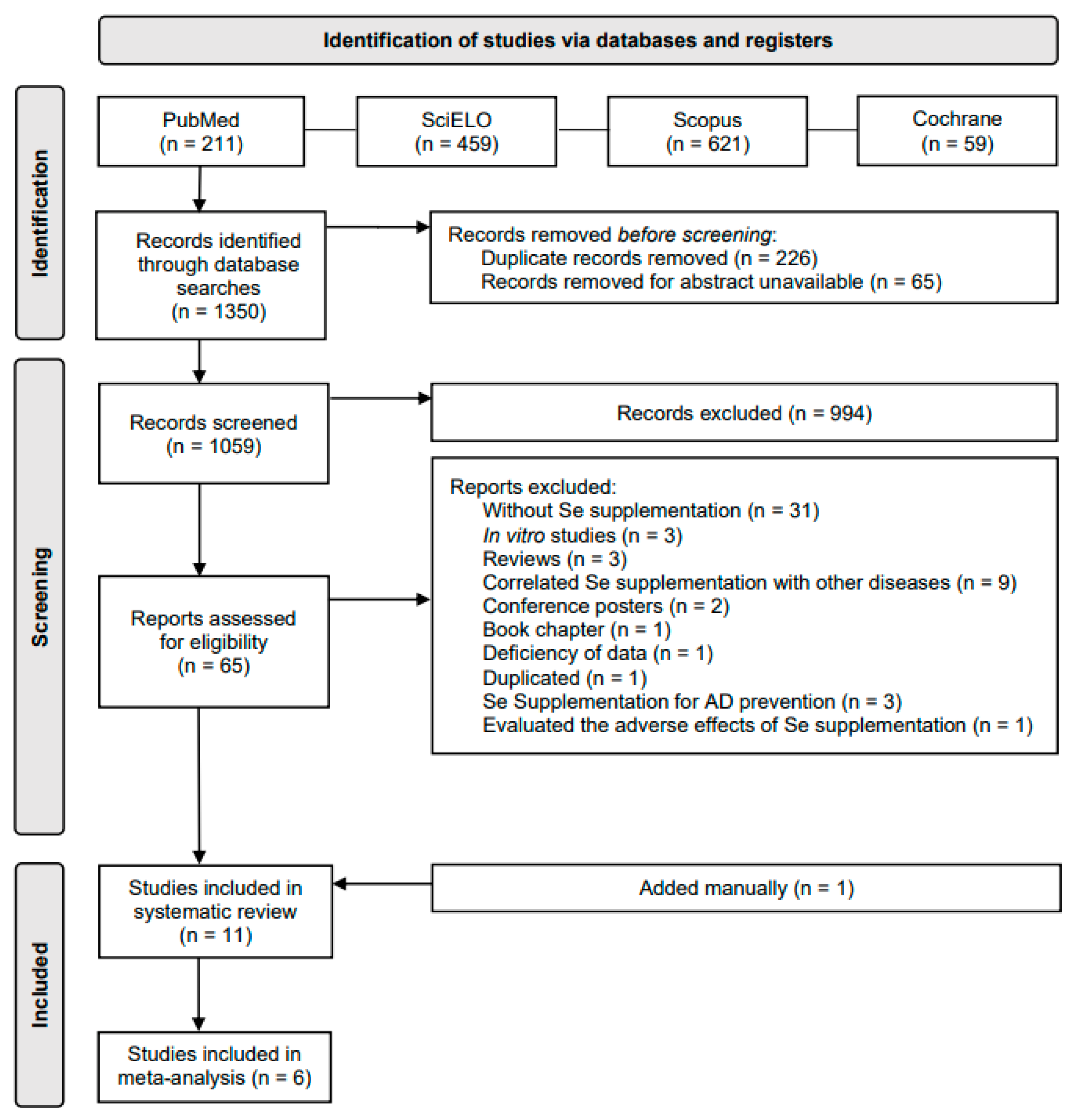
3.1. Selection of Papers
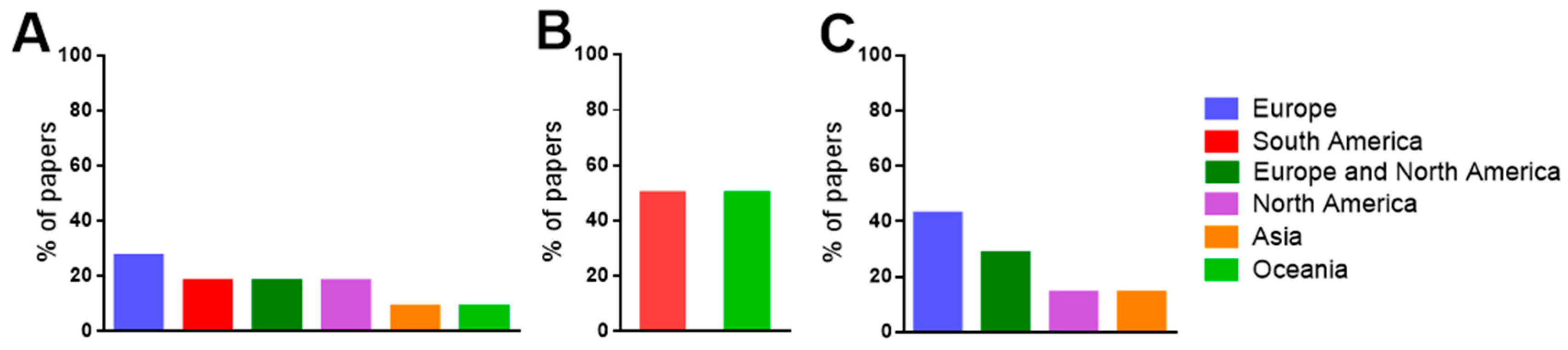
3.2. Characteristics of the Systematically Selected Papers
3.3. Patient Characteristics
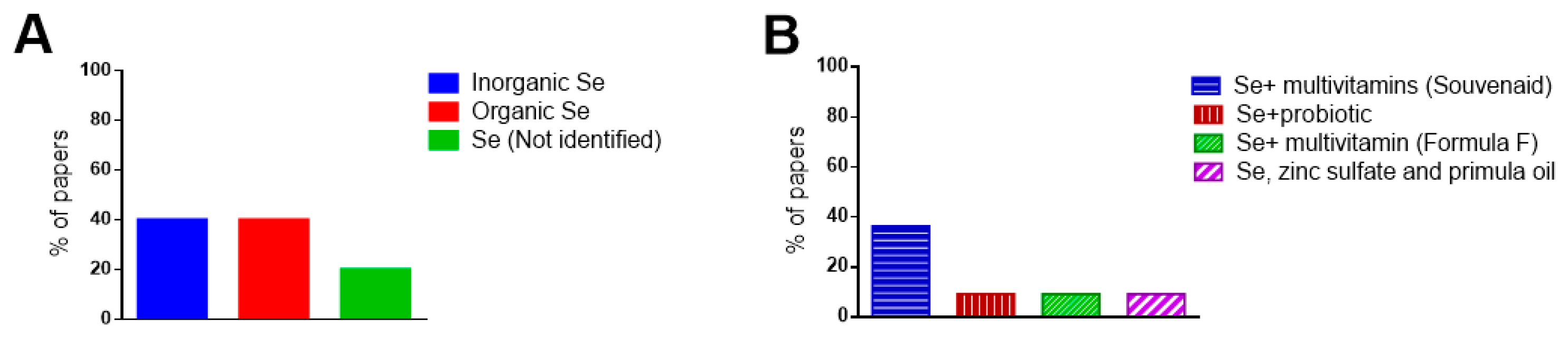
3.4. Selenium Supplementation
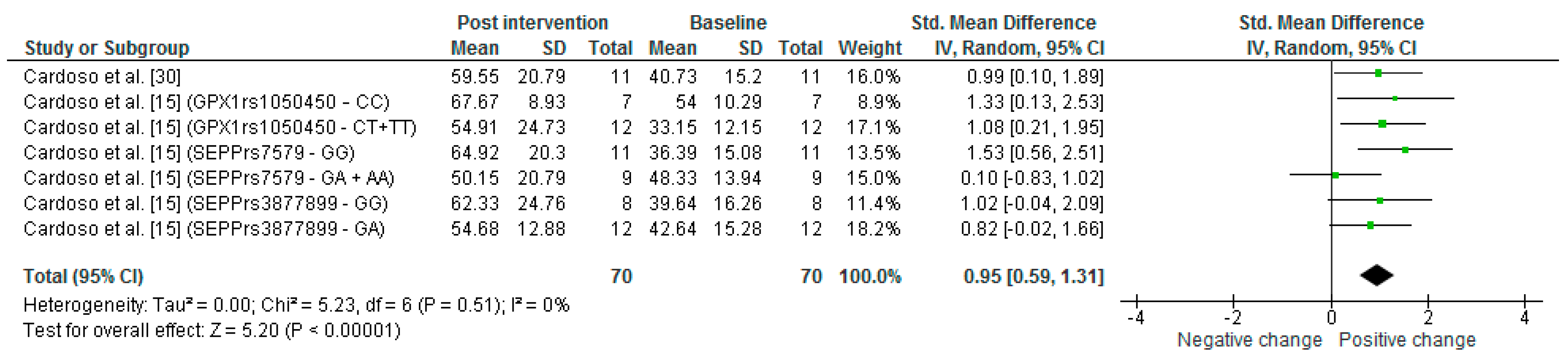
3.5. Improvement of Se Levels
3.6. Oxidative Stress
3.7. Cognitive Performance
3.8. Risk of Bias
4. Discussion
4.1. Improvement of Se Levels
4.2. Oxidative Stress
4.3. Cognitive Performance
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, C.S.; Piccoli, B.C.; Aschner, M.; Rocha, J.B.T. Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In Neurotoxicity of Metals; Aschner, M., Costa, L., Eds.; Springer: Cham, Switzerland, 2017; Volume 18, pp. 53–83. [Google Scholar] [CrossRef]
- Rocha, J.B.T.; Piccoli, B.C.; Oliveira, C.S. Biological and chemical interest in selenium: A brief historical account. Arkivoc 2016, 2017, 457–491. [Google Scholar] [CrossRef]
- Rocha, J.B.T.; Oliveira, C.S.; Nogara, P.A. Toxicology and anticancer activity of synthetic organoselenium compounds. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Jain, V.K., Priyadarsini, I., Eds.; Royal Society of Chemistry: London, UK, 2017; pp. 342–376. [Google Scholar] [CrossRef]
- Nogara, P.A.; Oliveira, C.S.; Rocha, J.B.T. Chemistry and pharmacology of synthetic organoselenium compounds. In Organoselenium Chemistry; Ranu, B.C., Banerjee, B., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 305–346. [Google Scholar] [CrossRef]
- Nogara, P.A.; Schimitz, G.L.; Costa, N.S.; Kandem, J.P.; Rocha, J.B.; Oliveira, C.S. The evolution of selenium and mercury research from 1700 to 2017 based on bibliometric analysis. Res. Soc. Dev. 2020, 9, e150922177. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Piccoli, B.C.; Nogara, P.A.; Pereira, M.E.; Carvalho, K.A.T.; Skalny, A.V.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T. Selenium neuroprotection in neurodegenerative disorders. In Handbook of Neurotoxicity, 2nd ed.; Kostrzewa, R.M., Ed.; Springer: Cham, Switzerland, 2021; Volume 1, p. 35. [Google Scholar] [CrossRef]
- Peters, M.M.; Hill, K.E.; Burk, R.F.; Weeber, E.J. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol. Neurodegener. 2006, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Byrns, C.N.; Pitts, M.W.; Gilman, C.A.; Hashimoto, A.C.; Berry, M.J. Mice lacking selenoprotein P and selenocysteine lyase exhibit severe neurological dysfunction, neurodegeneration, and audio-genic seizures. J. Biol. Chem. 2014, 289, 9662–9674. [Google Scholar] [CrossRef] [PubMed]
- Rueli, R.H.; Parubrub, A.C.; Dewing, A.S.; Hashimoto, A.C.; Bellinger, M.T.; Weeber, E.J.; Uyehara-Lock, J.H.; White, L.R.; Berry, M.J.; Bellinger, F.P. Increased selenoprotein P in choroid plexus and cerebrospinal fluid in Alzheimer’s disease brain. J. Alzheimer’s Dis. 2015, 44, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Garlet, Q.I.; Haskel, M.; Pereira, R.P.; Silva, W.; Rocha, J.; Oliveira, C.S.; Bonini, J.S. Delta-aminolevulinate dehydratase and glutathione peroxidase activity in Alzheimer’s disease: A case-control study. EXCLI J. 2019, 18, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Ayton, S.; Bush, A.I. The essential elements of Alzheimer’s disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s Disease: Implications for therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Busse, A.L.; Hare, D.J.; Cominetti, C.; Horst, M.A.; McColl, G.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Pro198Leu polymorphism affects the selenium status and GPx activity in response to Brazil nut intake. Food Funct. 2016, 7, 825–833. [Google Scholar] [CrossRef]
- Gomersall, T.; Smith, S.K.; Blewett, C.; Astell, A. ‘It’s definitely not Alzheimer’s’: Perceived benefits and drawbacks of a mild cognitive impairment diagnosis. Br. J. Health Psychol. 2017, 22, 786–804. [Google Scholar] [CrossRef]
- Radanovic, M.; Stella, F.; Fortaleza, O.V. Comprometimento cognitivo leve. Rev. De Med. 2015, 94, 162–168. [Google Scholar] [CrossRef][Green Version]
- Ganguli, M.; Jia, Y.; Hughes, T.F.; Snitz, B.E.; Chang, C.H.; Berman, S.B.; Sullivan, K.J.; Kamboh, M.I. Mild cognitive impairment that does not progress to dementia: A Population-based study. J. Am. Geriatr. Soc. 2019, 67, 232–238. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.P.; Pilotto, A. Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev. Neurother. 2011, 11, 677–708. [Google Scholar] [CrossRef]
- Correia, A.; Filipe, J.; Santos, A.; Graça, P. Nutrição e Doença de Alzheimer. Programa Nacional para a Promoção da Alimentação Saudável Nutrição e Doença de Alzheimer; Direção-Geral da Saúde: Lisbon, Portugal, 2015; pp. 1–77. [Google Scholar]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; Jager, C.A. Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef]
- Fenech, M. Vitamins associated with brain aging, mild cognitive impairment, and Alzheimer Disease: Biomarkers, epidemiological and experimental evidence, plausible mechanisms, and knowledge gaps. Adv. Nutr. 2017, 8, 958–970. [Google Scholar] [CrossRef]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s Disease—Review of the latest reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- Yan, X.; Liu, K.; Sun, X.; Qin, S.; Wu, M.; Qin, L.; Wang, Y.; Li, Z.; Zhong, X.; Wei, X. A cross-sectional study of blood selenium concentration and cognitive function in elderly Americans: National Health and Nutrition Examination Survey 2011–2014. Ann. Hum. Biol. 2020, 47, 610–619. [Google Scholar] [CrossRef]
- Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; Brodtmann, A.; Collins, S.; et al. A phase IIa randomized control trial of VEL015 (sodium selenate) in mild-moderate Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 54, 223–232. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Bernardo, W.M.; Nobre, M.R.C.; Jatene, F.B. A prática clínica baseada em evidências: Parte II—buscando as evidências em fontes de informação. Rev. Bras. Reumatol. 2004, 50, 104–108. [Google Scholar] [CrossRef]
- Alarcon-Gil, M.T.; Osorio Toro, S.; Baena-Caldas, G.P. The evidence-based medicine PICO strategy applied to dentistry using MeSH, Emtree and DeCS. Rev. Fac. Odontol. Univ. Antioq. 2019, 31, 91–101. [Google Scholar] [CrossRef]
- Cochrane Collaboration. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials; Cochrane: London, UK, 2021. [Google Scholar]
- Cardoso, B.R.; Apolinário, D.; Bandeira, V.S.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2015, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; et al. Supranutritional sodium selenate supplementation delivers selenium to the central nervous system: Results from a randomized controlled pilot trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, A.G.; Prior, C.A.; Corrigan, F.M. Dietary supplementation with zinc sulphate, sodium selenite and fatty acids in early dementia of Alzheimer’s Type. J. Nutr. Med. 1990, 1, 259–266. [Google Scholar] [CrossRef]
- Cornelli, U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener. Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Kamphuis, P.J.; Verhey, F.R.; Olde Rikkert, M.G.; Wurtman, R.J.; Wilkinson, D.; Twisk, J.W.; Kurz, A. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimer’s Dement. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Scheltens, P.; Twisk, J.W.; Blesa, R.; Scarpini, E.; von Arnim, C.A.; Bongers, A.; Harrison, J.; Swinkels, S.H.; Stam, C.J.; de Waal, H.; et al. Efficacy of Souvenaid in mild Alzheimer’s disease: Results from a randomized, controlled trial. J. Alzheimer’s Dis. 2012, 31, 225–236. [Google Scholar] [CrossRef]
- Shah, R.C.; Kamphuis, P.J.; Leurgans, S.; Swinkels, S.H.; Sadowsky, C.H.; Bongers, A.; Rappaport, S.A.; Quinn, J.F.; Wieggers, R.L.; Scheltens, P.; et al. The S-connect study: Results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 59. [Google Scholar] [CrossRef]
- Rijpma, A.; Meulenbroek, O.; van Hees, A.M.; Sijben, J.W.; Vellas, B.; Shah, R.C.; Bennett, D.A.; Scheltens, P.; Olde Rikkert, M.G. Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 51. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004, 292, 2237–2242. [Google Scholar] [CrossRef]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Ong, T.P.; Jacob-Filho, W.; Jaluul, O.; Freitas, M.I.; Cozzolino, S.M. Nutritional status of selenium in Alzheimer’s disease patients. Br. J. Nutr. 2010, 103, 803–806. [Google Scholar] [CrossRef]
- Reddy, V.S.; Bukke, S.; Dutt, N.; Rana, P.; Pandey, A.K. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer’s disease: A metal meta-analysis (AMMA study-I). J. Trace Elem. Med. Biol. 2017, 42, 68–75. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium-a fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F.; Campbell, D.R.; Rea, H.M. Effect of prolonged supplementation with daily supplements of selenomethionine and sodium selenite on glutathione peroxidase activity in blood of New Zealand residents. Am. J. Clin. Nutr. 1982, 36, 24–31. [Google Scholar] [CrossRef]
- Rea, H.M.; Thomson, C.D.; Campbell, D.R.; Robinson, M.F. Relation between erythrocyte selenium concentrations and glutathione peroxidase (EC 1.11.1.9) activities of New Zealand residents and visitors to New Zealand. Br. J. Nutr. 1979, 42, 201–208. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Gustaw-Rothenberg, K.; Kowalczuk, K.; Stryjecka-Zimmer, M. Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 2010, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Markesbery, W.R.; Shao, C.; Lovell, M.A. Seleno-L-methionine protects against β-amyloid and iron/hydrogen peroxide-mediated neuron death. Antioxid. Redox Signal. 2007, 9, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; Yamashita, E.T.; Chiappetta, A.L.M.L. Teste de fluência verbal no adulto e no idoso: Verificação da aprendizagem verbal. Rev. CEFAC 2008, 10, 443–451. [Google Scholar] [CrossRef][Green Version]
- Cecato, J.F.; Melo, B.; Moraes, G.C.; Martinelli, J.E.; Montiel, J.M. Accuracy of praxis test from Cambridge Cognitive Examination (CAMCOG) for Alzheimer’s disease: A cross-sectional study. Sao Paulo Med. 2018, 136, 390–397. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]


| Reference | Country | Pathology | Groups | Study Type | Characteristics of the Participants | Se Levels (μg/L) | Cognitive Tests (Score) |
|---|---|---|---|---|---|---|---|
| Cardoso et al. [30] | Brazil | MCI | Se supplementation group consumed one Brazil nut daily for 24 weeks. | Randomized clinical trial | Sex Control Female = 6 Male = 3 Se supplementation Female = 8 Male = 3 Age Control 77.6 ± 6.6 Se supplementation 77.7 ± 4.3 | Plasma Control Pre: 50.0 ± 15.5 Post: 47.8 ± 11.7 Se supplementation Pre: 56.2 ± 18.3 Post: 290.6 ± 74.6 Erythrocyte Control Pre: 50.8 ± 21.0 Post: 33.5 ± 16.1 Se supplementation Pre: 59.5 ± 20.6 Post: 574.6 ± 181.4 | COWAT Control Pre: 16.3 ± 3.7 Post: 14.1 ± 3.9 Se supplementation Pre: 12.8 ± 3.3 Post: 14.1 ± 3.9 Constructional praxis Control Pre: 8.7 ± 2.6 Post: 8.3 ± 2.4 Se supplementation Pre: 7.7 ± 2.3 Post: 9.2 ± 2.2 |
| Cardoso et al. [15] | Brazil | MCI | Se supplementation group consumed one Brazil nut daily for 24 weeks. | Randomized clinical trial | Sex GPX1-rs1050450 CC Female = 7 Male = 1 CT + CT Female = 7 Male = 5 SEPP-rs7579 GG Female = 6 Male = 5 GA + AA Female = 7 Male = 1 SEPP-rs3877899 GG Female = 8 Male = 4 GA Female = 6 Male = 2 Age GPX1rs1050450 CC 77.3 ± 7.1 CT + TT 77.83 ± 4.1 SEPPrs7579 GG 77.4 ± 4.4 GA + AA 78.0 ± 6.5 S3877899 GG 76.7 ± 3.6 GA 79.1 ± 7.2 | Plasma GPX1-rs1050450 CC Pre: 49.9 ± 10.3 Post: 246.2 ± 54.0 CT + CT Pre: 59.9 ± 21.6 Post: 315.9 ± 75.8 SEPP-rs7579 GG Pre: 59.6 ± 21.3 Post: 277.8 ± 62.1 GA + AA Pre: 50.3 ± 11.7 Post: 312.9 ± 98.9 SEPP-rs3877899 GG Pre: 52.8 ± 12.0 Post: 299.9 ± 68.7 GA Pre: 62.3 ± 27.5 Post: 274.2 ± 92.4 Erythrocyte GPX1-rs1050450 CC Pre: 65.1 ± 13.6 Post: 519.2 ± 239.8 CT + TT Pre: 56.4 ± 24.2 Post: 606.2 ± 151.2 SEPP-rs7579 GG Pre: 58.2 ± 22.5 Post: 536.6 ± 116.9 GA + AA Pre: 61.9 ± 19.8 Post: 640.9 ± 270.4 SEPP-rs3877899 GG Pre: 57.1 ± 16.9 Post: 607.4 ± 177.6 GA Pre: 63.7 ± 28.4 Post: 517.0 ± 199.1 | - |
| Malpas et al. [25] | Australia | AD | Se nutritional dose (control): 320 μg sodium selenate 3 times a day for 24 weeks. Se supranutritional dose: 10 mg sodium selenate 3 times a day for 24 weeks. | Phase IIa randomized control trial | Sex Control Female = 8 Male = 12 Supranutritional Female = 9 Male = 11 Age Control 71 Supranutritional 70 | - | MMSE Control Pre: 20 Post: 19 Supranutritional Pre: 20 Post: 19 ADAS-Cog Control Pre: 22.1 Post: 22.2 Supranutritional Pre:19.7 Post: 22.3 COWAT Control Pre: 29 Post: 28 Supranutritional Pre: 25 Post: 20 |
| Cardoso et al. [31] | Australia | AD | Se nutritional dose (control): 320 μg sodium selenate 3 times a day for 24 weeks. Se supranutritional dose: 10 mg sodium selenate 3 times a day for 24 weeks. | Randomized, double-blind, placebo-controlled pilot study | Sex Nutritional Female = 4 Male = 4 Supranutritional Female = 15 Male = 4 Age Nutritional 73.4 ± 5.5 Supranutritional 69.5 ± 8.3 | Serum Nutritional Pre: 122.2 ± 26.3 Post: 176.7 ± 46.2 Supranutritional Pre: 145.4 ± 28.8 Post: 858.3 ± 447.1 Cerebrospinal fluid Nutritional Pre: 1.6± 0.6 Post: 2.5± 0.7 Supranutritional Pre: 1.4 ± 0.5 Post: 20.2 ± 9.1 | - |
| Tamtaji et al. [12] | Iran | AD | Placebo: placebo (starch) for 12 weeks. Selenium: received selenium 200mg/day for 12 weeks. | Randomized, double-blind, controlled clinical trial | Sex Uninformed Age Placebo 78.5 ± 8.0 Selenium 78.8 ± 10.2 | - | MMSE Placebo Pre:9.3 ± 4.1 Post: 9.1 ± 4.4 Selenium Pre:9.9 ± 4.0 Post:10.4 ± 4.2 |
| Reference | Country | Pathology | Groups | Study Type | Characteristics of the Participants | Se Levels (μg/L) | Cognitive Test (Score) |
|---|---|---|---|---|---|---|---|
| Van Rhijn et al. [32] | United Kingdom | MCI | Olive Oil (control): six capsules of olive oil and one placebo tablet for 20 weeks. EPO/Zn/Se: six primrose oil capsule (500 mg) and one tablet (200 mg zinc sulphate and 1 mg sodium selenite) for 20 weeks. | Randomized, double-blind, placebo-controlled trial | Sex Olive Oil Female = 11 Male = 4 EPO/Zn/Se Female = 12 Male = 3 Age Olive Oil 83.4 ± 5.4 EPO/Zn/Se 78.7 ± 8.2 | - | ASTR Olive Oil Pre: 100.4 ± 17.1 Post: 106.9 ± 19.0 EPO/Zn/Se Pre: 72.6 ± 28.8 Post: 87.5 ± 36.7 CPM Olive Oil Pre: 17.2 ± 6.2 Post: 18.4 ± 9.0 EPO/Zn/Se Pre: 12.2 ± 6.7 Post: 15.8 ± 8.6 GNT Olive Oil Pre: 9.4 ± 7.8 Post: 10.6 ± 8.5 EPO/Zn/Se Pre: 7.2 ± 5.2 Post: 9.8 ± 5.4 DCT Olive Oil Pre: 63.8 ± 14.9 Post: 69.8 ± 18.8 EPO/Zn/Se Pre: 61.2 ± 20.0 Post: 69.7 ± 22.2 CAMCOG Olive Oil Pre: 65.1 ± 17.3 Post: 67.8 ± 17.7 EPO/Zn/Se Pre: 62.50 ± 16.60 Post: 68.30 ± 17.70 |
| Cornelli [33] | United States | AD | Placebo: 500 mg of fructose and flavoring one ampoule/day for 24 weeks. Formula F: Formula F * containing 27.5 µg of Se one ampoule/day for 24 weeks. | Randomized double-blind clinical trial | Sex Placebo Female = 15 Male = 10 Formula F Female = 14 Male = 9 Age Placebo 74 ± 4.9 Formula F 75 ± 4.2 | - | MMSE Placebo Pre: 23.9 ± 1.0 Post: 24.2 ± 1.3 Formula F Pre: 23.2 ± 1.1 Post: 24.3 ± 1.4 |
| Scheltens et al. [34] | The Netherlands, Germany, Belgium, United Kingdom and United States | AD | Control: 125 mL tetrapackages without actives once a day for 24 weeks. Active: 125 mL tetrapackages with Souvenaid ** containing 60 µg of Se once a day for 24 weeks. | Randomized, double-blind, controlled, multicenter trial | Sex Control Female = 54 Male = 52 Active Female = 52 Male = 54 Age Control 73.3 ± 7.8 Active 74.1 ± 7.2 | - | MMSE Control Pre: 24.0 ± 2.5 Post: 24.0 ± 3.4 Active Pre: 23.8 ± 2.7 Post: 24.1 ± 3.5 ADAS-Cog Control Pre: 25.5 ± 8.8 Post: 25.8 ± 7.8 Active Pre: 25.9 ± 7.6 Post: 25.9 ± 7.7 |
| Scheltens et al. [35] | The Netherlands, Germany, Belgium, Spain, Italy, and France | AD | Control: 125 mL tetrapackages without actives once a day for 24 weeks. Active: 125 mL tetrapackages with Souvenaid ** containing 60 µg of Se once a day for 24 weeks. | Randomized, controlled, double-blind, parallel-group trial | Sex Control Female = 65 Male = 64 Active Female = 62 Male = 68 Age Control 73.2 ± 8.4 Active 74.4 ± 6.9 | - | NTB Control Pre: 0.1 ± 0.1 Post: 0.1 ± 0.5 Active Pre: 0.1 ± 0.8 Post: 0.2 ± 0.4 ADAS-Cog Control Pre: 1.2 ± 1.5 Post: 1.4 ± 1.4 Active Pre: 1.6 ± 1.7 Post: 1.7 ± 1.6 |
| Shah et al. [36] | United States | AD | Control: 125 mL tetrapackages without actives once a day for 24 weeks. Active: 125 mL tetrapackages with Souvenaid ** containing 60 µg of Se once a day for 24 weeks. | Randomized, double-blind clinical trial | Sex Control Female = 135 Male = 127 Active Female = 139 Male = 126 Age Control 76.9 ± 8.2 Active 76.6 ± 8.2 | - | ADAS-Cog Control Pre: 23.4 ± 9.3 Post: 24.4 ± 10.9 Active Pre: 23.9 ± 9.6 Post: 25.4 ± 11.6 |
| Rijpma et al. [37] | The Netherlands, Germany, Belgium, United Kingdom and United States | AD | Control: 125 mL tetrapackages without actives once a day for 24 weeks. Active: 125 mL tetrapackages with Souvenaid ** containing 60 µg of Se once a day for 24 weeks. | Randomized double-blind, multicenter, controlled trial | Sex Control Female = 54 Male = 52 Active Female = 52 Male = 54 Age Control 73.3 ± 7.8 Active 74.1 ± 7.2 | Plasma Control Pre: 86.8 Post: 78.9 Active Pre: 86.8 Post: 102.6 | - |
| Rijpma et al. [37] | The Netherlands, Germany, Belgium, Spain, Italy, and France | AD | Control: 125 mL tetrapackages without actives once a day for 24 weeks. Active: 125 mL tetrapackages with Souvenaid ** containing 60 µg of Se once a day for 24 weeks. | Randomized double-blind, multicenter, controlled trial | Sex Control Female = 65 Male = 64 Active Female = 62 Male = 68 Age Control 73.2 ± 8.4 Active 74.4 ± 6.9 | Plasma Control Pre: 86.8 Post: 86.8 Active Pre: 86.8 Post: 110.5 | - |
| Tamtaji et al. [12] | Iran | AD | Placebo: placebo (starch) for 12 weeks. Selenium: 200 mg of Se plus probiotic *** every day for 12 weeks. | Randomized, double-blind, controlled clinical trial | Sex Uninformed Age Placebo 78.5 ± 8.0 Probiotic plus selenium 76.2 ± 8.1 | - | MMSE Placebo Pre: 9.3 ± 4.1 Post: 9.1 ± 4.4 Selenium plus probiotic Pre: 9.4 ± 3.5 Post:10.9 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.E.; Souza, J.V.; Galiciolli, M.E.A.; Sare, F.; Vieira, G.S.; Kruk, I.L.; Oliveira, C.S. Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3205. https://doi.org/10.3390/nu14153205
Pereira ME, Souza JV, Galiciolli MEA, Sare F, Vieira GS, Kruk IL, Oliveira CS. Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(15):3205. https://doi.org/10.3390/nu14153205
Chicago/Turabian StylePereira, Meire Ellen, Júlia Vicentin Souza, Maria Eduarda Andrade Galiciolli, Fernanda Sare, Giovanna Scorsin Vieira, Isabeli Lopes Kruk, and Cláudia Sirlene Oliveira. 2022. "Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis" Nutrients 14, no. 15: 3205. https://doi.org/10.3390/nu14153205
APA StylePereira, M. E., Souza, J. V., Galiciolli, M. E. A., Sare, F., Vieira, G. S., Kruk, I. L., & Oliveira, C. S. (2022). Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutrients, 14(15), 3205. https://doi.org/10.3390/nu14153205








