Sex–Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
Endoscopic Procedures and Histological Classification
2.2. Serology, CD-Specific Antibodies, and Nutritional Evaluations
2.3. GI Symptoms and GFD Adherence Assessment
2.4. Statistical Analyses
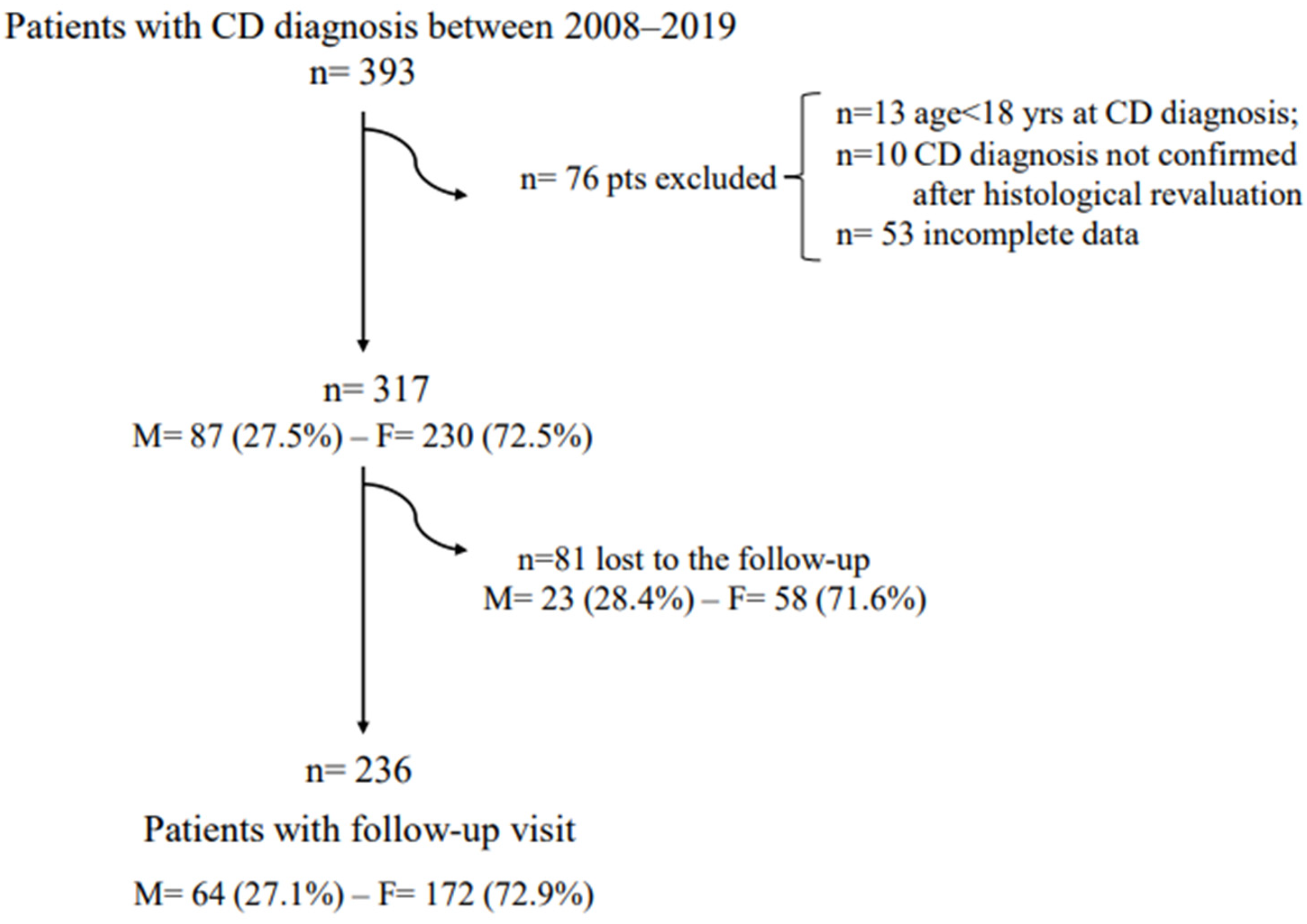
3. Results
3.1. Celiac Disease Patients at Diagnosis
3.2. Celiac Disease Patients at the GFD Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol. Nutr. Food Res. 2019, 63, e1800870. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Dilaghi, E.; Cingolani, S.; Pivetta, G.; Dottori, L.; Esposito, G.; Marzinotto, I.; Lampasona, V.; Buzzetti, R.; Annibale, B. Gender-sex differences in autoimmune atrophic gastritis. Transl. Res. 2022, 22, S1931–S5244. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Watson, T.; Clearman, B.; Mitros, F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am. J. Clin. Nutr. 2004, 79, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Kurppa, K.; Lauronen, O.; Collin, P.; Ukkola, A.; Laurila, K.; Huhtala, H.; Mäki, M.; Kaukinen, K. Factors associated with dietary adherence in celiac disease: A nationwide study. Digestion 2012, 86, 309–314. [Google Scholar] [CrossRef]
- Cohen, M.E.; Jaffe, A.; Strauch, C.B.; Lewis, S.K.; Lebwohl, B.; Green, P.H.R. Determinants of Follow-up Care for Patients with Celiac Disease. J. Clin. Gastroenterol. 2018, 52, 784–788. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; Hujoel, I.A.; West, C.P.; Taneja, V.; Prokop, L.J.; Rubio-Tapia, A.; Murray, J.A. Sex Difference in Celiac Disease in Undiagnosed Populations: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1954–1968. [Google Scholar] [CrossRef]
- Bai, D.; Brar, P.; Holleran, S.; Ramakrishnan, R.; Green, P.H. Effect of gender on the manifestations of celiac disease: Evidence for greater malabsorption in men. Scand. J. Gastroenterol. 2005, 40, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Fredella, C.; Saladino, V.; Trovato, C.; Cesana, B.M.; Quatrini, M.; Prampolini, L. Gluten intolerance: Gender- and age-related differences in symptoms. Scand. J. Gastroenterol. 2005, 40, 15–19. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; King, K.S.; Larson, J.J.; Van Dyke, C.T.; Murray, J.A.; Rubio-Tapia, A. Gender-Based Differences in a Population-Based Cohort with Celiac Disease: More Alike than Unalike. Dig. Dis. Sci. 2018, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.F.; Maria da Silva Kotze, L.; Kotze, L.R.; Chrisostomo, K.R.; Nisihara, R. Gender-Related Differences in Celiac Patients at Diagnosis. Arch. Med. Res. 2019, 50, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.L.; Withoff, S.; Kolkman, J.J.; Wijmenga, C.; Weersma, R.K.; Visschedijk, M.C. Non-classical clinical presentation at diagnosis by male celiac disease patients of older age. Eur. J. Intern. Med. 2021, 83, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Cirillo, M.; Sollazzo, R.; Savino, G.; Sabbatini, F.; Mazzacca, G. Gender and clinical presentation in adult celiac disease. Scand. J. Gastroenterol. 1995, 30, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Di Sabatino, A. Disease- and gender-related characteristics of coeliac disease influence diagnostic delay. Eur. J. Intern. Med. 2021, 83, 12–13. [Google Scholar] [CrossRef]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The Histopathology of Coeliac Disease: Time for a Standardized Report Scheme for Pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Report No.: WHO/NMH/NHD/MNM/11.1; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 22 June 2022).
- WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Levels. Summary Meeting Report; World Health Organization: Brussels, Belgium, 2004. [Google Scholar]
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 12, 600. [Google Scholar] [CrossRef]
- Carabotti, M.; Lahner, E.; Esposito, G.; Sacchi, M.C.; Severi, C.; Annibale, B. Upper Gastrointestinal Symptoms in Autoimmune Gastritis: A Cross-Sectional Study. Medicine 2017, 96, e5784. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 16, 00222–00225. [Google Scholar] [CrossRef] [Green Version]
- Biagi, F.; Andrealli, A.; Bianchi, P.I.; Marchese, A.; Klersy, C.; Corazza, G.R. A gluten-free diet score to evaluate dietary compliance in patients with coeliac disease. Br. J. Nutr. 2009, 102, 882–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, R.M.; Ford, A.C. Effect of gender on prevalence of irritable bowel syndrome in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 991–1000. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Anderson, B.; Bharucha, A.E. Sex- and Gender-Related Differences in Common Functional Gastroenterologic Disorders. Mayo Clin. Proc. 2021, 96, 1071–1089. [Google Scholar] [CrossRef]
- Aziz, I.; Palsson, O.S.; Tornblom, H.; Sperber, A.D.; Whitehead, W.E.; Simren, M. The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: A cross-sectional general population study in three countries. Am. J. Gastroenterol. 2018, 113, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Aro, P.; Storskrubb, T.; Johansson, S.E.; Lind, T.; BollingSternevald, E.; Graffner, H.; Vieth, M.; Stolte, M.; Engstrand, L.; et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: A Kalixanda study report. Scand. J. Gastroenterol. 2005, 40, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Iijima, K.; Shimosegawa, T. Gender difference in gastro-esophageal reflux diseases. World J. Gastroenterol. 2016, 22, 1800–1810. [Google Scholar] [CrossRef]
- Vannella, L.; Aloe Spiriti, M.A.; Di Giulio, E.; Lahner, E.; Corleto, V.D.; Monarca, B.; Delle Fave, G.; Annibale, B. Upper and lower gastrointestinal causes of iron deficiency anemia in elderly compared with adult outpatients. Minerva Gastroenterol. Dietol. 2010, 56, 397–404. [Google Scholar] [PubMed]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Collin, P.; Mäki, M.; Kaukinen, K. Factors associated with long diagnostic delay in celiac disease. Scand. J. Gastroenterol. 2014, 49, 1304–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vavricka, S.R.; Vadasz, N.; Stotz, M.; Lehmann, R.; Studerus, D.; Greuter, T.; Frei, P.; Zeitz, J.; Scharl, M.; Misselwitz, B.; et al. Celiac disease diagnosis still significantly delayed—Doctor’s but not patients’ delay responsive for the increased total delay in women. Dig. Liver Dis. 2016, 48, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Lahner, E.; Conti, L.; Esposito, G.; Sacchi, M.C.; Annibale, B. Risk factors associated with osteoporosis in a cohort of prospectively diagnosed adult coeliac patients. United Eur. Gastroenterol. J. 2018, 6, 1161–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.D.; Williams, J.; Lewis, S.K.; Bai, J.C.; Lebwohl, B.; Green, P.H.R. Measurement of Forearm Bone Density by Dual Energy X-Ray Absorptiometry Increases the Prevalence of Osteoporosis in Men with Celiac Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 99–106. [Google Scholar] [CrossRef]
- Parkes, M.; Cortes, A.; van Heel, D.A.; Brown, M.A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 2013, 14, 661–673. [Google Scholar] [CrossRef]
- Pekki, H.; Kurppa, K.; Mäki, M.; Huhtala, H.; Laurila, K.; Ilus, T.; Kaukinen, K. Performing routine follow-up biopsy 1 year after diagnosis does not affect long-term outcomes in coeliac disease. Aliment. Pharmacol Ther. 2017, 45, 1459–1468. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: Adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Dana, Z.Y.; Lena, B.; Vered, R.; Haim, S.; Efrat, B. Factors associated with non adherence to a gluten free diet in adult with celiac disease: A survey assessed by BIAGI score. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 762–767. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; West, N.P.; Robins, G.G.; Howdle, P.D. Long-term histological follow-up of people with coeliac disease in a UK teaching hospital. QJM 2010, 103, 511–517. [Google Scholar] [CrossRef]
- Leffler, D.A.; Edwards-George, J.; Dennis, M.; Schuppan, D.; Cook, F.; Franko, D.L.; Blom-Hoffman, J.; Kelly, C.P. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig. Dis. Sci. 2008, 53, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Herrera, A.; Reyes-Andrade, J.; Rubio-Escudero, C. Rationale for Timing of Follow-Up Visits to Assess Gluten-Free Diet in Celiac Disease Patients Based on Data Mining. Nutrients 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Deng, M.; Knowles, S.R.; Sainsbury, K.; Mullan, B.; Tye-Din, J.A. Food knowledge and psychological state predict adherence to a gluten-free diet in a survey of 5310 Australians and New Zealanders with coeliac disease. Aliment. Pharmacol. Ther. 2018, 48, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Bai, J.C. Follow-up of Celiac Disease. Gastroenterol. Clin. N. Am. 2019, 48, 127–136. [Google Scholar] [CrossRef]
- Galli, G.; Esposito, G.; Lahner, E.; Pilozzi, E.; Corleto, V.D.; Di Giulio, E.; Aloe Spiriti, M.A.; Annibale, B. Histological recovery and gluten-free diet adherence: A prospective 1-year follow-up study of adult patients with coeliac disease. Aliment Pharmacol. Ther. 2014, 40, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekki, H.; Kurppa, K.; Mäki, M.; Huhtala, H.; Sievänen, H.; Laurila, K.; Collin, P.; Kaukinen, K. Predictors and Significance of Incomplete Mucosal Recovery in Celiac Disease After 1 Year on a Gluten-Free Diet. Am. J. Gastroenterol. 2015, 110, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Zini, E.; Tomba, C.; Bardella, M.T.; Bosari, S.; Conte, D.; Runza, L.; Roncoroni, L.; Ferrero, S. Histological evaluation of duodenal biopsies from coeliac patients: The need for different grading criteria during follow-up. BMC Gastroenterol. 2015, 15, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, A.; Rad, N.; Ashtari, S.; Rostami-Nejad, M.; Moradi, A.; Haghbin, M.; Rostami, K.; Volta, U.; Zali, M.R. The value of a biopsy in celiac disease follow up: Assessment of the small bowel after 6 and 24 months treatment with a gluten free diet. Rev. Esp. Enferm. Dig. 2020, 112, 101–108. [Google Scholar] [CrossRef]
- Lebwohl, B.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.; Ludvigsson, J.F. Predictors of persistent villous atrophy in coeliac disease: A population-based study. Aliment. Pharmacol. Ther. 2014, 39, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Hære, P.; Høie, O.; Schulz, T.; Schönhardt, I.; Raki, M.; Lundin, K.E. Long-term mucosal recovery and healing in celiac disease is the rule—Not the exception. Scand. J. Gastroenterol. 2016, 51, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; Delle Fave, G. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
| Male Patients | Female Patients | p | |
|---|---|---|---|
| n = 87 | n = 230 | ||
| Median age, years (range) | 43 (18–72) | 36 (18–76) | 0.001 |
| Median BMI * kg/m2 (range) | 23.5 (15.9–35.7) | 21.6 (16.1–38.2) | 0.001 |
| Underweight (<18 kg/m2) | 9.5% | 15.0% | 0.26 |
| Comorbidities | |||
| Autoimmune | 31.0% | 30.4% | 1 |
| Other # | 25.3% | 14.8% | 0.03 |
| 1st degree family history of CD § | 21.8% | 17.4% | 0.41 |
| ≥3 years duration of symptoms/signs before the CD diagnosis | 21.1% | 45.4% | <0.0001 |
| tTg IgA Ab ≥ 10 ULN | 40.5% | 51.7% | 0.09 |
| Clinical presentation Classical symptoms | 30.2% | 22.2% | 0.14 |
| GI ° symptoms | |||
| Total of pts with GI symptoms | 62.1% | 85.0% | <0.001 |
| Nausea/vomit | 10.3% | 25.6% | 0.003 |
| Heartburn | 19.5% | 31.7% | 0.03 |
| Regurgitation | 12.6% | 22.0% | 0.07 |
| Dysphagia | 6.9% | 10.1% | 0.51 |
| Postprandial fullness/early satiety | 24.1% | 47.6% | <0.001 |
| Abdominal pain | 34.5% | 52.4% | 0.005 |
| Abdominal bloating | 39.1% | 61.7% | <0.001 |
| Constipation | 3.4% | 20.7% | <0.0001 |
| Diarrhoea | 27.6% | 24.7% | 0.66 |
| Signs of malabsorption | 50.0% | 72.5% | 0.02 |
| Anaemia | 16.0% | 48.4% | <0.0001 |
| Hypoferritinaemia | 25.0% | 63.9% | <0.0001 |
| Hypocholesterolaemia | 15.0% | 8.0% | 0.01 |
| Hypotriglyceridaemia | 1.0% | 10.3% | 0.01 |
| Hypoproteinaemia | 9.0% | 6.0% | 0.12 |
| Osteopenia/osteoporosis | 66.2% | 49.2% | <0.01 |
| Marsh 3C at diagnosis time | 51.7% | 56.9% | 0.44 |
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| Age # | 0.80 | 0.37–1.68 | 0.55 |
| Body mass index # | 0.88 | 0.79–0.98 | 0.02 |
| Non-autoimmune comorbidities § | 0.42 | 0.16–1.09 | 0.07 |
| >3 years duration of symptoms/signs before CD diagnosis | 3.39 | 1.52–7.56 | 0.002 |
| Gastrointestinal symptoms * | 0.70 | 0.20–2.34 | 0.56 |
| Nausea/vomit | 3.53 | 1.14–10.93 | 0.02 |
| Heartburn | 2.99 | 1.11–8.05 | 0.02 |
| Dyspepsia | 2.70 | 1.12–6.46 | 0.02 |
| Abdominal pain | 0.98 | 0.39–2.44 | 0.98 |
| Abdominal bloating | 2.22 | 0.92–5.32 | 0.07 |
| Constipation | 4.84 | 1.19–19.63 | 0.02 |
| Signs of malabsorption * | 3.39 | 1.44–7.97 | 0.005 |
| Osteopenia/osteoporosis | 0.30 | 0.13–0.72 | 0.007 |
| Male Patients | Female Patients | p-Value | |
|---|---|---|---|
| n = 64 | n = 172 | ||
| Median months of GFD * (range) | 15 (12–30) | 14 (12–30) | 0.32 |
| Adequate GFD | 92.2% | 93.4% | 0.77 |
| Median BMI § kg/m2 (range) | 23.9 (16–32.8) | 22.2 (16.6–36.6) | 0.01 |
| Underweight (BMI < 18 kg/m2) | 3.3% | 4.5% | 1 |
| GI ° symptoms | |||
| Total of pts with GI symptoms | 31.2% | 32.1% | 1 |
| -Nausea/vomit | 0 | 3.0% | |
| -Heartburn | 10.9% | 5.9% | 0.25 |
| -Regurgitation | 6.2% | 3.6% | 0.46 |
| -Dysphagia | 1.5% | 0.6% | 0.47 |
| -Postprandial fullness/early satiety | 3.2% | 6.5% | 0.52 |
| -Abdominal pain | 9.4% | 15.5% | 0.28 |
| -Abdominal bloating | 14.1% | 16.7% | 0.84 |
| -Constipation | 9.4% | 9.0% | 1 |
| -Diarrhoea | 12.5% | 5.4% | 0.08 |
| Antibodies positivity | 18.0% | 24.8% | 0.37 |
| Signs of malabsorption | 23.4% | 34.5% | 0.115 |
| Anaemia | 6.8% | 16.3% | 0.055 |
| Hypoferritinaemia | 14.7% | 33.3% | <0.01 |
| Hypocholesterolaemia | 5.0% | 1.8% | 0.346 |
| Hypotriglyceridaemia | 5.0% | 11.6% | 0.22 |
| Hypoproteinaemia | 1.8% | 2.7% | 1 |
| Histological control | 93.7% | 81.4% | 0.02 |
| Marsh 3 (atrophic disease) | 30.0% | 28.6% | 0.86 |
| Marsh score | |||
| Marsh 0 | 63.3% | 55.0% | 0.39 |
| Marsh 1 | 8.3% | 15.0% | 0.25 |
| Marsh 2 | 0 | 2.9% | |
| Marsh 3A | 21.6% | 18.6% | 0.69 |
| Marsh 3B | 5.0% | 7.1% | 0.75 |
| Marsh 3C | 1.6% | 1.4% | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, G.; Amici, G.; Conti, L.; Lahner, E.; Annibale, B.; Carabotti, M. Sex–Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up. Nutrients 2022, 14, 3192. https://doi.org/10.3390/nu14153192
Galli G, Amici G, Conti L, Lahner E, Annibale B, Carabotti M. Sex–Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up. Nutrients. 2022; 14(15):3192. https://doi.org/10.3390/nu14153192
Chicago/Turabian StyleGalli, Gloria, Giulia Amici, Laura Conti, Edith Lahner, Bruno Annibale, and Marilia Carabotti. 2022. "Sex–Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up" Nutrients 14, no. 15: 3192. https://doi.org/10.3390/nu14153192
APA StyleGalli, G., Amici, G., Conti, L., Lahner, E., Annibale, B., & Carabotti, M. (2022). Sex–Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up. Nutrients, 14(15), 3192. https://doi.org/10.3390/nu14153192







