Efficacy and Safety of Omija (Schisandra chinensis) Extract Mixture on the Improvement of Hyperglycemia: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiments
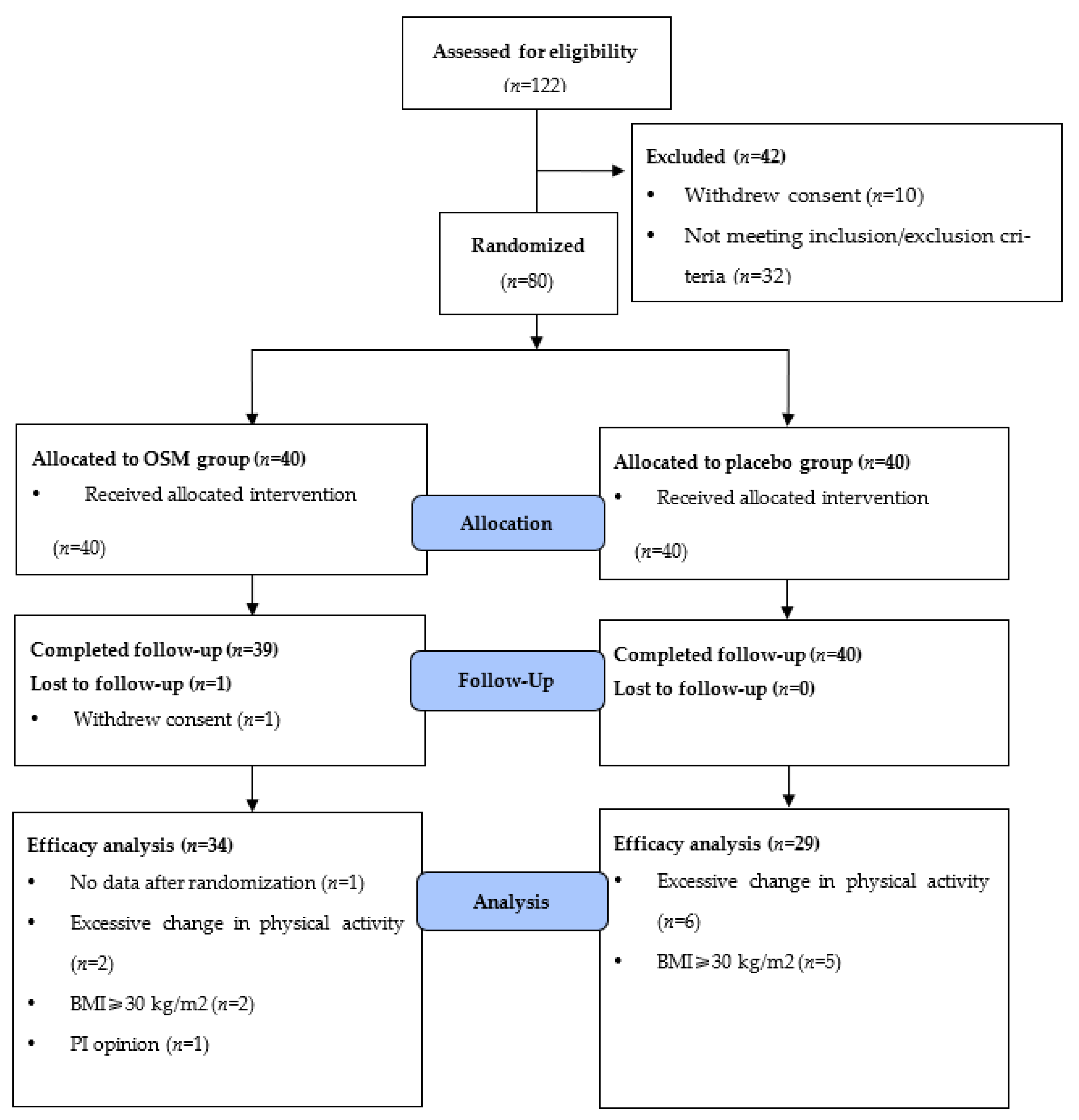
2.2. Registration and Randomization of Participants
2.3. Intervention
2.4. Efficacy Outcomes
2.5. Safety Outcomes
2.6. Diet and Global Physical Activity Questionnaire (GPAQ)
2.7. Diabetic Family History and Anthropometric Assessments
2.8. Sample Size and Statistical Analysis
3. Results
3.1. Characteristics of the Participants
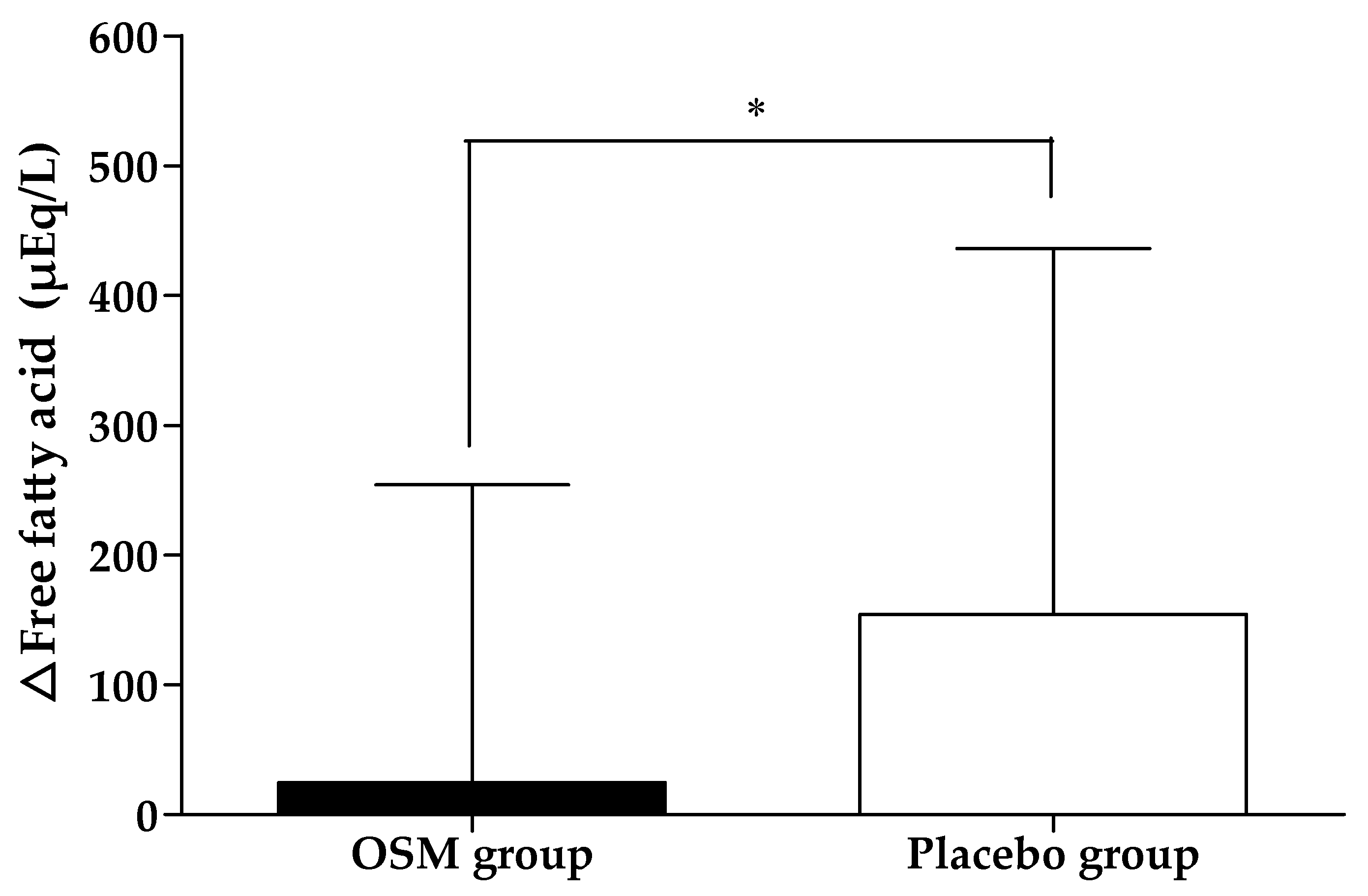
3.2. Efficacy Outcome
3.3. Diet and Global Physical Activity Questionnaire (GPAQ)
3.4. Safety Analysis
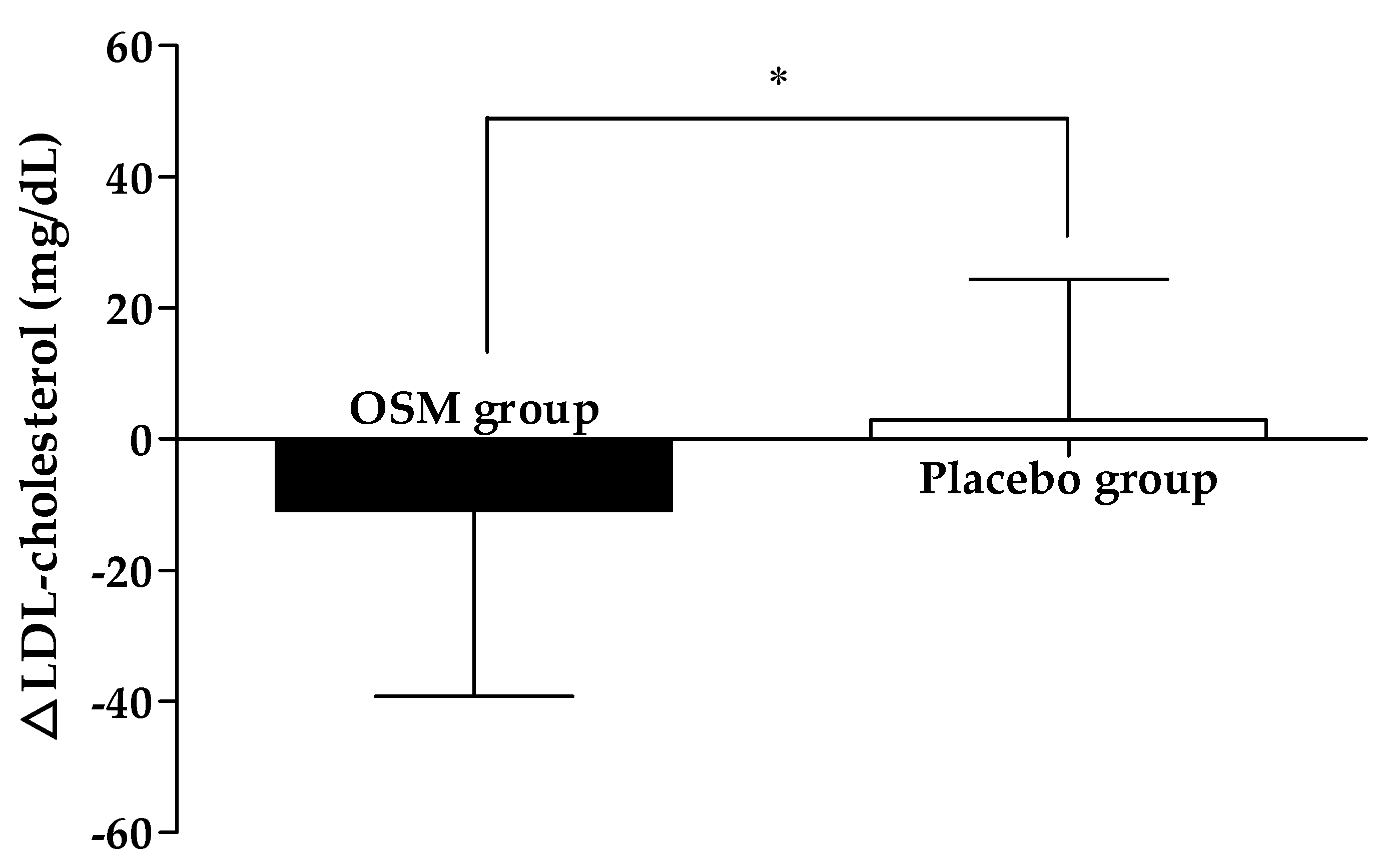
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.; Burmester, G.; Combeet, B.; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530, —In this Article, Catherine Pelletier. 2016. [Google Scholar]
- Zimmet, P.; Alberti, K.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Barr, E.L.; Zimmet, P.Z.; Welborn, T.A.; Jolley, D.; Magliano, D.J.; Dunstan, D.W.; Cameron, A.J.; Dwyer, T.; Taylor, H.R.; Tonkin, A.M. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007, 116, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Oizumi, T.; Daimon, M.; Jimbu, Y.; Wada, K.; Kameda, W.; Susa, S.; Yamaguchi, H.; Ohnuma, H.; Tominaga, M.; Kato, T. Impaired glucose tolerance is a risk factor for stroke in a Japanese sample—The Funagata study. Metabolism 2008, 57, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Ryden, L.; Ferrari, R.; Malmberg, K.; Pyörälä, K.; Simoons, M.; Standl, E.; Soler-Soler, J.; Öhrvik, J. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: The Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2004, 25, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Int. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Ju, I.-S.; Kim, B.-C.; Lee, W.-S.; Kim, M.-J.; Lee, B.-G.; An, B.-J.; Kim, J.-H.; Kwon, O.-J. Biological activity of Omija (Schizandra chinensis Baillon) extracts. Appl. Biol. Chem. 2007, 50, 198–203. [Google Scholar]
- Kim, C.-H.; Kwon, M.-C.; Kim, H.-S.; Ahn, J.-H.; Chio, G.-P.; Choi, Y.-B.; Ko, J.-R.; Lee, H.-Y. Enhancement of immune activities of Kadsura japonica Dunal. through conventional fermentation process. Korean J. Med. Crop Sci. 2007, 15, 162–169. [Google Scholar]
- Lee, S.-H.; Lee, Y.-C.; Yoon, S.-K. Isolation of the antimicrobial compounds from Omija (Schizandra chinensis) extract. Korean J. Food Sci. Technol. 2003, 35, 483–487. [Google Scholar]
- Mok, C. Quality characteristics of instant tea prepared from spray-dried Omija (Schizandra chinensis Baillon) extract/grape juice mixture. Food Eng. Prog. 2005, 9, 226–230. [Google Scholar]
- Oh, S.-L.; Kim, S.-S.; Min, B.-Y.; Chung, D.-H. Composition of free sugars, free amino acids, non-volatile organic acids and tannins in the extracts of L. chinensis M., A. acutiloba K., S. chinensis B. and A. sessiliflorum S. Korean J. Food Sci. Technol. 1990, 22, 76–81. [Google Scholar]
- Kim, D.; Kim, M.; Kim, H.; Park, J.; Lim, J. Hong S: Herb Medicinal Pharmacognosy; Shinlilsangsa: Seoul, Korea, 2005; p. 407. [Google Scholar]
- Kim, O.-C.; Jang, H.-J. Volatile components of Schizandra chinensis Bullion. Appl. Biol. Chem. 1994, 37, 30–36. [Google Scholar]
- Gao, X.X.; Meng, X.Z.; Li, J.H.; Tong, H.B. Hypoglycemic effects of a watersoluble polysaccharide isolated from Schisandra chinensis (Turcz.) Baill in alloxaninduced diabetic mice. J. Biotechnol. 2008, 136, S725. [Google Scholar]
- Gao, X.X.; Meng, X.Z.; Li, J.H.; Tong, H.B. Isolation, characterization and hypoglycemic activity of an acid polysaccharide isolated from Schisandra chinensis (Turcz.) Baill. Lett. Org. Chem. 2009, 6, 428–433. [Google Scholar] [CrossRef]
- Jin, D.; Zhao, T.; Feng, W.W.; Mao, G.H.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; Wang, X.T.; Yang, L.Q.; et al. Schisandra polysaccharide increased glucose consumption by up-regulating the expression of GLUT-4. Int. J. Biol. Macromol. 2016, 87, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Du, X.X.; Tao, X.; Liang, S.; Che, J.Y.; Yang, S.; Li, H.; Chen, J.G.; Wang, C.M. Hypoglycemic Effect of Acidic Polysaccharide from Schisandra chinensis on T2D Rats Induced by High-Fat Diet Combined with STZ. Biol. Pharm. Bull. 2019, 42, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.-H.; Ha, K.-S.; Moon, K.-S.; Lee, O.-H.; Jang, H.-D.; Kwon, Y.-I. In vitro and in vivo anti-hyperglycemic effects of Omija (Schizandra chinensis) fruit. Int. J. Mol. Sci. 2011, 12, 1359–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.-Y.; Qin, L.-Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M. Soy consumption and the risk of prostate cancer: An updated systematic review and meta-analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, C.; Wada, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef]
- Dong, J.Y.; Tong, X. Effect of soya protein on blood pressure: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2011, 106, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Beavers, D.P.; Beavers, K.M. Exposure to isoflavone-containing soy products and endothelial function: A Bayesian meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 182–191. [Google Scholar] [CrossRef]
- Villegas, R.; Gao, Y.-T.; Yang, G.; Li, H.-L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Takahashi, Y.; Kirii, K.; Inoue, M.; Noda, M.; Tsugane, S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J. Nutr. 2010, 140, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Park, K.-H.; Yang, H.J.; Jang, D.J.; Lee, H.Y.; Park, Y.M.; Kim, B.S.; Shin, D.Y. Ameliorative Effects of Schizandra chinensis Extracts and Their Soybean Powder Blends to Diabetes Mellitus. J. Food Nutr. Res. 2022, 10, 8–18. [Google Scholar]
- Chi, X.-X.; Zhang, T.; Zhang, D.-J.; Yu, W.; Wang, Q.-Y.; Zhen, J.-L. Effects of isoflavones on lipid and apolipoprotein levels in patients with type 2 diabetes in Heilongjiang Province in China. J. Clin. Biochem. Nutr. 2016, 59, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Fuller, J.H.; Stevens, L.K. Epidemiology of hypertension in diabetic patients and implications for treatment. Diabetes Care 1991, 14, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Kim, K.H.; Kim, T.H.; Lee, H.J. Analysis and characterization of aroma-active compounds of Schizandra chinensis (omija) leaves. J. Sci. Food Agric. 2005, 85, 161–166. [Google Scholar] [CrossRef]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M.; Holman, R.R.; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: A model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin. Sci. 2001, 101, 671–679. [Google Scholar] [CrossRef]
- Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jędrych, M. Effects of soy isoflavones on glycemic control and lipid profile in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 1886. [Google Scholar] [CrossRef] [PubMed]
- Ta, N.T.; Ngo, H.T.T.; Nguyen, P.M.; Truong, T.T.; Nguyen, G.H.; Dinh, H.T.D.; Nguyen, L.T.; Le, H.T.; Nguyen, K.C.; Yamamoto, S. Effectiveness of Textured Soybean Protein on Blood Biochemistry in Vietnamese Type 2 Diabetes Mellitus Patients. J. Nutr. Sci. Vitaminol. 2022, 68, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, M.; Lazzarini, D.; Fumelli, P.; Carle, F.; Kosmidis, A. Bioelectrical impedance analysis and diabetes mellitus: Which correlation among fructosamine, glycosylated haemoglobin and exchangeable potassium. Minerva Med. 2007, 98, 633–638. [Google Scholar] [PubMed]
| Component | OSM Supplement (%) | Placebo Supplement (%) |
|---|---|---|
| OSM (Omija extract and soybean mixture) | 35.7 | 0.0 |
| Crystalline cellulose | 62.3 | 96.8 |
| Red food coloring | 0.0 | 1.2 |
| Silicon dioxide | 1.0 | 1.0 |
| Magnesium stearate | 1.0 | 1.0 |
| Total | 100.0 | 100.0 |
| OSM Group (n = 34) | Placebo Group (n = 29) | Total (n = 63) | p-Value (1) | |
|---|---|---|---|---|
| Sex (M/F) | 18/16 | 13/16 | 31/32 | 0.521 (2) |
| Age (years) | 50.82± 9.55 | 49.38 ± 10.74 | 50.16 ± 10.06 | 0.574 |
| Height (cm) | 165.26 ± 11.08 | 163.14 ± 8.26 | 164.29 ± 9.86 | 0.398 |
| Weight (kg) | 68.74 ± 13.10 | 65.72 ± 11.20 | 67.35 ± 12.26 | 0.334 |
| BMI (kg/m2) | 24.96 ± 2.43 | 24.54 ± 2.53 | 24.77 ± 2.46 | 0.507 |
| SBP (mmHg) | 127.09 ± 10.78 | 126.28 ± 10.34 | 126.71 ± 10.50 | 0.762 |
| DBP (mmHg) | 79.50 ± 8.57 | 79.31 ± 9.66 | 79.41 ± 9.01 | 0.935 |
| Pulse (beats/minute) | 77.18 ± 9.66 | 80.34 ± 10.27 | 78.63 ± 9.99 | 0.212 |
| FPG (mg/dL) | 116.62 ± 10.55 | 109.00 ± 8.19 | 113.11 ± 10.20 | 0.003 ** |
| HbA1c (%) | 5.83 ± 0.29 | 5.67 ± 0.34 | 5.76 ± 0.32 | 0.048 * |
| Alcohol (n, %) | 13 (38.24) | 12 (41.38) | 25 (39.68) | 0.799 (2) |
| Alcohol (units/week) | 10.54± 5.17 | 14.53± 5.30 | 12.45± 5.51 | 0.070 |
| Smoking (n, %) | 5 (14.71) | 3 (10.34) | 8 (12.70) | 0.604 (2) |
| Smoking (cigarette/day) | 10.60± 2.61 | 10.67± 4.04 | 10.63± 2.92 | 0.978 |
| Diabetic family history (n, %) | 13 (38.24) | 8 (27.59) | 21 (33.3) | 0.372 (2) |
| OSM Group (n = 34) | Placebo Group (n = 29) | p-Value (2) | Adj. p-Value (3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Change in the Value | p-Value (1) | Baseline | 12 Weeks | Change in the Value | p-Value (1) | ||||
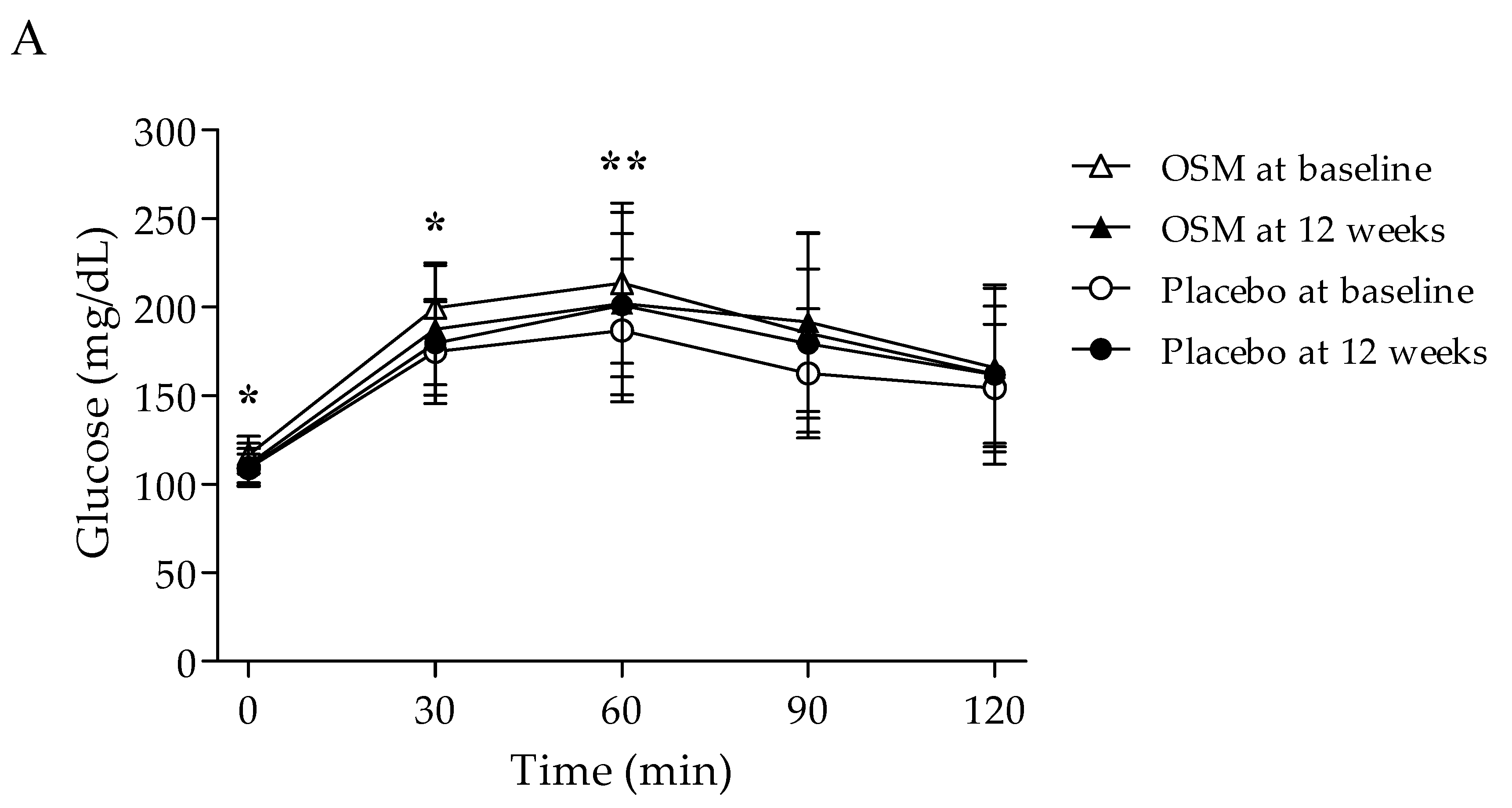
| FPG (mg/dL) | 0 min | 116.62 ± 10.55 | 111.00 ± 12.07 | −5.62 ± 9.73 | 0.002 | 109.00 ± 8.19 | 109.66 ± 10.38 | 0.66 ± 12.25 | 0.775 | 0.027 * | 0.020 * |
| PPG (mg/dL) | 30 min | 199.56 ± 24.04 | 187.74 ± 37.42 | −11.82 ± 28.98 | 0.023 | 174.93 ± 29.37 | 179.59 ± 23.47 | 4.66 ± 26.36 | 0.350 | 0.022 * | 0.022 * |
| 60 min | 213.62 ± 45.16 | 202.15 ± 51.56 | −11.47 ± 31.36 | 0.041 | 186.76 ± 40.25 | 201.14 ± 40.45 | 14.38 ± 32.84 | 0.026 | 0.002 ** | 0.002 ** | |
| 90 min | 185.41 ± 56.13 | 191.62 ± 50.41 | 6.21 ± 38.68 | 0.356 | 162.59 ± 36.46 | 179.48 ± 42.14 | 16.90 ± 30.08 | 0.005 | 0.232 | 0.232 | |
| 120 min | 162.00 ± 50.61 | 165.82 ± 44.72 | 3.82 ± 38.22 | 0.564 | 154.41 ± 35.99 | 161.90 ± 38.72 | 7.48 ± 31.91 | 0.217 | 0.685 | 0.685 | |
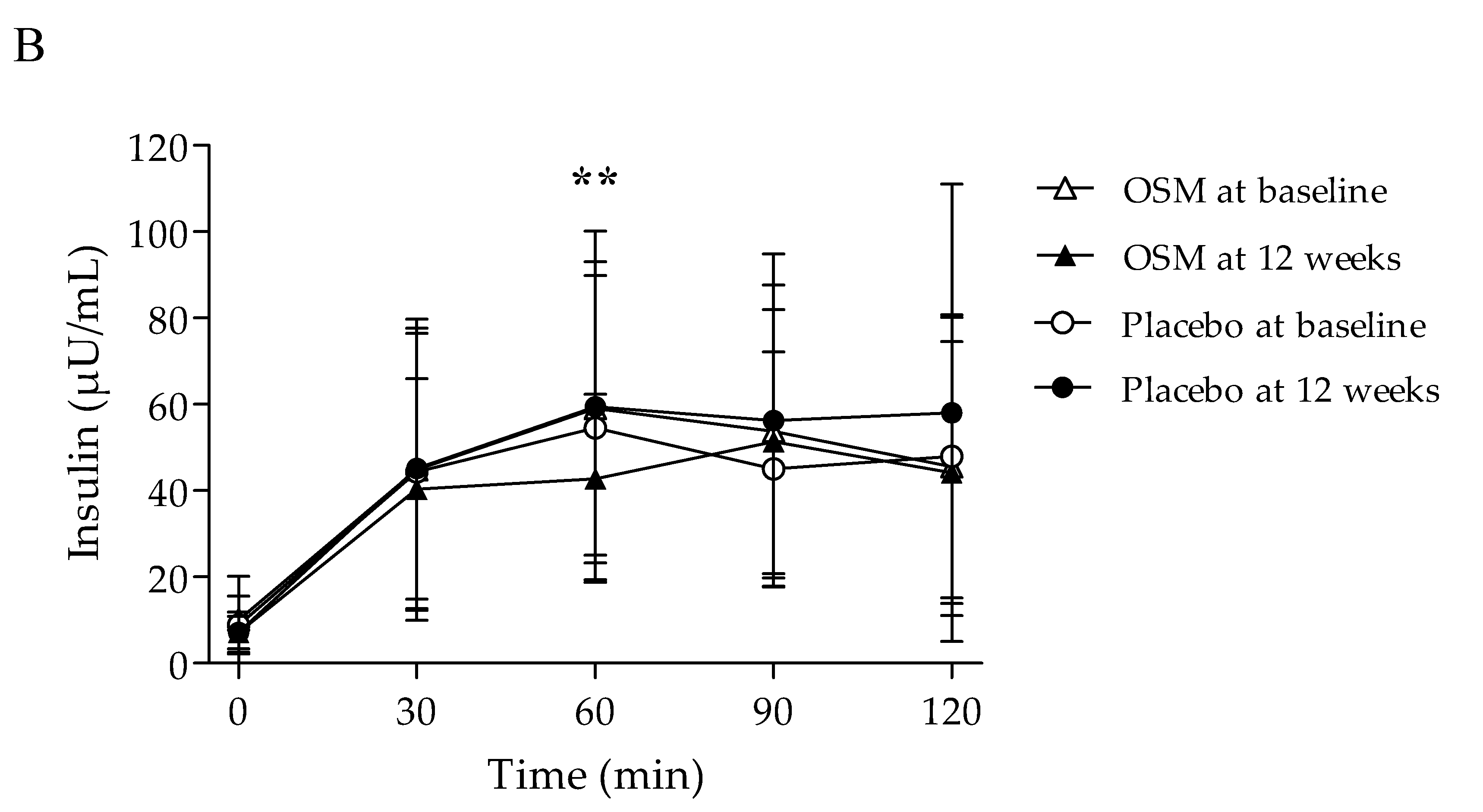
| FPI (μU/mL) | 0 min | 10.08 ± 10.07 | 7.17 ± 4.65 | −2.91 ± 8.45 | 0.053 | 8.85 ± 6.72 | 7.13 ± 3.74 | −1.72 ± 5.99 | 0.134 | 0.527 | 0.527 |
| PPI (μU/mL) | 30 min | 44.85 ± 34.93 | 40.38 ± 25.53 | −4.46 ± 20.94 | 0.223 | 44.38 ± 32.10 | 45.17 ± 32.49 | 0.78 ± 19.44 | 0.830 | 0.310 | 0.310 |
| 60 min | 59.06 ± 34.03 | 42.77 ± 19.53 | −16.29 ± 27.34 | 0.002 | 54.60 ± 35.22 | 59.42 ± 40.71 | 4.82 ± 32.01 | 0.424 | 0.006 ** | 0.006 ** | |
| 90 min | 53.71 ± 33.93 | 51.39 ± 30.60 | −2.32 ± 27.10 | 0.621 | 45.00 ± 27.19 | 56.26 ± 38.59 | 11.26 ± 31.00 | 0.061 | 0.068 | 0.068 | |
| 120 min | 45.60 ± 34.53 | 44.18 ± 30.37 | −1.41 ± 20.84 | 0.695 | 47.97 ± 32.78 | 58.02 ± 53.02 | 10.05 ± 37.13 | 0.156 | 0.147 | 0.129 | |
| AUC | Glucose (mg × min/dL) | 8168.82 ± 3679.67 | 8281.76 ± 3570.23 | 112.94 ± 2608.49 | 0.802 | 6614.48 ± 2944.75 | 7720.86 ± 3043.42 | 1106.38 ± 2217.94 | 0.012 | 0.112 | 0.112 |
| Insulin (μU × min/mL) | 4357.84 ± 2200.15 | 3948.49 ± 1996.45 | −409.35 ± 1374.66 | 0.092 | 4110.19 ± 2524.17 | 4947.18 ± 3684.09 | 836.99 ± 2345.70 | 0.065 | 0.016 * | 0.011 * | |
| HOMA-IR | 3.01 ± 3.26 | 2.00 ± 1.43 | −1.02 ± 2.78 | 0.041 | 2.43 ± 2.04 | 1.96 ± 1.19 | −0.47 ± 2.00 | 0.218 | 0.381 | 0.381 | |
| HOMA-β | 64.56 ± 54.45 | 55.01 ± 32.55 | −9.55 ± 45.11 | 0.226 | 68.69 ± 45.12 | 55.86 ± 27.53 | −12.83 ± 32.01 | 0.040 | 0.744 | 0.744 | |
| HbA1c (%) | 5.83 ± 0.29 | 5.92 ± 0.24 | 0.09 ± 0.16 | 0.002 | 5.67 ± 0.34 | 5.81 ± 0.31 | 0.14 ± 0.17 | <.0001 | 0.227 | 0.195 | |
| C-peptide (ng/mL) | 2.86 ± 1.43 | 2.30 ± 0.93 | −0.56 ± 1.11 | 0.006 | 2.68 ± 1.13 | 2.25 ± 0.82 | −0.42 ± 0.96 | 0.025 | 0.615 | 0.615 | |
| Fructosamine (μmol/L) | 262.88 ± 24.46 | 254.97 ± 20.90 | −7.91 ± 13.78 | 0.002 | 254.62 ± 18.93 | 253.79 ± 15.86 | −0.83 ± 11.67 | 0.706 | 0.033 * | 0.033 * | |
| AMPK (ng/mL) | 7.73 ± 6.91 | 8.08 ± 5.35 | 0.35 ± 5.61 | 0.722 | 7.68 ± 2.98 | 11.40 ± 8.45 | 3.72 ± 7.58 | 0.013 | 0.047 * | 0.047 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Baek, H.-I.; Ha, K.-C.; Cha, Y.-S.; Park, S.-J. Efficacy and Safety of Omija (Schisandra chinensis) Extract Mixture on the Improvement of Hyperglycemia: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3159. https://doi.org/10.3390/nu14153159
Kim D-S, Baek H-I, Ha K-C, Cha Y-S, Park S-J. Efficacy and Safety of Omija (Schisandra chinensis) Extract Mixture on the Improvement of Hyperglycemia: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients. 2022; 14(15):3159. https://doi.org/10.3390/nu14153159
Chicago/Turabian StyleKim, Da-Som, Hyang-Im Baek, Ki-Chan Ha, Youn-Soo Cha, and Soo-Jung Park. 2022. "Efficacy and Safety of Omija (Schisandra chinensis) Extract Mixture on the Improvement of Hyperglycemia: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial" Nutrients 14, no. 15: 3159. https://doi.org/10.3390/nu14153159
APA StyleKim, D.-S., Baek, H.-I., Ha, K.-C., Cha, Y.-S., & Park, S.-J. (2022). Efficacy and Safety of Omija (Schisandra chinensis) Extract Mixture on the Improvement of Hyperglycemia: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Nutrients, 14(15), 3159. https://doi.org/10.3390/nu14153159






