Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients
Abstract
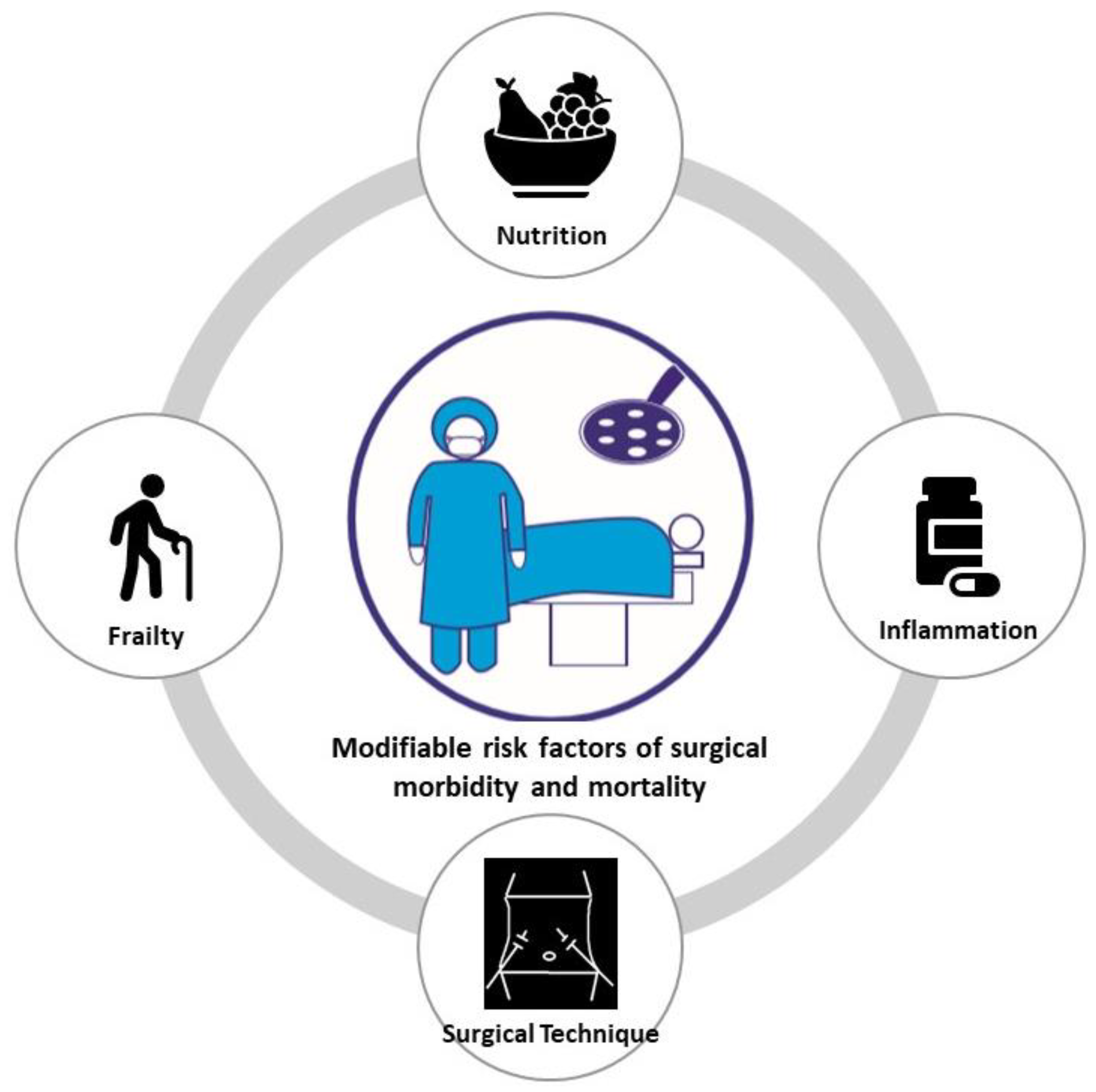
:1. Introduction
2. Methodology
3. Nutrition
3.1. Preoperative Nutritional Status and Its Influence on the Outcomes of Surgery
3.2. Nutritional Risk Screening and Assessment
3.3. Nutritional Support
4. Inflammation
4.1. Prognostic Effect of the Preoperative Inflammation on Short-Term Outcomes
4.2. Prognostic Effect of the Preoperative Inflammation on Long-Term Outcomes
5. Frailty
6. Minimally Invasive Surgery
6.1. Surgical Stress Response after Minimally Invasive Surgery
6.2. Enhanced Recovery after Surgery (ERAS) Programs for Minimally Invasive Surgery
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moonesinghe, S.R.; Harris, S.; Mythen, M.G.; Rowan, K.M.; Haddad, F.S.; Emberton, M.; Grocott, M.P. Survival after postoperative morbidity: A longitudinal observational cohort study. Br. J. Anaesth. 2014, 113, 977–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, D.L.; Bowe, E.A.; Henderson, W.G.; Khuri, S.F.; Mentzer, R.M.J. National Surgical Quality Improvement Program (NSQIP) risk factors can be used to validate American Society of Anesthesiologists’ Physical Status Classification (ASA PS) levels. Ann. Surg. 2006, 243, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar] [PubMed]
- Yuan, P.; Wu, Z.; Li, Z.; Bu, Z.; Wu, A.; Wu, X.; Zhang, L.; Shi, J.; Ji, J. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019, 19, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann. Surg. Oncol. 2013, 20, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.G.; Li, P.; Tang, D.; Chen, J.; Wang, D.R. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J. Gastroenterol. 2013, 19, 4060–4065. [Google Scholar] [CrossRef]
- Slankamenac, K.; Slankamenac, M.; Schlegel, A.; Nocito, A.; Rickenbacher, A.; Clavien, P.A.; Turina, M. Impact of postoperative complications on readmission and long-term survival in patients following surgery for colorectal cancer. Int. J. Color Dis. 2017, 32, 805–811. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Czaykowski, P.M.; Gill, S.; Kennecke, H.F.; Gordon, V.L.; Turner, D. Adjuvant chemotherapy for stage III colon cancer: Does timing matter? Dis. Colon Rectum 2011, 54, 1082–1089. [Google Scholar] [CrossRef]
- Bayraktar, U.D.; Chen, E.; Bayraktar, S.; Sands, L.R.; Marchetti, F.; Montero, A.J.; Rocha-Lima, C.M.S. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer 2011, 117, 2364–2370. [Google Scholar] [CrossRef]
- Sasse, P.; Bergmann, A.; Afonso, W.; Ladas, E.J.; Ferman, S. Malnutrition at diagnosis and throughout therapy in pediatric patients with solid tumors: A single-institution study in a developing country. Pediatr. Blood Cancer 2021, 68, e29317. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- da Silva Nunes, L.F.P.; Gadelha, P.C.F.P.; de Souza Costa, M.D.; de Amorim, A.C.R.; da Silva, M.D.G.B. Nutritional status and its impact on time and relocation in postoperative complications of abdominal patients undergoing surgery. Nutr. Hosp. 2014, 30, 629–635. [Google Scholar]
- van Stijn, M.F.; Korkic-Halilovic, I.; Bakker, M.S.; van der Ploeg, T.; van Leeuwen, P.A.; Houdijk, A.P. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: A systematic review. J. Parenter. Enter. Nutr. 2013, 37, 37–43. [Google Scholar] [CrossRef]
- Kwag, S.J.; Kim, J.G.; Kang, W.K.; Lee, J.K.; Oh, S.T. The nutritional risk is a independent factor for postoperative morbidity in surgery for colorectal cancer. Ann. Surg. Treat Res. 2014, 86, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Lee, J.S. Impact of preoperative malnutrition, based on albumin level and body mass index, on operative outcomes in patients with pancreatic head cancer. J. Hepatobiliary Pancreat. Sci. 2021, 12, 1069–1075. [Google Scholar] [CrossRef]
- Laky, B.; Janda, M.; Cleghorn, G.; Obermair, A. Comparison of Different Nutritional Assessments and Body-Composition Measurements in Detecting Malnutrition Among Gynecologic Cancer Patients. Am. J. Clin. Nutr. 2008, 87, 1678–1685. [Google Scholar] [CrossRef]
- Jin, J.; Xiong, G.; Wang, X.; Peng, F.; Zhu, F.; Wang, M.; Qin, R. The impact of preoperative and postoperative malnutrition on outcomes for ampullary carcinoma after pancreaticoduodenectomy. Front. Oncol. 2021, 11, 748341. [Google Scholar] [CrossRef]
- Chen, S.B.; Liu, D.T.; Chen, Y.P. The impact of preoperative nutritional status on the survival of patients with esophageal squamous cell carcinoma. Front. Surg. 2021, 8, 752792. [Google Scholar] [CrossRef]
- Lee, B.; Han, H.S.; Yoon, Y.S. Impact of preoperative malnutrition on postoperative long-term outcomes of patients with pancreatic head cancer. Ann. Surg. Open 2021, 2, e047. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Shou, J.A.; Sigal, R.; Ziegler, M.; Daly, J.M. The influence of protein malnutrition on T cell, natural killer cell, and lymphokine-activated killer cell function, and on biological responsiveness to high-dose interleukin-2. Cell Immunol. 1990, 128, 569–577. [Google Scholar] [CrossRef]
- Good, R.A.; West, A.; Day, N.K.; Dong, Z.W.; Fernandes, G. Effects of undernutrition of host cell and organ function. Cancer Res. 1982, 42, 737s–746s. [Google Scholar]
- Di Fiore, F.; Lecleire, S.; Pop, D.; Rigal, O.; Hamidou, H.; Paillot, B.; Ducrotté, P.; Lerebours, E.; Michel, P. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am. J. Gastroenterol. 2007, 102, 2557–2563. [Google Scholar] [CrossRef]
- Dhungel, B.; Diggs, B.S.; Hunter, J.G.; Sheppard, B.C.; Vetto, J.T.; Dolan, J.P. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J. Gastrointest. Surg. 2010, 14, 1492–1501. [Google Scholar] [CrossRef]
- Migita, K.; Takayama, T.; Matsumoto, S.; Wakatsuki, K.; Tanaka, T.; Ito, M.; Kunishige, T.; Nakade, H.; Nakajima, Y. Impact of being underweight on the long-term outcomes of patients with gastric cancer. Gastric Cancer. 2016, 19, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional risk screening and assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [Green Version]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Elia, M. Screening for malnutrition: A multidisciplinary responsibility. In Development and Use of the Malnutrition Universal Screening Tool (‘MUST’) for Adults; BAPEN: Redditch, UK, 2003. [Google Scholar]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Charney, P. Nutrition screening vs. nutrition assessment: How do they differ? Nutr. Clin. Pract. 2008, 23, 366–372. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Mazaki, T.; Ebisawa, K. Enteral versus parenteral nutrition after gastrointestinal surgery: A systematic review and meta-analysis of randomized controlled trials in the English literature. J. Gastrointest. Surg. 2008, 12, 739–755. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Moyes, L.H.; Leitch, E.F.; McKee, R.F.; Anderson, J.H.; Horgan, P.G.; McMillan, D.C. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resections for colorectal cancer. Br. J. Cancer 2009, 100, 1236–1239. [Google Scholar] [CrossRef] [Green Version]
- Kubo, T.; Ono, S.; Ueno, H.; Shinto, E.; Yamamoto, J.; Hase, K. Elevated preoperative C-reactive protein levels are a risk factor for the development of postoperative infectious complications following elective colorectal surgery. Langenbecks Arch. Surg. 2013, 398, 965–971. [Google Scholar] [CrossRef]
- González-Martínez, S.; Olona Tabueña, N.; Martín Baranera, M.; Martí-Saurí, I.; Moll, J.L.; García, M.Á.M.; Zurdo, J.M. Proteínas mediadoras de la respuesta inflamatoria como predictores de resultados adversos postoperatorios en pacientes quirúrgicos octogenarios: Estudio prospectivo observacional. Cirugia Española. 2015, 93, 166–173. [Google Scholar] [CrossRef]
- Neal, C.P.; Mann, C.D.; Garcea, G.; Briggs, C.D.; Dennison, A.R.; Berry, D.P. Preoperative systemic inflammation and infectious complications after resection of colorectal liver metastases. Arch. Surg. 2011, 146, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- De Magistris, L.; Paquette, B.; Orry, D.; Facy, O.; Di Giacomo, G.; Rat, P.; Binquet, C.; Ortega-Deballon, P. Preoperative inflammation increases the risk of infection after elective colorectal surgery: Results from a prospective cohort. Int. J. Colorectal Dis. 2016, 31, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Srinivasa, S.; Kahokehr, A.A.; Yu, T.C.; Hill, A.G. Preoperative glucocorticoid use in major abdominal surgery: Systematic review and meta-analysis of randomized trials. Ann. Surg. 2011, 254, 183–191. [Google Scholar] [CrossRef]
- Chapman, S.J.; Glasbey, J.; Kelly, M.; Khatri, C.; Nepogodiev, D.; Fitzgerald, J.E.; Bhangu, A.; Harrison, E.; Adams, R. Impact of postoperative non-steroidal anti-inflammatory drugs on adverse events after gastrointestinal surgery. Br. J. Surg. 2014, 101, 1413–1423. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, W.; Yu, N.; Yang, G.; Xu, H.; Liu, H. Systemic inflammatory response predicts poor prognoses in Barcelona Clinic Liver Cancer stage B/C hepatocellular carcinoma with transarterial chemoembolization: A prospective study. Transl. Cancer Res. 2019, 8, 2552–2563. [Google Scholar] [CrossRef]
- Saad, M.R.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Lee, J.S.; Shehta, A. Impact of Acute Inflammation on the Survival Outcomes of Patients with Resected Pancreatic Ductal Adenocarcinoma. Dig. Surg. 2021, 38, 343–351. [Google Scholar] [CrossRef]
- Hausmann, S.; Kong, B.; Michalski, C.; Erkan, M.; Friess, H. The role of inflammation in pancreatic cancer. Adv. Exp. Med. Biol. 2014, 816, 129–151. [Google Scholar]
- D'Silva, M.; Han, H.S.; Yoon, Y.S. Role of cholangitis in predicting survival in patients with carcinoma of the ampulla of vater. Surg. Oncol. 2020, 35, 34–38. [Google Scholar] [CrossRef]
- Han, H.S.; Cho, J.Y.; Yoon, Y.S.; Ahn, K.S.; Kim, H. Preoperative inflammation is a prognostic factor for gallbladder carcinoma. Br. J. Surg. 2011, 98, 111–116. [Google Scholar] [CrossRef]
- Akita, M.; Ajiki, T.; Matsumoto, T.; Shinozaki, K.; Goto, T.; Asari, S.; Toyama, H.; Kido, M.; Fukumoto, T.; Ku, Y. Preoperative cholangitis affects survival outcome in patients with extrahepatic bile duct cancer. J. Gastrointest. Surg. 2017, 21, 983–989. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Hwang, D.W.; Jung, K.; Kim, J.H.; Kwon, Y.; Kim, H. Preoperative cholangitis and metastatic lymph node have a negative impact on survival after resection of extrahepatic bile duct cancer. World J. Surg. 2012, 36, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Hwang, J.Y.; Han, H.S.; Kim, S.T.; Hwang, I.; Chun, Y.O. The impact of acute inflammation on progression and metastasis in pancreatic cancer animal model. Surg. Oncol. 2018, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.J.; Sharma, M.D.; Chandler, P.R.; Duhadaway, J.B.; Everhart, M.E.; Johnson, B.A., 3rd; Kahler, D.J.; Pihkala, J.; Soler, A.P.; Munn, D.H. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc. Natl. Acad. Sci. USA 2008, 105, 17073–17078. [Google Scholar] [CrossRef] [Green Version]
- Jänne, P.A.; Mayer, R.J. Chemoprevention of colorectal cancer. N. Engl. J. Med. 2000, 342, 1960–1968. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Coombes, R.C.; Tovey, H.; Kilburn, L.; Mansi, J.; Palmieri, C.; Bartlett, J.; Hicks, J.; Makris, A.; Evans, A.; Loibl, S.; et al. Randomized European Celecoxib Trial (REACT) trial management group and investigators. Effect of celecoxib vs placebo as adjuvant therapy on disease-free survival among patients with breast cancer: The REACT Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1291–1301. [Google Scholar] [CrossRef]
- Huang, Q.; Qin, H.; Xiao, J.; He, X.; Xie, M.; He, X.; Yao, Q.; Lan, P.; Lian, L. Association of tumor differentiation and prognosis in patients with rectal cancer undergoing neoadjuvant chemoradiation therapy. Gastroenterol. Rep. 2019, 7, 283–290. [Google Scholar] [CrossRef]
- Arico, S.; Pattingre, S.; Bauvy, C.; Gane, P.; Barbat, A.; Codogno, P.; Ogier-Denis, E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J. Biol. Chem. 2002, 277, 27613–27621. [Google Scholar] [CrossRef] [Green Version]
- Grubbs, C.J.; Lubet, R.A.; Koki, A.T.; Leahy, K.M.; Masferrer, J.L.; Steele, V.E.; Kelloff, G.J.; Hill, D.L.; Seibert, K. Celecoxib inhibits Nbutyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000, 60, 5599–5602. [Google Scholar]
- Meyerhardt, J.A.; Shi, Q.; Fuchs, C.S.; Meyer, J.; Niedzwiecki, D.; Zemla, T.; Kumthekar, P.; Guthrie, K.A.; Couture, F.; Kuebler, P.; et al. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage iii colon cancer: The CALGB/SWOG 80702 (Alliance) Randomized Clinical Trial. JAMA 2021, 325, 1277–1286. [Google Scholar] [CrossRef]
- Rockwood, K.; Andrew, M.; Mitnitski, A. A comparison of two approaches to measuring frailty in elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 738–743. [Google Scholar] [CrossRef]
- Ji, L.; Jazwinski, S.M.; Kim, S. Frailty and Biological Age. Ann. Geriatr. Med. Res. 2021, 25, 141–149. [Google Scholar] [CrossRef]
- Panayi, A.C.; Orkaby, A.R.; Sakthivel, D.; Endo, Y.; Varon, D.; Roh, D.; Orgill, D.P.; Neppl, R.L.; Javedan, H.; Bhasin, S.; et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am. J. Surg. 2019, 218, 393–400. [Google Scholar] [CrossRef]
- Carli, F.; Zavorsky, G.S. Optimizing functional exercise capacity in the elderly surgical population. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 23–32. [Google Scholar] [CrossRef]
- Hijazi, Y.; Gondal, U.; Aziz, O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int. J. Surg. 2017, 39, 156–162. [Google Scholar] [CrossRef]
- Yi, L.; Zhang, W.; Zhang, H.; Shen, J.; Zou, J.; Luo, P.; Zhang, J. Systematic review and meta-analysis of the benefit of celecoxib in treating advanced non-small-cell lung cancer. Drug Des. Devel. Ther. 2018, 12, 2455–2466. [Google Scholar] [CrossRef] [Green Version]
- Blum, C.A.; Adams, D.B. Who did the first laparoscopic cholecystectomy? J. Minim. Access Surg. 2011, 7, 165–168. [Google Scholar] [CrossRef]
- Jaschinski, T.; Mosch, C.G.; Eikermann, M.; Neugebauer, E.A.; Sauerland, S. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst. Rev. 2018, 11, CD001546. [Google Scholar] [CrossRef]
- Buia, A.; Stockhausen, F.; Hanisch, E. Laparoscopic surgery: A qualified systematic review. World J. Methodol. 2015, 5, 238–254. [Google Scholar] [CrossRef]
- Stipancic, I.; Zarkovic, N.; Servis, D.; Sabolović, S.; Tatzber, F.; Busic, Z. Oxidative stress markers after laparoscopic and open cholecystectomy. J. Laparoendosc Adv. Surg. Tech. A 2005, 15, 347–352. [Google Scholar] [CrossRef]
- Mishra, V. Oxidative stress and role of antioxidant supplementation in critical illness. Clin. Lab. 2007, 53, 199–209. [Google Scholar]
- Kehlet, H. Surgical stress response: Does endoscopicsurgery confer an advantage? World J. Surg. 1999, 23, 801–807. [Google Scholar] [CrossRef]
- Whelan, R.L.; Franklin, M.; Holubar, S.D.; Donahue, J.; Fowler, R.; Munger, C.; Doorman, J.; Balli, J.E.; Glass, J.; Gonzalez, J.J.; et al. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg. Endosc. 2003, 17, 972–978. [Google Scholar] [CrossRef]
- Arsalani-Zadeh, R.; Ullah, S.; Khan, S.; MacFie, J. Oxidative stress in laparoscopic versus open abdominal surgery: A systematic review. J Surg. Res. 2011, 169, e59–e68. [Google Scholar] [CrossRef]
- Bardram, L.; Funch-Jensen, P.; Jensen, P.; Crawford, M.E.; Kehlet, H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995, 345, 763–764. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Multimodal strategies to improve surgical outcome. Am. J. Surg. 2002, 183, 630–641. [Google Scholar] [CrossRef]
- Grant, M.C.; Yang, D.; Wu, C.L.; Makary, M.A.; Wick, E.C. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: Results from a systematic review and meta-analysis. Ann. Surg. 2017, 265, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Q.; Bai, B.; Ji, G.; Liu, Y. Enhanced recovery after surgery programs for laparoscopic abdominal surgery: A systematic review and meta-analysis. World J. Surg. 2018, 42, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Kehlet, H. Management of patients in fast track surgery. BMJ 2001, 322, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H. Fast-track colonic surgery: Status and perspectives. Recent Results Cancer Res. 2005, 165, 8–13. [Google Scholar]
- Tanaka, R.; Lee, S.W.; Kawai, M.; Tashiro, K.; Kawashima, S.; Kagota, S.; Honda, K.; Uchiyama, K. Protocol for enhanced recovery after surgery improves short-term outcomes for patients with gastric cancer: A randomized clinical trial. Gastric Cancer 2017, 20, 861–871. [Google Scholar] [CrossRef]
- Visioni, A.; Shah, R.; Gabriel, E.; Attwood, K.; Kukar, M.; Nurkin, S. Enhanced recovery after surgery for noncolorectal surgery? A systematic review and meta-analysis of major abdominal surgery. Ann. Surg. 2018, 267, 57–65. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, G.; Zhang, W.; Zhang, F.; Lv, S.; Wang, A.; Fang, Z. Enhanced recovery after surgery programs for liver resection: A meta-analysis. J. Gastrointest. Surg. 2017, 21, 472–486. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, K.H.; Guan, Q.L.; Cao, N.; Chen, Y.; Zhao, P.; Chen, Y.L.; Yao, L. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: An update meta-analysis based on randomized controlled trials. Surg. Endosc. 2013, 27, 2466–2480. [Google Scholar] [CrossRef]
- Spanjersberg, W.R.; van Sambeeck, J.D.; Bremers, A.; Rosman, C.; van Laarhoven, C.J. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg. Endosc. 2015, 29, 3443–3453. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Han, H.-S. Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients. Nutrients 2022, 14, 3107. https://doi.org/10.3390/nu14153107
Lee B, Han H-S. Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients. Nutrients. 2022; 14(15):3107. https://doi.org/10.3390/nu14153107
Chicago/Turabian StyleLee, Boram, and Ho-Seong Han. 2022. "Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients" Nutrients 14, no. 15: 3107. https://doi.org/10.3390/nu14153107
APA StyleLee, B., & Han, H.-S. (2022). Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients. Nutrients, 14(15), 3107. https://doi.org/10.3390/nu14153107





