Associations between Body Composition and Vitamin D Status in Children with Overweight and Obesity Participating in a 1-Year Lifestyle Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Subjects
2.2. Inclusion and Exclusion Criteria
2.2.1. Included
2.2.2. Excluded
2.3. Biochemistry
2.4. Anthropometry
2.5. Assessment of Vitamin D Sources
2.6. Pubertal Status
2.7. Socio-Economic/Demographic Assessment
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
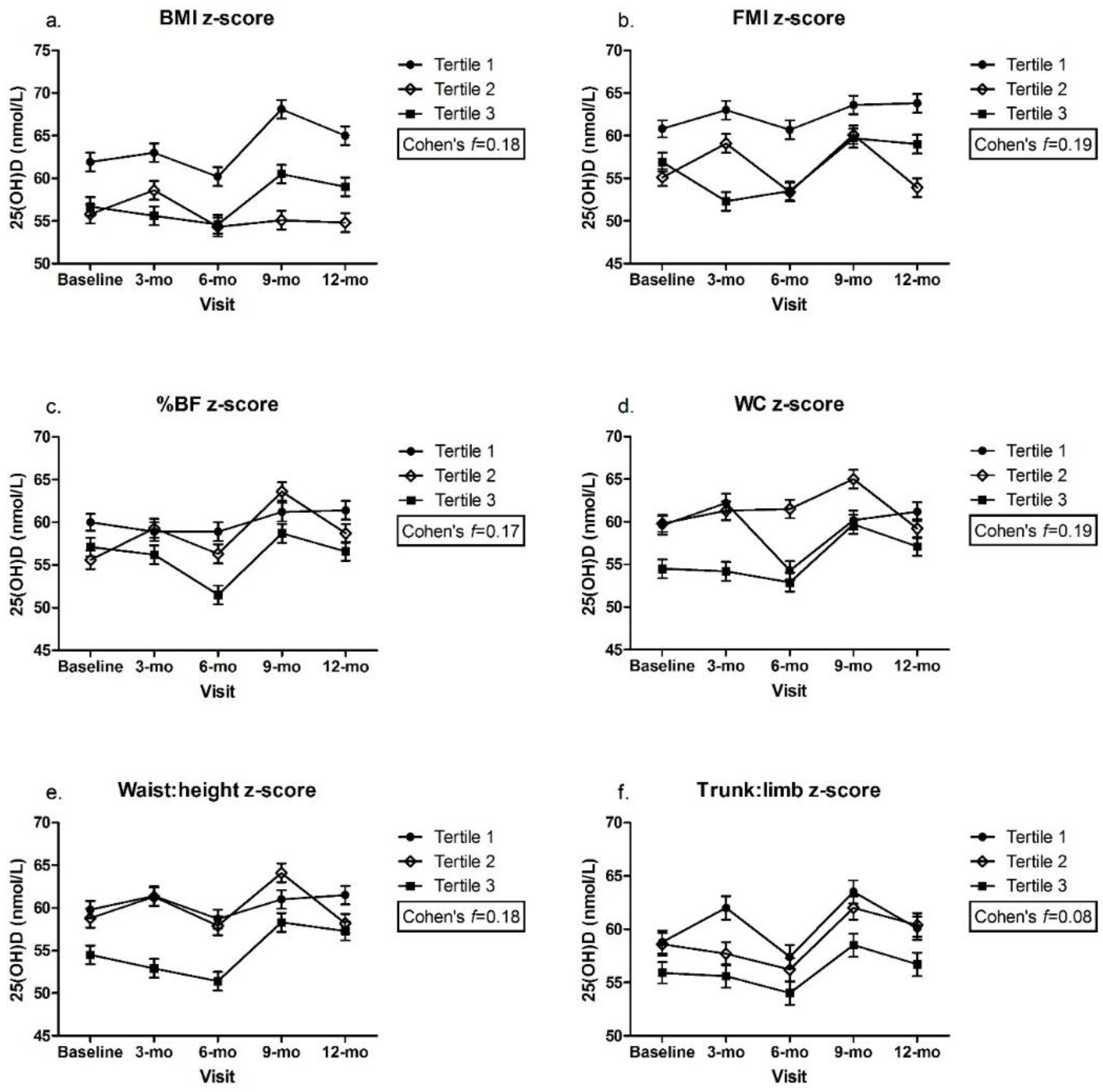
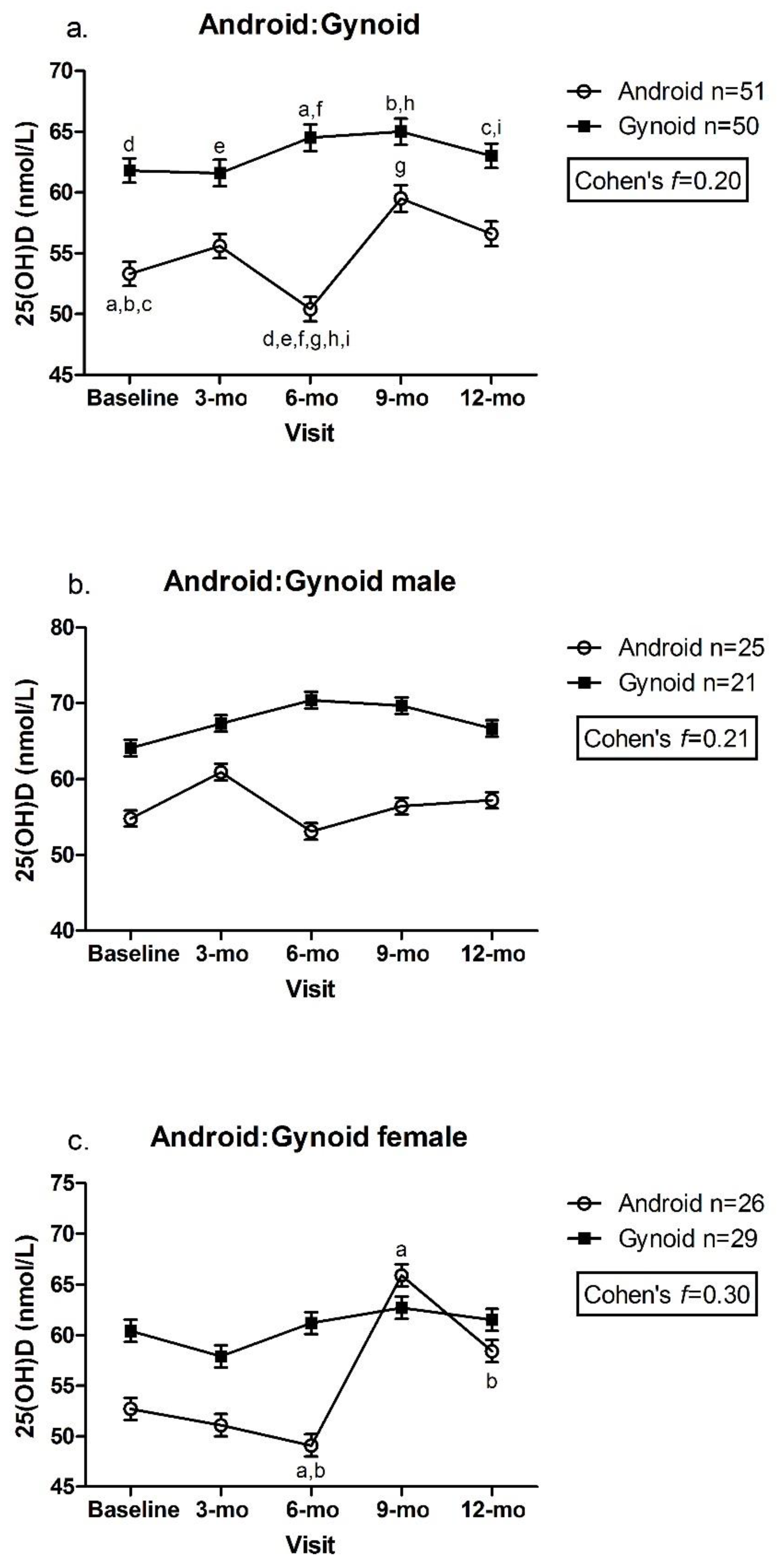
3.2. Adiposity Indicators by Tertile and Change in 25(OH)D over Time
3.3. Effect of Body Composition Change between Baseline and 12-mo on 25(OH)D Concentrations
4. Discussion
Strengths and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, D.P.; Kropac, E.; Do, M.T.; Roberts, K.C.; Jayaraman, G.C. Childhood overweight and obesity trends in Canada. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2016, 36, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Statistics Canada. Table 13-10-0795-01 Measured Children and Youth Body Mass Index (BMI) (World Health Organization Classification), by Age Group and Sex, Canada and Provinces, Canadian Community Health Survey—Nutrition; Statistics Canada: Ottawa, ON, Canada, 2017. [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016; NCHS Data Brief (288); National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8.
- Biro, F.M.; Wien, M. Childhood obesity and adult morbidities. Am. J. Clin. Nutr. 2010, 91, 1499S–1505S. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Sievers, M.L.; Bennett, P.H.; Looker, H.C. Childhood Obesity, Other Cardiovascular Risk Factors, Premature Death. N. Engl. J. Med. 2010, 362, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Mei, Z.; Srinivasan, S.R.; Berenson, G.S.; Dietz, W.H. Cardiovascular Risk Factors and Excess Adiposity Among Overweight Children and Adolescents: The Bogalusa Heart Study. J. Pediatr. 2007, 150, 12–17.e2. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, R.; Kichler, J.; Babar, G.; Calhoun, M. Hypovitaminosis D in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008, 57, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Arunabh, S.; Pollack, S.; Yeh, J.; Aloia, J.F. Body Fat Content and 25-Hydroxyvitamin D Levels in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 157–161. [Google Scholar] [CrossRef]
- Çizmecioğlu, F.M.; Etiler, N.; Görmüş, U.; Hamzaoğlu, O.; Hatun, Ş. Hypovitaminosis D in obese and overweight schoolchildren. J. Clin. Res. Pediatr. Endocrinol. 2008, 1, 89–96. [Google Scholar] [CrossRef]
- Goldner, W.S.; Stoner, J.A.; Thompson, J.; Taylor, K.; Larson, L.; Erickson, J.; McBride, C. Prevalence of Vitamin D Insufficiency and Deficiency in Morbidly Obese Patients: A Comparison with Non-Obese Controls. Obes. Surg. 2008, 18, 145–150. [Google Scholar] [CrossRef]
- Liel, Y.; Ulmer, E.; Shary, J.; Hollis, B.W.; Bell, N.H. Low circulating vitamin D in obesity. Calcif. Tissue Int. 1988, 43, 199–201. [Google Scholar] [CrossRef]
- Greene-Finestone, L.S.; Garriguet, D.; Brooks, S.; Langlois, K.; Whiting, S.J. Overweight and obesity are associated with lower vitamin D status in Canadian children and adolescents. Paediatr. Child Health 2017, 22, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, P.; Karlsson, T.; Svensson, H.; Lönn, M.; Carlsson, N.-G.; Sandberg, A.-S.; Jennische, E.; Osmancevic, A.; Holmäng, A. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: A pilot study. J. Photochem. Photobiol. B. 2014, 138, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D storage in adipose tissue of obese and normal weight women. J. Bone. Miner. Res. 2017, 3, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drincic, A.T.; Armas, L.A.G.; van Diest, E.E.; Heaney, R.P. Volumetric Dilution, Rather Than Sequestration Best Explains the Low Vitamin D Status of Obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Pannu, P.K.; Zhao, Y.; Soares, M.J. Reductions in body weight and percent fat mass increase the vitamin D status of obese subjects: A systematic review and metaregression analysis. Nutr. Res. 2016, 36, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Coupaye, M.; Breuil, M.C.; Rivière, P.; Castel, B.; Bogard, C.; Dupré, T.; Msika, S.; LeDoux, S. Serum Vitamin D Increases with Weight Loss in Obese Subjects 6 Months After Roux-en-Y Gastric Bypass. Obes. Surg. 2013, 23, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.L. Vitamin D and intestinal calcium transport after bariatric surgery. J. Steroid Biochem. Mol. Biol. 2017, 173, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Toninello, P.; Montanari, A.; Bassetto, F.; Vindigni, V.; Paoli, A. Nutritional Support for Bariatric Surgery Patients: The Skin beyond the Fat. Nutrients 2021, 13, 1565. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Morano, A.B.; Chowdhury, H.A.; Shapses, S.A. Energy restriction reduces fractional calcium absorption in mature obese and lean rats. J. Nutr. 2002, 132, 2660–2666. [Google Scholar] [CrossRef] [Green Version]
- Kouda, K.; Nakamura, H.; Fujita, Y.; Ohara, K.; Iki, M. Vitamin D status and body fat measured by dual-energy X-ray absorptiometry in a general population of Japanese children. Nutrition 2013, 29, 1204–1208. [Google Scholar] [CrossRef]
- Cohen, T.R.; Hazell, T.J.; Vanstone, C.A.; Rodd, C.; Weiler, H.A. A family-centered lifestyle intervention for obese six- to eight-year-old children: Results from a one-year randomized controlled trial conducted in Montreal, Canada. Can. J. Public Health 2016, 107, e453–e460. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.R.; Mak, I.L.; Loiselle, S.-E.; Kasvis, P.; Hazell, T.J.; Vanstone, C.A.; Rodd, C.; Weiler, H.A. Changes in Adiposity without Impacting Bone Health in Nine- to Twelve-Year-Old Children with Overweight and Obesity after a One-Year Family-Centered Lifestyle Behavior Intervention. Child Obes. 2022. [Google Scholar] [CrossRef]
- Canadian Public Health Agency of Canada. 24 Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. 2011. Available online: https://www.canada.ca/en/public-health/services/health-promotion/healthy-living/physical-activity/24-hour-movement-guidelines-children-youth.html (accessed on 26 July 2022).
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height, and Body Mass Index-for-Age: Methods and Development; World Health Organization, Department of Nutrition for Health and Development: Geneva, Switzerland, 2006. [Google Scholar]
- Cohen, T.R.; Hazell, T.J.; Vanstone, C.A.; Plourde, H.; Rodd, C.J.; Weiler, H.A. A family-centered lifestyle intervention to improve body composition and bone mass in overweight and obese children 6 through 8 years: A randomized controlled trial study protocol. BMC Public Health 2013, 13, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D; National Academy Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Sharma, A.K.; Metzger, D.L.; Daymont, C.; Hadjiyannakis, S.; Rodd, C.J. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: Association with cardio-metabolic risks. Pediatr. Res. 2015, 78, 723–729. [Google Scholar] [CrossRef]
- Ofenheimer, A.; Breyer-Kohansal, R.; Hartl, S.; Burghuber, O.C.; Krach, F.; Schrott, A.; Franssen, F.M.E.; Wouters, E.F.M.; Breyer, M. Reference charts for body composition parameters by dual-energy X-ray absorptiometry in European children and adolescents aged 6 to 18 years—Results from the Austrian LEAD (Lung, Heart, Social, Body) cohort. Pediatr. Obes. 2021, 16, e12695. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The lms method and penalized likelihood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, A.; Fischer, T.; Lahti, A.; Wilhelm, K.P.; Takiwaki, H.; Serup, J. Guidelines for measurement skin colour and erythema A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1996, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. Soleil et peau. J. Med. Esthet. 1975, 2, 33–34. [Google Scholar]
- Statistics Canada. Canadian Community Health Survey, Cycle 4.1. 2008. Available online: https://www23.statcan.gc.ca/imdb/p3Instr.pl?Function=getInstrumentList&Item_Id=33186&UL=1V& (accessed on 3 February 2022).
- Lund, C.C. The estimation of areas of burns. Surg. Gynecol. Obste. 1944, 79, 352–358. [Google Scholar]
- Kimlin, M.G. Geographic location and vitamin D synthesis. Mol. Asp. Med. 2008, 29, 453–461. [Google Scholar] [CrossRef]
- Health Canada. Canadian Community Health Survey, Cycle 2.2, Nutrition (2004): A Guide to Accessing and Interpreting the Data. Ottawa: Office of Nutrition Policy and Promotion. 2006. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/hpfb-dgpsa/pdf/surveill/cchs-guide-escc-eng.pdf (accessed on 3 February 2022).
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Health Canada. Canadian Community Health Survey, Cycle 2.2, Nutrition, 2004. Nutrient Intakes from food: Provincial, Regional and National Summary Data Tables. 2008; Volume 2. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/health-nutrition-surveys/canadian-community-health-survey-cchs/canadian-community-health-survey-cycle-2-2-nutrition-focus-food-nutrition-surveillance-health-canada.html (accessed on 26 July 2022).
- Forbes, G.B.; Welle, S.L. Lean body mass in obesity. Int. J. Obes. 1983, 7, 99–107. [Google Scholar] [PubMed]
- Freedman, D.S.; Wang, J.; Maynard, L.M.; Thornton, J.C.; Mei, Z.; Pierson, R.N.; Dietz, W.H. Relation of BMI to fat and fat-free mass among children and adolescents. Int. J. Obes. 2005, 29, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xiao, P.; Cheng, H.; Zhao, X.; Yan, Y.; Liu, J.; Xie, X.; Mi, J. Central body fat deposits are associated with poor vitamin D status in Chinese children and adolescents. Nutrition 2022, 99, 111651. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ma, X.; Shen, Y.; Ni, J.; Luo, Y.; Xiao, Y.; Bao, Y.; Jia, W. Associations of serum 25-hydroxyvitamin D3 levels with visceral adipose tissue in Chinese men with normal glucose tolerance. PLoS ONE 2014, 9, e86773. [Google Scholar] [CrossRef]
- Landau, Z.; Abiri, S.; Lebenthal, Y.; Jakubowicz, D.; Mor, N.; Lerner-Geva, L.; Boaz, M.; Wainstein, J.; Bar-Dayan, Y. Lifestyle intervention program benefits children with overweigh compared to children with obesity. Obes. Res. Clin. Pract. 2018, 12, 85–92. [Google Scholar] [CrossRef]
- Hannemann, A.; Thuesen, B.H.; Friedrich, N.; Völzke, H.; Steveling, A.; Ittermann, T.; Hegenscheid, K.; Nauck, M.; Linneberg, A.; Wallaschofski, H. Adiposity measures and vitamin D concentrations in Northeast Germany and Denmark. Nutr. Metab. 2015, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Gangloff, A.; Bergeron, J.; Pelletier-Beaumont, E.; Nazare, J.-A.; Smith, J.; Borel, A.-L.; Lemieux, I.; Tremblay, A.; Poirier, P.; Alméras, N.; et al. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: Results from a 1-year lifestyle intervention in viscerally obese men. Int. J. Obes. 2015, 39, 1638–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, H.J. Use of Percentiles and Z-Scores in Anthropometry. In Handbook of Anthropometry; Preedy, V., Ed.; Springer: New York, NY, USA, 2012; pp. 29–48. [Google Scholar]
- Eckhardt, L.; Mayer, J.A.; Creech, L.; Johnston, M.R.; Lui, K.J.; Sallis, J.F.; Elder, J.P. Assessing children’s ultraviolet radiation exposure: The potential usefulness of a colorimeter. Am. J. Public Health 1996, 86, 802–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pershing, L.K.; Tirumala, V.P.; Nelson, J.L.; Corlett, J.L.; Lin, A.G.; Meyer, L.J.; Leachmen, S.A. Reflectance Spectrophotometer: The Dermatologists’ Sphygmomanometer for Skin Phototyping? J. Investig. Dermatol. 2008, 128, 1633–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Females n = 55 | Males n = 46 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Min | Max | Mean ± SEM | Min | Max | p | ES | |
| Age (y) a | 9.0 ± 0.3 | 6.0 | 12.9 | 9.7 ± 0.3 | 6.8 | 12.9 | 0.111 | 0.34 |
| Height (cm) | 139.3 ± 1.9 | 111.5 | 178.9 | 142.4 ± 1.6 | 126.0 | 169.5 | 0.219 | 0.25 |
| Height z-score | 1.0 ± 0.1 | −0.7 | 3.8 | 1.1 ± 0.1 | −0.7 | 3.3 | 0.691 | 0.08 |
| Weight (kg) a | 51.3 ± 2.6 | 25.2 | 118.2 | 54.6 ± 2.0 | 35.0 | 90.0 | 0.062 | 0.20 |
| BMI (kg/m2) a | 25.5 ± 0.6 | 18.2 | 39.1 | 26.6 ± 0.5 | 19.5 | 36.0 | 0.085 | 0.25 |
| BMI z-score a | 2.8 ± 0.1 | 1.5 | 4.3 | 3.4 ± 0.2 | 1.9 | 6.8 | 0.002 | 0.70 |
| Overweight | 3 (5.5) | 2 (4.4) | ||||||
| Obese | 52 (94.6) | 44 (95.7) | ||||||
| WC (cm) | 85.0 ± 1.7 | 62.0 | 121.0 | 90.1 ± 1.5 | 71.5 | 116.5 | 0.028 | 0.44 |
| WC z-score | 2.1 ± 0.0 | 1.4 | 3.1 | 2.1 ± 0.1 | 1.2 | 2.6 | 0.194 | 0.29 |
| Waist:height | 0.61 ± 0.01 | 0.50 | 0.71 | 0.63 ± 0.01 | 0.53 | 0.75 | 0.010 | 0.52 |
| %BF | 38.4 ± 0.6 | 29.0 | 48.3 | 36.3 ± 0.7 | 22.2 | 48.7 | 0.025 | 0.46 |
| FM (kg) a | 20.0 ± 1.1 | 8.4 | 49.4 | 20.1 ± 1.0 | 9.1 | 41.4 | 0.551 | 0.02 |
| FMI (kg/m2) a | 9.9 ± 0.3 | 5.5 | 16.5 | 9.8 ± 0.4 | 5.0 | 17.1 | 0.959 | 0.05 |
| Trunk:limb FM | 0.65 ± 0.01 | 0.41 | 0.90 | 0.66 ± 0.02 | 0.46 | 1.00 | 0.863 | 0.03 |
| Android:gynoid | 0.98 ± 0.01 | 0.80 | 1.21 | 1.01 ± 0.02 | 0.70 | 1.18 | 0.145 | 0.29 |
| Android fat patterning | 26 (47) | 25 (54) | ||||||
| Gynoid fat patterning | 29 (53) | 21 (46) | ||||||
| LBM (kg) a,b | 30.0 ± 1.4 | 15.7 | 65 | 33.2 ± 1.1 | 22.9 | 57.3 | 0.018 | 0.34 |
| LMI (kg/m2) | 15.0 ± 0.3 | 11.4 | 22.0 | 16.1 ± 0.2 | 13.4 | 20.0 | 0.003 | 0.57 |
| 25(OH)D (nmol/L) a | 62.2 ± 2.4 | 30.7 | 108.0 | 62.2 ± 2.6 | 34.5 | 105.0 | 0.846 | 0.00 |
| ≥50 nmol/L | 40 (72.7) | 31 (67.4) | ||||||
| 30–49 nmol/L | 15 (27.3)) | 15 (32.3) | ||||||
| <30 nmol/L | 0 | 0 | ||||||
| Vitamin D intake (IU/d) a | 175.9 ± 13.5 | 13.0 | 477.9 | 200.7 ± 20.4 | 7.7 | 741.6 | 0.399 | 0.21 |
| ≥EAR | 2 (3.6) | 2 (4.3) | ||||||
| ≥RDA | 0 (0) | 1 (2.2) | ||||||
| Supplements used | 6 (10.9) | 1 (2.2) | ||||||
| Sun index (min/d) a | 8.3 ± 2.4 | 0.0 | 112.4 | 13.7 ± 4.0 | 0.0 | 168.0 | 0.013 | 0.25 |
| Sunscreen used | 22 (40.0) | 18 (39.1) | ||||||
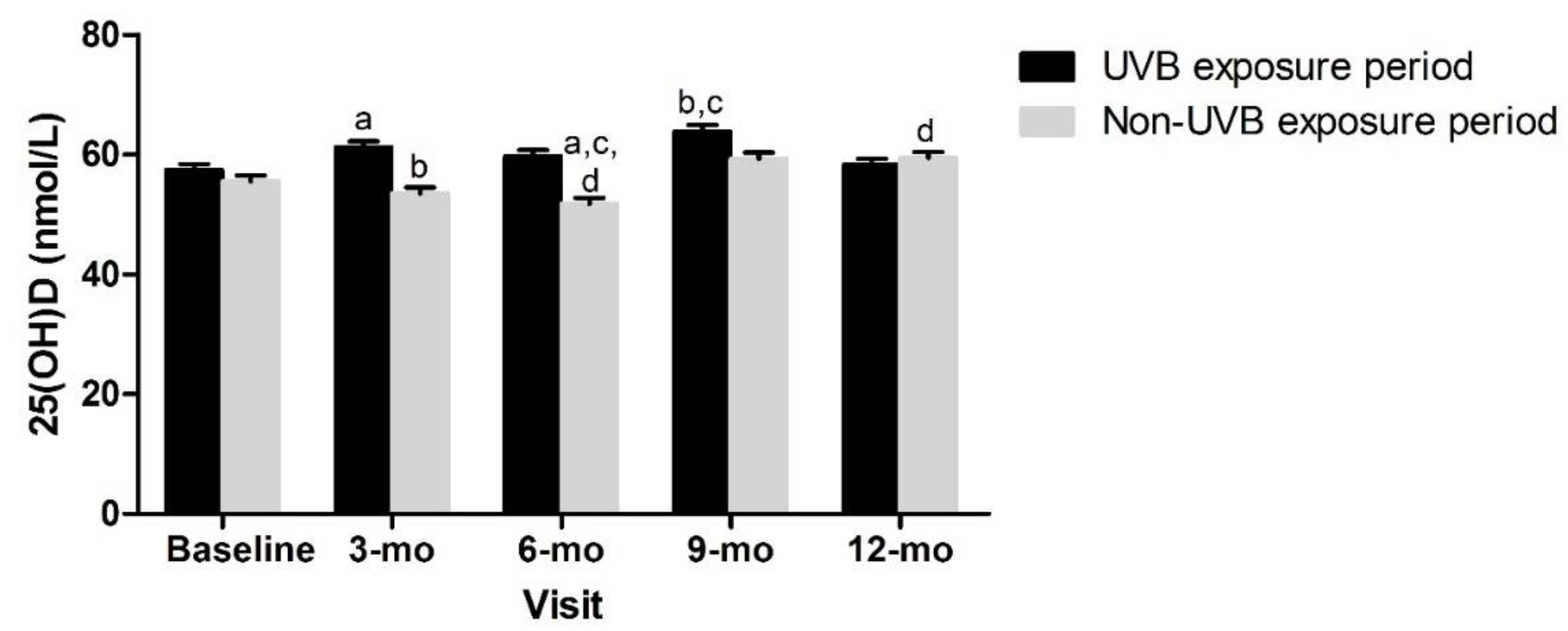
| UVB exposure period | 23 (41.8) | 31 (57.4) | ||||||
| Ethnicity | ||||||||
| White | 41 (74.6) | 31 (67.4) | ||||||
| Black | 6 (10.9) | 1 (2.2) | ||||||
| Hispanic | 2 (3.6) | 1 (2.2) | ||||||
| Mixed | 4 (7.3) | 10 (21.7) | ||||||
| Asian | 0 | 2 (4.4) | ||||||
| Other | 2 (3.6) | 1 (2.2) | ||||||
| Fitzpatrick skin type | ||||||||
| I-III | 42 (77.8) | 35 (76.1) | ||||||
| IV-VI | 12 (22.2) | 11 (23.9) | ||||||
| Pubertal status | ||||||||
| Tanner 1–3 | 50 (90.9) | 43 (93.5) | ||||||
| Tanner 4–5 | 5 (9.1) | 3 (6.5) | ||||||
| Mothers’ education level | ||||||||
| High school/college * | 27 (51.9) | 22 (48.9) | ||||||
| University | 24 (46.2) | 23 (51.1) | ||||||
| Refused to answer | 1 (1.9) | 0 | ||||||
| Family income | ||||||||
| < CAD 75,000 | 17 (32.1) | 17 (37.8) | ||||||
| ≥ CAD 75,000 | 30 (56.6) | 25 (55.6) | ||||||
| Declined to answer | 6 (11.3) | 3 (6.7) | ||||||
| Loss | Maintenance | Gain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z-Score | n | Baseline | 12-mo | ptime | N | Baseline | 12-mo | ptime | n | Baseline | 12-mo | ptime | ES(f) |
| BMI | 26 | 3.6 ± 0.2 a | 2.7 ± 0.2 | <0.001 | 47 | 3.0 ± 0.1 | 2.8 ± 0.1 | <0.001 | 28 | 2.7 ± 0.2 a | 2.9 ± 0.2 | 0.01 | 1.56 |
| FMI | 17 | 1.8 ± 0.1 | 1.0 ± 0.1 a,b | <0.001 | 62 | 1.7 ± 0.1 | 1.5 ± 1.1 a | <0.001 | 22 | 1.5 ± 0.1 | 1.7 ± 0.1 b | <0.001 | 1.70 |
| %BF | 15 | 0.9 ± 0.1 | 0.1 ± 0.1 a,b | <0.001 | 58 | 1.1 ± 0.1 | 0.9 ± 0.1 a | <0.001 | 28 | 0.8 ± 0.1 | 1.0 ± 0.1 b | <0.001 | 1.69 |
| WC | 8 | 2.0 ± 0.1 | 1.4 ± 0.1 a,b | <0.001 | 70 | 2.2 ± 0.0 c | 2.0 ± 0.0 b | <0.001 | 23 | 1.9 ± 0.1 c | 2.0 ± 0.1 a | <0.001 | 1.57 |
| Waist:height | 10 | 1.8 ± 0.1 | 1.1 ± 0.1 a,b | <0.001 | 60 | 2.0 ± 0.0 | 1.9 ± 0.0 a | <0.001 | 31 | 1.8 ± 0.1 | 2.0 ± 0.1 b | <0.001 | 1.59 |
| Trunk:limb | 31 | 0.3 ± 0.1 a | −0.5 ± 0.1 b | <0.001 | 45 | 0.0 ± 0.1 | −0.2 ± 0.1 | <0.001 | 25 | −0.2 ± 0.1 a | 0.1 ± 0.1 b | <0.001 | 1.92 |
| Android:gynoid † | 19 | 1.0 ± 0.0 | 0.9 ± 0.0 a,b | <0.001 | 40 | 1.0 ± 0.0 | 1.0 ± 0.0 b | 0.30 | 42 | 1.0 ± 0.0 | 1.0 ± 0.0 a | <0.001 | 2.10 |
| LMI | 29 | 3.4 ± 0.2 | 2.6 ± 0.2 | <0.001 | 42 | 3.1 ± 0.1 | 2.9 ± 0.1 | <0.001 | 30 | 2.9 ± 0.2 | 3.2 ± 0.2 | <0.001 | 2.07 |
| 25(OH)D (nmol/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decrease | Maintenance | Increase | ES(f) | |||||||
| Z-Score | n | Baseline | 12-mo | n | Baseline | 12-mo | n | Baseline | 12-mo | |
| BMI | 26 | 57.1 ± 1.1 | 59.5 ± 1.1 | 47 | 55.5 ± 1.0 | 55.4 ± 1.0 | 28 | 57.2 ± 1.1 | 56.1 ± 1.1 | 0.15 |
| FMI | 17 | 62.7 ± 1.1 | 64.0 ± 1.1 | 62 | 56.1 ± 1.0 | 56.1 ± 1.0 | 22 | 53.4 ± 1.1 | 54.3 ± 1.1 | 0.14 |
| %BF | 15 | 63.0 ± 1.1 | 65.8 ± 1.1 * | 58 | 57.7 ± 1.0 | 56.8 ± 1.0 | 28 | 52.2 ± 1.1 | 53.1 ± 1.1 * | 0.14 |
| WC | 8 | 63.9 ± 1.1 | 64.6 ± 1.1 | 70 | 55.2 ± 1.0 | 57.1 ± 1.0 | 23 | 57.1 ± 1.1 | 53.4 ± 1.1 | 0.21 |
| Waist:height | 10 | 63.3 ± 1.1 | 65.6 ± 1.1 | 60 | 54.4 ± 1.0 | 55.4 ± 1.0 | 31 | 58.7 ± 1.1 | 56.8 ± 1.1 | 0.16 |
| Trunk:limb | 31 | 55.4 ± 1.1 | 58.6 ± 1.1 | 45 | 56.3 ± 1.0 | 56.2 ± 1.1 | 25 | 58.3 ± 1.1 | 55.3 ± 1.1 | 0.27 |
| Android:gynoid † | 19 | 54.4 ± 1.1 | 59.6 ± 1.1 | 40 | 52.1 ± 1.0 | 55.8 ± 1.1 | 42 | 57.3 ± 1.0 | 56.4 ± 1.0 | 0.22 |
| LMI | 29 | 55.3 ± 1.1 | 57.4 ± 1.1 | 42 | 56.2 ± 1.0 | 56.8 ± 1.0 | 30 | 57.3 ± 1.1 | 55.5 ± 1.1 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasvis, P.; Cohen, T.R.; Loiselle, S.-È.; Hazell, T.J.; Vanstone, C.A.; Weiler, H.A. Associations between Body Composition and Vitamin D Status in Children with Overweight and Obesity Participating in a 1-Year Lifestyle Intervention. Nutrients 2022, 14, 3153. https://doi.org/10.3390/nu14153153
Kasvis P, Cohen TR, Loiselle S-È, Hazell TJ, Vanstone CA, Weiler HA. Associations between Body Composition and Vitamin D Status in Children with Overweight and Obesity Participating in a 1-Year Lifestyle Intervention. Nutrients. 2022; 14(15):3153. https://doi.org/10.3390/nu14153153
Chicago/Turabian StyleKasvis, Popi, Tamara R. Cohen, Sarah-Ève Loiselle, Tom J. Hazell, Catherine A. Vanstone, and Hope A. Weiler. 2022. "Associations between Body Composition and Vitamin D Status in Children with Overweight and Obesity Participating in a 1-Year Lifestyle Intervention" Nutrients 14, no. 15: 3153. https://doi.org/10.3390/nu14153153
APA StyleKasvis, P., Cohen, T. R., Loiselle, S.-È., Hazell, T. J., Vanstone, C. A., & Weiler, H. A. (2022). Associations between Body Composition and Vitamin D Status in Children with Overweight and Obesity Participating in a 1-Year Lifestyle Intervention. Nutrients, 14(15), 3153. https://doi.org/10.3390/nu14153153





