Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cases Ascertainment and Follow-Up Period
2.3. Adherence to Dietary Patterns
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorrectal Cancer. Available online: dietandcancerreport.org (accessed on 23 March 2022).
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar] [PubMed]
- Tucker, K.L. Dietary patterns, approaches, and multicultural perspective. Appl. Physiol. Nutr. Metab. 2010, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Boucher, K.; Caan, B.; Potter, J.; Ma, K.-N. Eating Patterns and Risk of Colon Cancer. Am. J. Epidemiol. 1998, 148, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Hu, F.B.; Hansen, H.; Wolk, A. Prospective study of major dietary patterns and colorectal cancer risk in women. Am. J. Epidemiol. 2001, 154, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.; Hu, F.B.; Fuchs, C.; Giovannucci, E.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Major dietary patterns and the risk of colorectal cancer in women. Arch. Intern. Med. 2003, 163, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Hu, F.B.; Fuchs, C.; Rimm, E.B.; Willett, W.C.; Giovannucci, E. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Causes Control 2004, 15, 853–862. [Google Scholar] [CrossRef]
- Kim, M.K.; Sasaki, S.; Otani, T.; Tsugane, S.; the Japan Public Health Center-based Prospective Study Group. Dietary patterns and subsequent colorectal cancer risk by subsite: A prospective cohort study. Int. J. Cancer 2005, 115, 790–798. [Google Scholar] [CrossRef]
- Kesse, E.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Dietary Patterns and Risk of Colorectal Tumors: A Cohort of French Women of the National Education System (E3N). Am. J. Epidemiol. 2006, 164, 1085–1093. [Google Scholar] [CrossRef]
- Butler, L.M.; Wang, R.; Koh, W.-P.; Yu, M.C. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br. J. Cancer 2008, 99, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Flood, A.; Rastogi, T.; Wirfält, E.; Mitrou, P.N.; Reedy, J.; Subar, A.F.; Kipnis, V.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.; et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am. J. Clin. Nutr. 2008, 88, 176–184. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, E.; Deneo-Pellegrini, H.; Boffetta, P.; Ronco, A.L.; Aune, D.; Acosta, G.; Mendilaharsu, M.; Brennan, P.; Ferro, G. Dietary patterns and risk of cancer: A factor analysis in Uruguay. Int. J. Cancer 2009, 124, 1391–1397. [Google Scholar] [CrossRef]
- Miller, P.E.; Lazarus, P.; Lesko, S.M.; Muscat, J.E.; Harper, G.; Cross, A.J.; Sinha, R.; Ryczak, K.; Escobar, G.; Mauger, D.T.; et al. Diet Index-Based and Empirically Derived Dietary Patterns Are Associated with Colorectal Cancer Risk. J. Nutr. 2010, 140, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, B.; Bastos, J.; Lunet, N. Dietary patterns and colorectal cancer: A case-control study from Portugal. Eur. J. Cancer Prev. 2011, 20, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Safari, A.; Shariff, Z.M.; Kandiah, M.; Rashidkhani, B.; Fereidooni, F. Dietary patterns and risk of colorectal cancer in Tehran Province: A case–control study. BMC Public Health 2013, 13, 222. [Google Scholar] [CrossRef] [Green Version]
- Nimptsch, K.; Malik, V.S.; Fung, T.T.; Pischon, T.; Hu, F.B.; Willett, W.C.; Fuchs, C.S.; Ogino, S.; Chan, A.T.; Giovannucci, E.; et al. Dietary patterns during high school and risk of col-orectal adenoma in a cohort of middle-aged women. Int. J. Cancer 2014, 134, 2458–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, P.P.; Woodrow, J.; Zhu, Y.; Roebothan, B.; McLaughlin, J.R.; Parfrey, P.S. Dietary patterns and colorectal cancer: Results from a Canadian population-based study. Nutr. J. 2015, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Azizi, H.; Asadollahi, K.; Esmaeili, E.D.; Mirzapoor, M. Iranian Dietary Patterns and Risk of Colorectal Cancer. Health Promot. Perspect. 2015, 5, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary patterns and colorectal cancer risk in a Korean population. Medicine 2016, 95, e3759. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef] [Green Version]
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Agraib, L.M.; Abu-Mweis, S.S.; Al-Jaberi, T.; Al-Nusairr, M.; Bani-Hani, K.E.; Heath, D.D. Dietary patterns and colorectal cancer. Clin. Nutr. 2016, 36, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Saito, E.; Sawada, N.; Ishihara, J.; Takachi, R.; Nanri, A.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; et al. Dietary patterns and colorectal cancer risk in middle-aged adults: A large population-based prospective cohort study. Clin. Nutr. 2018, 37, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Houshyari, M.; Jafari, S.; Rafiei, P.; Mazandaranian, M.; Hekmatdoost, A.; Sadeghi, A.; Hejazi, E. Dietary patterns and the risk of colorectal cancer and adenoma: A case control study in Iran. Gastroenterol. Hepatol. Bed Bench 2019, 12, 217–225. [Google Scholar] [PubMed]
- Castelló, A.; Amiano, P.; de Larrea, N.F.; Martín, V.; Alonso, M.H.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Olmedo-Requena, R.; Guevara, M.; Fernandez-Tardon, G.; et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr. 2018, 58, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Munoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Castelló, A.; Buijsse, B.; Martín, M.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Pastor-Barriuso, R.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Evaluating the Applicability of Data-Driven Dietary Patterns to Independent Samples with a Focus on Measurement Tools for Pattern Similarity. J. Acad. Nutr. Diet. 2016, 116, 1914–1924.e6. [Google Scholar] [CrossRef]
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Dierssen-Sotos, T.; Tardón, A.; Moreno, V.; et al. Adherence to the Western, Prudent and Medi-terranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Castelló, A.; de Larrea, N.F.; Martín, V.; Dávila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castaño-Vinyals, G.; Gómez-Acebo, I.; Fernández-Tardón, G.; et al. High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer 2017, 21, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Solans, M.; Castelló, A.; Benavente, Y.; Marcos-Gragera, R.; Amiano, P.; Gracia-Lavedan, E.; Costas, L.; Robles, C.; Gonzalez-Barca, E.; de la Banda, E.; et al. Adherence to the Western, Prudent, and Mediterranean dietary patterns and chronic lymphocytic leukemia in the MCC-Spain study. Haematologica 2018, 103, 1881–1888. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Kaaks, R. The EPIC Project: Rationale and study design. European Prospective Investigation into Cancer and Nu-trition. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Navarro, C.; Martínez, C.; Quirós, J.R.; Dorronsoro, M.; Barricarte, A.; Tormo, M.J.; Agudo, A.; Chirlaque, M.D.; Amiano, P.; et al. The European prospective investi-gation about cancer and nutrition (EPIC). Rev. Esp. Salud Publica 2004, 78, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S91–S99. [CrossRef] [Green Version]
- Relative validity and reproducibility of a diet history questionnaire in Spain. II. Nutrients. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S100–S109. [CrossRef]
- Panel de Consumo Alimentario. Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/ (accessed on 23 September 2021).
- Castaño-Vinyals, G.; Aragonés, N.; Pérez-Gómez, B.; Martín, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; de Sanjosé, S.; Jiménez-Moleón, J.J.; et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): Rationale and study design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Harrel, F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2015. [Google Scholar]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and “Western-Lifestyle” Inflammatory Diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Ashmore, J.H.; Rogers, C.J.; Kelleher, S.L.; Lesko, S.M.; Hartman, T.J. Dietary Iron and Colorectal Cancer Risk: A Review of Human Population Studies. Crit. Rev. Food Sci. Nutr. 2015, 56, 1012–1020. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Dissabandara, L.; Gopalan, V. A critical overview on the biological and molecular features of red and processed meat in colorectal carcinogenesis. J. Gastroenterol. 2016, 52, 407–418. [Google Scholar] [CrossRef]
- Santarelli, R.L.; Pierre, F.; Corpet, D. Processed Meat and Colorectal Cancer: A Review of Epidemiologic and Experimental Evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Giovannucci, E.; Willett, W.C. Dietary factors and risk of colon cancer. Ann. Med. 1994, 26, 443–452. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [Green Version]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Marino, M.; Masella, R.; Bulzomi, P.; Campesi, I.; Malorni, W.; Franconi, F. Nutrition and human health from a sex–gender perspective. Mol. Asp. Med. 2011, 32, 1–70. [Google Scholar] [CrossRef]
- Huyghe, J.R.; Harrison, T.A.; Bien, S.A.; Hampel, H.; Figueiredo, J.C.; Schmit, S.L.; Conti, D.V.; Chen, S.; Qu, C.; Lin, Y.; et al. Genetic architectures of proximal and distal colorectal cancer are partly distinct. Gut 2021, 70, 1325–1334. [Google Scholar] [CrossRef]
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014, 25, 1995–2001. [Google Scholar] [CrossRef]
- Bernardo, D.; Durant, L.; Mann, E.R.; Bassity, E.; Montalvillo, E.; Man, R.; Vora, R.; Reddi, D.; Bayiroglu, F.; Fernández-Salazar, L.; et al. Chemokine (C-C Motif) Receptor 2 Mediates Dendritic Cell Recruitment to the Human Colon but Is Not Responsible for Differences Observed in Dendritic Cell Subsets, Phenotype, and Function Between the Proximal and Distal Colon. Cell. Mol. Gastroenterol. Hepatol. 2015, 2, 22–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povey, A.C.; Hall, C.N.; Badawi, A.F.; Cooper, D.P.; O’Connor, P.J. Elevated levels of the pro-carcinogenic adduct, O6-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut 2000, 47, 362–365. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Deneo-Pellegrini, H.; Ronco, A.L.; Correa, P.; Boffetta, P.; Aune, D.; Acosta, G.; Mendilaharsu, M.; Luaces, M.E.; Lando, G.; et al. Dietary Patterns and Risk of Colorectal Cancer: A Factor Analysis in Uruguay. Asian Pac. J. Cancer Prev. 2011, 12, 753–759. [Google Scholar]
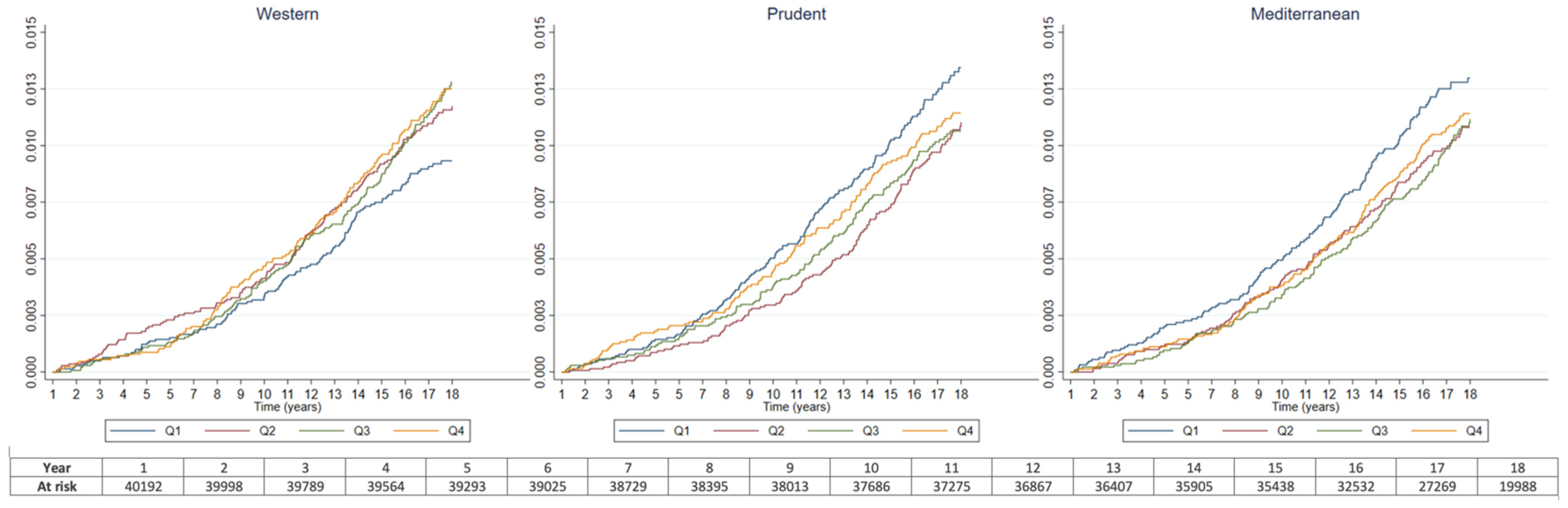


| Western | Prudent | Mediterranean | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p a | Q1 | Q2 | Q3 | Q4 | p a | Q1 | Q2 | Q3 | Q4 | p a | |
| n = 10,187 | n = 10,267 | n = 10,301 | n = 10,143 | n = 10,183 | n = 10,226 | n = 10,272 | n = 10,217 | n = 10,161 | n = 10,305 | n = 10,283 | n = 10,149 | ||||
| Alcohol (gr/day) median (IQR) b | 1.60 (0.00; 11.04) | 4.75 (0.01; 22.22) | 7.36 (0.36; 29.50) | 10.28 (1.43; 34.22) | <0.001 | 3.84 (0.00; 23.77) | 6.29 (0.04; 28.46) | 5.46 (0.10; 23.22) | 5.70 (0.59; 20.90) | <0.001 | 1.34 (0.00; 10.94) | 3.59 (0.00; 17.09) | 6.44 (0.49; 25.89) | 14.97 (2.63; 40.52) | <0.001 |
| Energy (Kcal/day) mean (sd) | 1625 (1335; 1988) | 1928 (1584; 2335) | 2124 (1784; 2536) | 2541 (2129; 3027) | <0.001 | 1830 (1478; 2264) | 2092 (1669; 2574) | 2067 (1657; 2572) | 2185 (1783; 2672) | <0.001 | 1587 (1297; 1952) | 1875 (1579; 2255) | 2122 (1797; 2526) | 2600 (2222; 3054) | <0.001 |
| BMI (kg/m2) mean (sd) | 28.23 (25.61; 31.33) | 27.94 (25.46; 30.85) | 27.71 (25.22; 30.41) | 27.44 (24.98; 30.15) | <0.001 | 28.07 (25.46; 30.86) | 27.90 (25.37; 30.78) | 27.81 (25.30; 30.71) | 27.49 (25.09; 30.33) | <0.001 | 28.07 (25.35; 31.24) | 27.80 (25.19; 30.73) | 27.67 (25.22; 30.51) | 27.77 (25.48; 30.34) | <0.001 |
| Age at recruitment (years) median (IQR) | 50.89 (44.44; 57.92) | 49.28 (43.26; 56.33) | 48.25 (42.55; 54.95) | 46.31 (41.60; 52.48) | <0.001 | 48.91 (42.84; 56.69) | 48.58 (42.91; 55.54) | 48.72 (42.67; 55.58) | 48.35 (42.66; 54.93) | <0.001 | 49.73 (42.97; 57.49) | 48.70 (42.52; 55.98) | 48.10 (42.54; 54.75) | 48.32 (43.11; 54.38) | <0.001 |
| Colorectal Cancer | 0.140 | 0.229 | 0.107 | ||||||||||||
| No | 10,067 (98.82%) | 10,120 (98.57%) | 10,141 (98.45%) | 10,002 (98.61%) | 10,021 (98.41%) | 10,087 (98.64%) | 10,141 (98.72%) | 10,081 (98.67%) | 10,019 (98.60%) | 10,178 (98.77%) | 10,148 (98.69%) | 9985 (98.38%) | |||
| Yes | 120 (1.18%) | 147 (1.43%) | 160 (1.55%) | 141 (1.39%) | 162 (1.59%) | 139 (1.36%) | 131 (1.28%) | 136 (1.33%) | 142 (1.40%) | 127 (1.23%) | 135 (1.31%) | 164 (1.62%) | |||
| Subtype | 0.535 | 0.842 | 0.216 | ||||||||||||
| Proximal | 37 (0.36%) | 36 (0.35%) | 39 (0.38%) | 37 (0.36%) | 40 (0.39%) | 38 (0.37%) | 34 (0.33%) | 37 (0.36%) | 35 (0.34%) | 39 (0.38%) | 31 (0.30%) | 44 (0.43%) | |||
| Distal | 43 (0.42%) | 54 (0.53%) | 62 (0.60%) | 46 (0.45%) | 58 (0.57%) | 49 (0.48%) | 49 (0.48%) | 49 (0.48%) | 55 (0.54%) | 44 (0.43%) | 54 (0.53%) | 52 (0.51%) | |||
| Rectum | 37 (0.36%) | 48 (0.47%) | 51 (0.50%) | 47 (0.46%) | 54 (0.53%) | 42 (0.41%) | 44 (0.43%) | 43 (0.42%) | 40 (0.39%) | 39 (0.38%) | 46 (0.45%) | 58 (0.57%) | |||
| Other colorectal tumours with location non-specified | 3 (0.03%) | 9 (0.09%) | 8 (0.08%) | 11 (0.11%) | 10 (0.10%) | 10 (0.10%) | 4 (0.04%) | 7 (0.07%) | 12 (0.12%) | 5 (0.05%) | 4 (0.04%) | 10 (0.10%) | |||
| Sex n (%) | <0.001 | <0.001 | <0.001 | ||||||||||||
| Male | 2340 (22.97%) | 3589 (34.96%) | 4318 (41.92%) | 5121 (50.49%) | 3576 (35.12%) | 4203 (41.10%) | 3840 (37.38%) | 3749 (36.69%) | 2243 (22.07%) | 3175 (30.81%) | 4117 (40.04%) | 5833 (57.47%) | |||
| Female | 7847 (77.03%) | 6678 (65.04%) | 5983 (58.08%) | 5022 (49.51%) | 6607 (64.88%) | 6023 (58.90%) | 6432 (62.62%) | 6468 (63.31%) | 7918 (77.93%) | 7130 (69.19%) | 6166 (59.96%) | 4316 (42.53%) | |||
| Physical Activity n (%) | <0.001 | <0.001 | <0.001 | ||||||||||||
| Inactive | 1048 (10.29%) | 1378 (13.42%) | 1417 (13.76%) | 1561 (15.39%) | 1175 (11.54%) | 1345 (13.15%) | 1383 (13.46%) | 1501 (14.69%) | 1116 (10.98%) | 1260 (12.23%) | 1364 (13.26%) | 1664 (16.40%) | |||
| Moderately inactive | 1785 (17.52%) | 2117 (20.62%) | 2185 (21.21%) | 2434 (24.00%) | 2064 (20.27%) | 2208 (21.59%) | 2107 (20.51%) | 2142 (20.97%) | 1911 (18.81%) | 2010 (19.51%) | 2108 (20.50%) | 2492 (24.55%) | |||
| Moderately active | 6406 (62.88%) | 5902 (57.49%) | 5810 (56.40%) | 5303 (52.28%) | 6117 (60.07%) | 5863 (57.33%) | 5863 (57.08%) | 5578 (54.60%) | 6310 (62.10%) | 6162 (59.80%) | 5871 (57.09%) | 5078 (50.03%) | |||
| Active | 948 (9.31%) | 870 (8.47%) | 889 (8.63%) | 845 (8.33%) | 827 (8.12%) | 810 (7.92%) | 919 (8.95%) | 996 (9.75%) | 824 (8.11%) | 873 (8.47%) | 940 (9.14%) | 915 (9.02%) | |||
| Smoking n (%) | <0.001 | 0.000 | <0.001 | ||||||||||||
| Never Smoker | 6609 (64.88%) | 5855 (57.03%) | 5485 (53.25%) | 4764 (46.97%) | 5703 (56.01%) | 5619 (54.95%) | 5707 (55.56%) | 5684 (55.63%) | <0.001 | 6290 (61.90%) | 5970 (57.93%) | 5587 (54.33%) | 4866 (47.95%) | ||
| Former Smoker | 1699 (16.68%) | 1806 (17.59%) | 1812 (17.59%) | 1905 (18.78%) | 1392 (13.67%) | 1713 (16.75%) | 1936 (18.85%) | 2181 (21.35%) | 1337 (13.16%) | 1643 (15.94%) | 1911 (18.58%) | 2331 (22.97%) | |||
| Current Smoker | 1873 (18.39%) | 2601 (25.33%) | 2998 (29.10%) | 3469 (34.20%) | 3083 (30.28%) | 2888 (28.24%) | 2624 (25.55%) | 2346 (22.96%) | 2530 (24.90%) | 2688 (26.08%) | 2776 (27.00%) | 2947 (29.04%) | |||
| Unknown | 6 (0.06%) | 5 (0.05%) | 6 (0.06%) | 5 (0.05%) | 5 (0.05%) | 6 (0.06%) | 5 (0.05%) | 6 (0.06%) | 4 (0.04%) | 4 (0.04%) | 9 (0.09%) | 5 (0.05%) | |||
| Education n (%) | <0.001 | <0.001 | <0.001 | ||||||||||||
| No formal Education | 4028 (39.54%) | 3613 (35.19%) | 3404 (33.05%) | 3026 (29.83%) | 3985 (39.13%) | 3528 (34.50%) | 3446 (33.55%) | 3112 (30.46%) | 4060 (39.96%) | 3714 (36.04%) | 3339 (32.47%) | 2958 (29.15%) | |||
| Primary School | 3727 (36.59%) | 3898 (37.97%) | 4042 (39.24%) | 4155 (40.96%) | 4054 (39.81%) | 4136 (40.45%) | 3986 (38.80%) | 3646 (35.69%) | 3838 (37.77%) | 3829 (37.16%) | 4126 (40.12%) | 4029 (39.70%) | |||
| Secondary/Technical School | 1267 (12.44%) | 1530 (14.90%) | 1553 (15.08%) | 1658 (16.35%) | 1290 (12.67%) | 1447 (14.15%) | 1531 (14.90%) | 1740 (17.03%) | 1222 (12.03%) | 1540 (14.94%) | 1523 (14.81%) | 1723 (16.98%) | |||
| University or more | 1101 (10.81%) | 1157 (11.27%) | 1230 (11.94%) | 1232 (12.15%) | 780 (7.66%) | 1037 (10.14%) | 1234 (12.01%) | 1669 (16.34%) | 984 (9.68%) | 1157 (11.23%) | 1215 (11.82%) | 1364 (13.44%) | |||
| Unknown | 64 (0.63%) | 69 (0.67%) | 72 (0.70%) | 72 (0.71%) | 74 (0.73%) | 78 (0.76%) | 75 (0.73%) | 50 (0.49%) | 57 (0.56%) | 65 (0.63%) | 80 (0.78%) | 75 (0.74%) | |||
| Previous history of CRC n (%) | 0.131 | 0.003 | 0.867 | ||||||||||||
| No | 9460 (92.86%) | 9584 (93.35%) | 9641 (93.59%) | 9500 (93.66%) | 9580 (94.08%) | 9559 (93.48%) | 9550 (92.97%) | 9496 (92.94%) | 9504 (93.53%) | 9619 (93.34%) | 9611 (93.46%) | 9451 (93.12%) | |||
| Mother/Father | 565 (5.55%) | 537 (5.23%) | 528 (5.13%) | 499 (4.92%) | 458 (4.50%) | 522 (5.10%) | 564 (5.49%) | 585 (5.73%) | 514 (5.06%) | 543 (5.27%) | 521 (5.07%) | 551 (5.43%) | |||
| Siblings | 122 (1.20%) | 111 (1.08%) | 92 (0.89%) | 97 (0.96%) | 98 (0.96%) | 109 (1.07%) | 116 (1.13%) | 99 (0.97%) | 100 (0.98%) | 104 (1.01%) | 111 (1.08%) | 107 (1.05%) | |||
| Unknown | 40 (0.39%) | 35 (0.34%) | 40 (0.39%) | 47 (0.46%) | 47 (0.46%) | 36 (0.35%) | 42 (0.41%) | 37 (0.36%) | 43 (0.42%) | 39 (0.38%) | 40 (0.39%) | 40 (0.39%) | |||
| Full Follow-Up (>1 year) n = 40,898 | Full Follow-Up (>1 year) n = 40,192 | 1–10 Years Follow-Up n = 40,192 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person/Years | Number of Events | HR a,b | (95%CI) | Person/Years | Number of Events | HR a,c | (95%CI) | Person/Years | Number of Events | HR a,d | (95%CI) | ||||
| LL | UL | LL | UL | LL | UL | ||||||||||
| 661,313 | 568 | 650,165 | 551 | 351,736 | 211 | ||||||||||
| WESTERN | |||||||||||||||
| Quartiles | |||||||||||||||
| Q1 | 164,274 | 120 | 1 | 161,552 | 117 | 1 | 87,484 | 45 | 1 | ||||||
| Q2 | 166,314 | 147 | 1.30 | 1.02 | 1.66 | 163,356 | 145 | 1.24 | 0.95 | 1.60 | 88,111 | 56 | 1.28 | 0.85 | 1.91 |
| Q3 | 166,147 | 160 | 1.47 | 1.16 | 1.87 | 163,148 | 151 | 1.29 | 0.98 | 1.71 | 88,449 | 53 | 1.24 | 0.81 | 1.89 |
| Q4 | 164,578 | 141 | 1.51 | 1.18 | 1.93 | 162,108 | 138 | 1.28 | 0.94 | 1.73 | 87,691 | 57 | 1.53 | 0.99 | 2.36 |
| p-trend | 0.001 | 0.143 | 0.087 | ||||||||||||
| 1SD-increase | 1.15 | 1.05 | 1.25 | 1.06 | 0.95 | 1.18 | 1.17 | 0.99 | 1.37 | ||||||
| PRUDENT | |||||||||||||||
| Quartiles | |||||||||||||||
| Q1 | 160,590 | 162 | 1 | 15,7643 | 158 | 1 | 87,125 | 67 | 1 | ||||||
| Q2 | 165,016 | 139 | 0.84 | 0.67 | 1.06 | 16,2226 | 132 | 0.80 | 0.63 | 1.01 | 88,070 | 40 | 0.58 | 0.39 | 0.86 |
| Q3 | 167,603 | 131 | 0.79 | 0.62 | 1.00 | 16,4622 | 128 | 0.80 | 0.63 | 1.02 | 88,475 | 49 | 0.73 | 0.50 | 1.07 |
| Q4 | 168,103 | 136 | 0.83 | 0.65 | 1.05 | 16,5675 | 133 | 0.87 | 0.67 | 1.13 | 88,066 | 55 | 0.85 | 0.57 | 1.25 |
| p-trend | 0.092 | 0.297 | 0.547 | ||||||||||||
| 1SD-increase | 0.92 | 0.85 | 1.01 | 0.94 | 0.85 | 1.04 | 0.94 | 0.81 | 1.09 | ||||||
| MEDITERRANEAN | |||||||||||||||
| Quartiles | |||||||||||||||
| Q1 | 161,982 | 142 | 1 | 159,681 | 140 | 1 | 87,295 | 59 | 1 | ||||||
| Q2 | 166,675 | 127 | 0.91 | 0.71 | 1.15 | 164,219 | 125 | 0.82 | 0.64 | 1.05 | 88,971 | 51 | 0.81 | 0.55 | 1.18 |
| Q3 | 167,169 | 135 | 0.98 | 0.77 | 1.24 | 164,108 | 129 | 0.80 | 0.62 | 1.04 | 88,406 | 45 | 0.69 | 0.46 | 1.02 |
| Q4 | 165,485 | 164 | 1.19 | 0.94 | 1.50 | 162,158 | 157 | 0.86 | 0.66 | 1.14 | 87,063 | 56 | 0.78 | 0.52 | 1.16 |
| p-trend | 0.107 | 0.315 | 0.165 | ||||||||||||
| 1SD-increase | 1.05 | 0.96 | 1.14 | 0.91 | 0.82 | 1.01 | 0.84 | 0.73 | 0.98 | ||||||
| Full Follow-Up (>1 year) | 1–10 Years Follow-Up | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male n = 15,096 | Female n = 25,096 | Male n = 15,096 | Female n = 25,096 | |||||||||||||||||||
| Person/Years | Number of Events | HR a,b | (95%CI) | Person/Years | Number of Events | HR a,b | (95%CI) | p-int (Sex) | Person/Years | Number of Events | HR a,c | (95%CI) | Person/Years | Number of Events | HR a,c | (95%CI) | p-int (Sex) | |||||
| LL | UL | LL | UL | LL | UL | LL | UL | |||||||||||||||
| 238,874 | 324 | 411,291 | 227 | 130,848 | 109 | 220,888 | 102 | |||||||||||||||
| WESTERN | ||||||||||||||||||||||
| Quartiles | 0.177 | 0.005 | ||||||||||||||||||||
| Q1 | 35,894 | 56 | 1 | 125,658 | 61 | 1 | 19,721 | 21 | 1 | 67,763 | 24 | 1 | ||||||||||
| Q2 | 55,480 | 72 | 0.94 | 0.66 | 1.35 | 107,877 | 73 | 1.59 | 1.12 | 2.26 | 30,332 | 22 | 0.80 | 0.43 | 1.46 | 57,780 | 34 | 1.98 | 1.16 | 3.36 | ||
| Q3 | 67,137 | 100 | 1.13 | 0.79 | 1.61 | 96,012 | 51 | 1.39 | 0.94 | 2.07 | 36,833 | 29 | 0.94 | 0.53 | 1.68 | 51,615 | 24 | 1.78 | 0.99 | 3.20 | ||
| Q4 | 80,364 | 96 | 1.05 | 0.73 | 1.53 | 81,744 | 42 | 1.55 | 1.01 | 2.38 | 43,961 | 37 | 1.27 | 0.72 | 2.24 | 43,730 | 20 | 2.16 | 1.16 | 4.02 | ||
| p-trend | 0.571 | 0.068 | 0.262 | 0.021 | ||||||||||||||||||
| 1SD-increase | 1.01 | 0.88 | 1.15 | 1.12 | 0.97 | 1.30 | 0.227 | 1.15 | 0.92 | 1.43 | 1.31 | 1.06 | 1.61 | 0.001 | ||||||||
| PRUDENT | ||||||||||||||||||||||
| Quartiles | 0.666 | 0.080 | ||||||||||||||||||||
| Q1 | 53,559 | 84 | 1 | 104,084 | 74 | 1 | 30,208 | 32 | 1.00 | 56,917 | 35 | 1 | ||||||||||
| Q2 | 65,259 | 84 | 0.86 | 0.63 | 1.17 | 96,967 | 48 | 0.72 | 0.50 | 1.04 | 35,879 | 21 | 0.58 | 0.34 | 1.01 | 52,191 | 19 | 0.61 | 0.35 | 1.07 | ||
| Q3 | 60,548 | 76 | 0.86 | 0.63 | 1.18 | 104,074 | 52 | 0.73 | 0.51 | 1.05 | 32,837 | 25 | 0.78 | 0.46 | 1.32 | 55,638 | 24 | 0.70 | 0.41 | 1.19 | ||
| Q4 | 59,508 | 80 | 0.99 | 0.71 | 1.37 | 106,166 | 53 | 0.73 | 0.50 | 1.07 | 31,924 | 31 | 1.03 | 0.62 | 1.73 | 56,142 | 24 | 0.68 | 0.39 | 1.18 | ||
| p-trend | 0.922 | 0.108 | 0.711 | 0.200 | ||||||||||||||||||
| 1SD-increase | 0.97 | 0.86 | 1.10 | 0.89 | 0.78 | 1.03 | 0.328 | 1.01 | 0.82 | 1.24 | 0.88 | 0.72 | 1.07 | 0.007 | ||||||||
| MEDITERRANEAN | ||||||||||||||||||||||
| Quartiles | 0.505 | 0.013 | ||||||||||||||||||||
| Q1 | 33,594 | 53 | 1 | 126,087 | 87 | 1 | 18,897 | 24 | 1 | 68,398 | 35 | 1 | ||||||||||
| Q2 | 49,202 | 72 | 0.93 | 0.65 | 1.33 | 115,017 | 53 | 0.72 | 0.51 | 1.02 | 27,113 | 23 | 0.68 | 0.38 | 1.21 | 61,858 | 28 | 0.96 | 0.58 | 1.59 | ||
| Q3 | 64,411 | 76 | 0.77 | 0.54 | 1.11 | 99,697 | 53 | 0.88 | 0.62 | 1.25 | 35,111 | 23 | 0.55 | 0.31 | 0.98 | 53,296 | 22 | 0.95 | 0.55 | 1.63 | ||
| Q4 | 91,667 | 123 | 0.90 | 0.63 | 1.28 | 70,491 | 34 | 0.83 | 0.54 | 1.27 | 49,727 | 39 | 0.70 | 0.41 | 1.19 | 37,336 | 17 | 1.13 | 0.62 | 2.08 | ||
| p-trend | 0.486 | 0.404 | 0.230 | 0.811 | ||||||||||||||||||
| 1SD-increase | 0.91 | 0.80 | 1.03 | 0.91 | 0.79 | 1.05 | 0.952 | 0.80 | 0.65 | 0.98 | 0.99 | 0.80 | 1.22 | 0.006 | ||||||||
| Full Follow-Up (>1 year) a | 1–10 Years Follow-Up a | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximal | Distal | Rectum | Proximal | Distal | Rectum | |||||||||||||||||||||||||
| n = 39,784 | n = 39,839 | n = 39,821 | n = 39,784 | n = 39,839 | n = 39,821 | |||||||||||||||||||||||||
| Person/Years | Number of Events | HR b,c | (95%CI) | Person/Years | Number of Events | HR b,c | (95%CI) | Person/Years | Number of Events | HR b,c | (95%CI) | Person/Years | Number of Events | HR b,d | (95%CI) | Person/Years | Number of Events | HR b,d | (95%CI) | Person/Years | Number of Events | HR b,d | (95%CI) | |||||||
| LL | UL | LL | UL | LL | UL | LL | UL | LL | UL | LL | UL | |||||||||||||||||||
| 646,116 | 143 | 646,676 | 198 | 646,429 | 180 | 348,663 | 53 | 349,070 | 73 | 348,905 | 69 | |||||||||||||||||||
| WESTERN | ||||||||||||||||||||||||||||||
| Quartiles | ||||||||||||||||||||||||||||||
| Q1 | 160,768 | 36 | 1 | 160,807 | 41 | 1 | 160,771 | 37 | 1 | 86,874 | 16 | 1 | 86,902 | 16 | 1 | 86,892 | 12 | 1 | ||||||||||||
| Q2 | 162,315 | 35 | 0.99 | 0.60 | 1.62 | 162,455 | 53 | 1.25 | 0.81 | 1.93 | 162,449 | 48 | 1.31 | 0.83 | 2.07 | 87,329 | 12 | 0.77 | 0.36 | 1.67 | 87,432 | 22 | 1.57 | 0.81 | 3.05 | 87,407 | 18 | 1.61 | 0.76 | 3.41 |
| Q3 | 161,907 | 35 | 1.01 | 0.59 | 1.74 | 162,205 | 60 | 1.39 | 0.88 | 2.20 | 162,062 | 48 | 1.29 | 0.79 | 2.12 | 87,532 | 14 | 1.02 | 0.47 | 2.22 | 87,749 | 19 | 1.44 | 0.70 | 2.93 | 87,641 | 16 | 1.46 | 0.66 | 3.25 |
| Q4 | 161,126 | 37 | 1.17 | 0.65 | 2.08 | 161,208 | 44 | 1.03 | 0.61 | 1.73 | 161,148 | 47 | 1.40 | 0.82 | 2.39 | 86,928 | 11 | 1.00 | 0.42 | 2.35 | 86,988 | 16 | 1.46 | 0.68 | 3.15 | 86,966 | 23 | 2.41 | 1.10 | 5.30 |
| p-trend | 0.595 | 0.897 | 0.277 | 0.858 | 0.439 | 0.046 | ||||||||||||||||||||||||
| 1SD-increase | 1.02 | 0.82 | 1.28 | 1.03 | 0.86 | 1.24 | 1.05 | 0.86 | 1.28 | 1.02 | 0.74 | 1.40 | 1.17 | 0.9 | 1.53 | 1.38 | 1.03 | 1.84 | ||||||||||||
| PRUDENT | ||||||||||||||||||||||||||||||
| Quartiles | ||||||||||||||||||||||||||||||
| Q1 | 156,521 | 39 | 1 | 156,643 | 56 | 1 | 156,627 | 53 | 1 | 86,242 | 14 | 1 | 86,364 | 27 | 1 | 86,330 | 21 | 1 | ||||||||||||
| Q2 | 161,139 | 35 | 0.85 | 0.53 | 1.35 | 161,339 | 47 | 0.79 | 0.53 | 1.17 | 161,241 | 41 | 0.74 | 0.49 | 1.12 | 87,296 | 13 | 0.87 | 0.40 | 1.87 | 87,407 | 11 | 0.42 | 0.21 | 0.85 | 87,349 | 13 | 0.6 | 0.30 | 1.21 |
| Q3 | 163,685 | 32 | 0.77 | 0.47 | 1.26 | 163,843 | 48 | 0.82 | 0.55 | 1.23 | 163,754 | 44 | 0.87 | 0.57 | 1.33 | 87,748 | 11 | 0.68 | 0.30 | 1.55 | 87,881 | 16 | 0.63 | 0.33 | 1.19 | 87,823 | 19 | 0.97 | 0.51 | 1.84 |
| Q4 | 164,771 | 37 | 0.88 | 0.52 | 1.48 | 164,851 | 47 | 0.82 | 0.53 | 1.28 | 164,807 | 42 | 0.91 | 0.58 | 1.45 | 87,376 | 15 | 0.84 | 0.38 | 1.86 | 87,418 | 19 | 0.78 | 0.41 | 1.49 | 87,404 | 16 | 0.86 | 0.42 | 1.73 |
| p-trend | 0.562 | 0.429 | 0.821 | 0.583 | 0.529 | 0.943 | ||||||||||||||||||||||||
| 1SD-increase | 1.02 | 0.84 | 1.24 | 0.89 | 0.75 | 1.05 | 0.98 | 0.82 | 1.17 | 1.01 | 0.75 | 1.36 | 0.89 | 0.69 | 1.15 | 0.98 | 0.75 | 1.27 | ||||||||||||
| MEDITERRANEAN | ||||||||||||||||||||||||||||||
| Quartiles | ||||||||||||||||||||||||||||||
| Q1 | 158,715 | 34 | 1 | 158,892 | 54 | 1 | 158,742 | 40 | 1 | 86,544 | 15 | 1 | 86,674 | 22 | 1 | 86,563 | 15 | 1 | ||||||||||||
| Q2 | 163,388 | 38 | 1.03 | 0.64 | 1.66 | 163,387 | 43 | 0.72 | 0.48 | 1.08 | 163,343 | 39 | 0.91 | 0.58 | 1.42 | 88,331 | 11 | 0.69 | 0.32 | 1.53 | 88,338 | 20 | 0.93 | 0.50 | 1.72 | 88,308 | 18 | 1.16 | 0.58 | 2.33 |
| Q3 | 163,069 | 30 | 0.79 | 0.47 | 1.33 | 163,273 | 51 | 0.78 | 0.52 | 1.18 | 163,253 | 45 | 1.00 | 0.63 | 1.57 | 87,627 | 9 | 0.56 | 0.24 | 1.31 | 87,795 | 20 | 0.96 | 0.51 | 1.81 | 87,762 | 14 | 0.88 | 0.41 | 1.88 |
| Q4 | 160,944 | 41 | 0.99 | 0.58 | 1.72 | 161,124 | 50 | 0.66 | 0.41 | 1.04 | 161,090 | 56 | 1.13 | 0.69 | 1.84 | 86,161 | 18 | 1.15 | 0.53 | 2.48 | 86,264 | 11 | 0.51 | 0.23 | 1.13 | 86,273 | 22 | 1.31 | 0.63 | 2.74 |
| p-trend | 0.744 | 0.121 | 0.543 | 0.805 | 0.157 | 0.62 | ||||||||||||||||||||||||
| 1SD-increase | 0.99 | 0.81 | 1.22 | 0.86 | 0.73 | 1.02 | 0.96 | 0.80 | 1.15 | 1.05 | 0.76 | 1.44 | 0.81 | 0.63 | 1.03 | 0.98 | 0.75 | 1.29 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.-D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C.; et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14, 3085. https://doi.org/10.3390/nu14153085
Castelló A, Rodríguez-Barranco M, Fernández de Larrea N, Jakszyn P, Dorronsoro A, Amiano P, Chirlaque M-D, Colorado-Yohar S, Guevara M, Moreno-Iribas C, et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients. 2022; 14(15):3085. https://doi.org/10.3390/nu14153085
Chicago/Turabian StyleCastelló, Adela, Miguel Rodríguez-Barranco, Nerea Fernández de Larrea, Paula Jakszyn, Ane Dorronsoro, Pilar Amiano, María-Dolores Chirlaque, Sandra Colorado-Yohar, Marcela Guevara, Conchi Moreno-Iribas, and et al. 2022. "Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain)" Nutrients 14, no. 15: 3085. https://doi.org/10.3390/nu14153085
APA StyleCastelló, A., Rodríguez-Barranco, M., Fernández de Larrea, N., Jakszyn, P., Dorronsoro, A., Amiano, P., Chirlaque, M.-D., Colorado-Yohar, S., Guevara, M., Moreno-Iribas, C., Pollán, M., & Sánchez, M.-J. (2022). Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients, 14(15), 3085. https://doi.org/10.3390/nu14153085







