Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome
Abstract
:1. Introduction
2. Materials and Methods
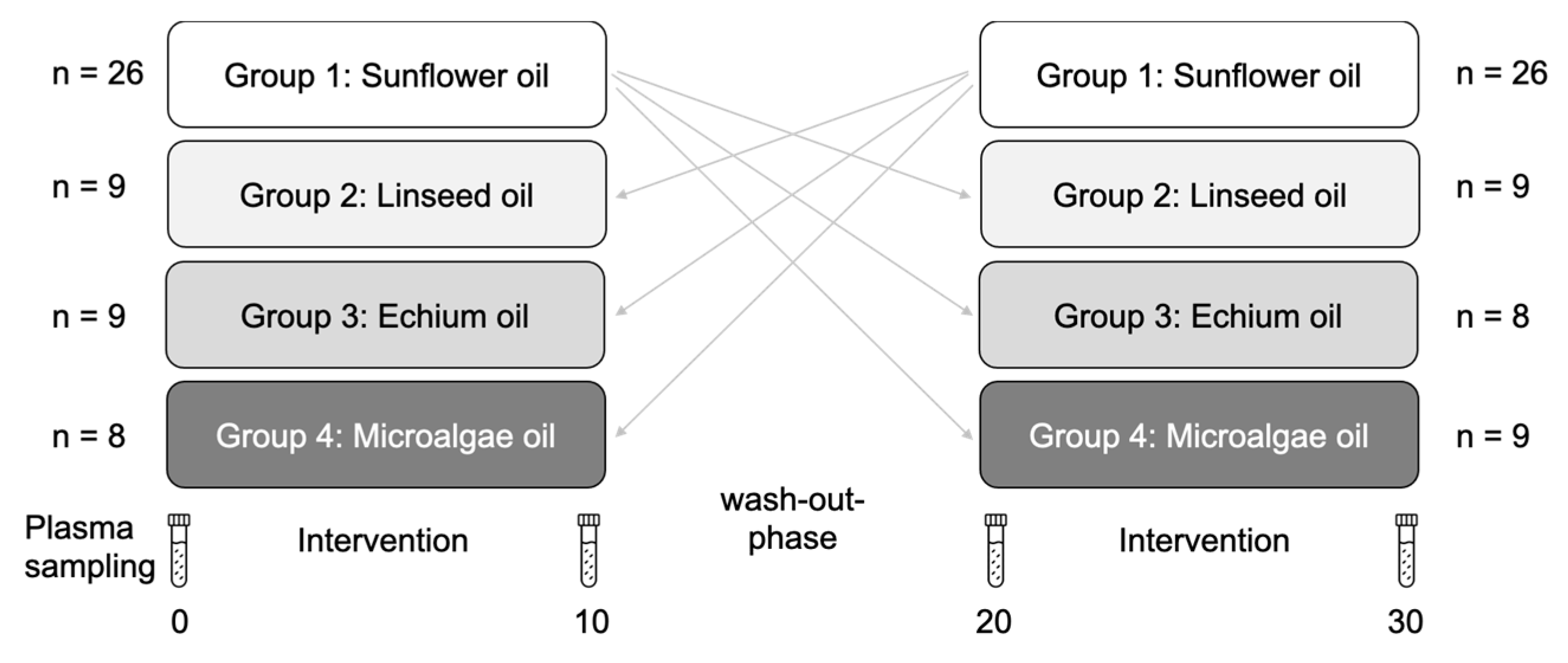
2.1. Study Design and Participants
2.2. Lipidomics
2.3. Lipidomic Data Processing and Statistical Analyses
3. Results and Discussion
3.1. Lipidomics and Data Analysis
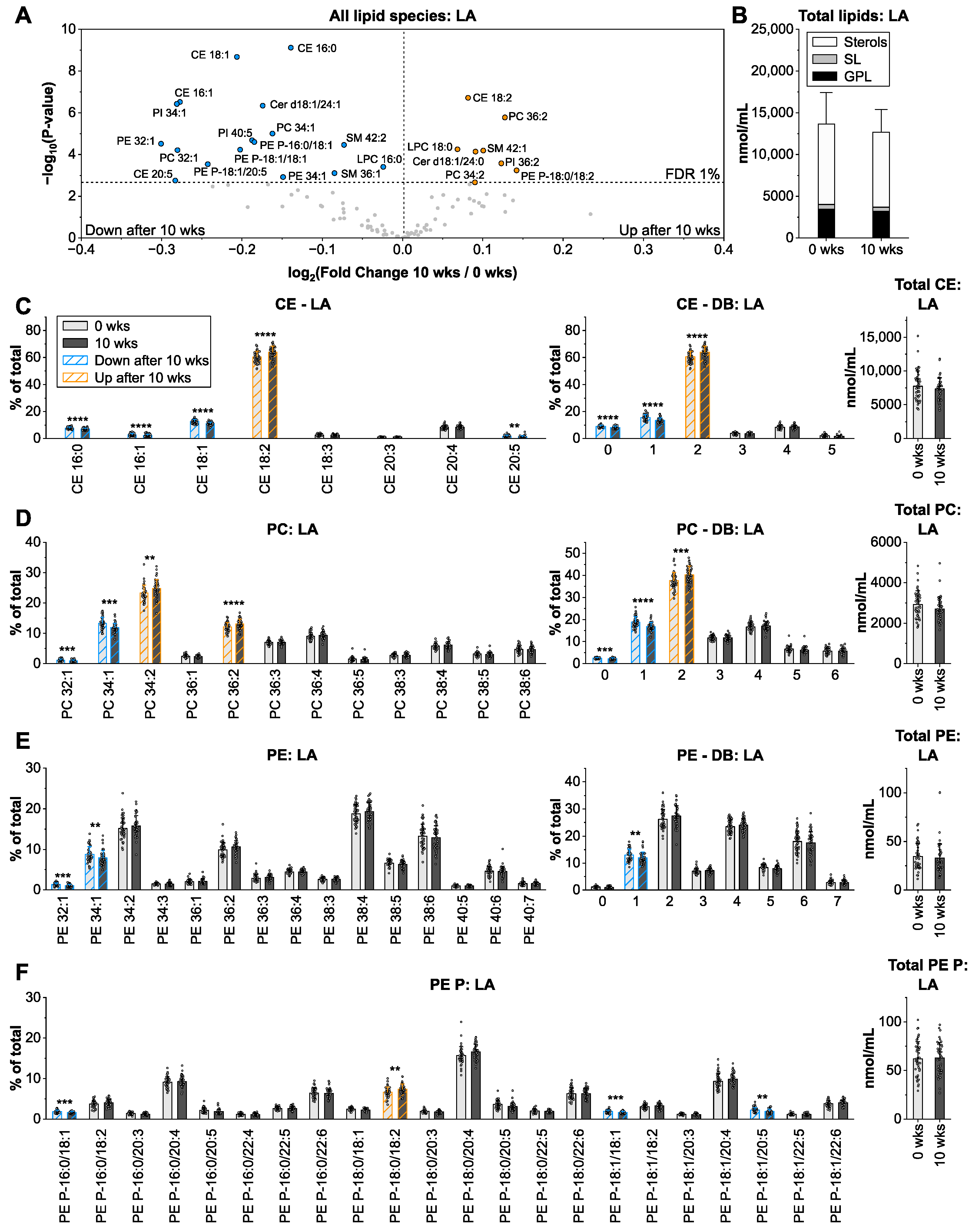
3.2. Linoleic Acid from Sunflower Oil Associates with Saturated Fatty Acids in Cholesteryl Ester and Glycerophospholipids
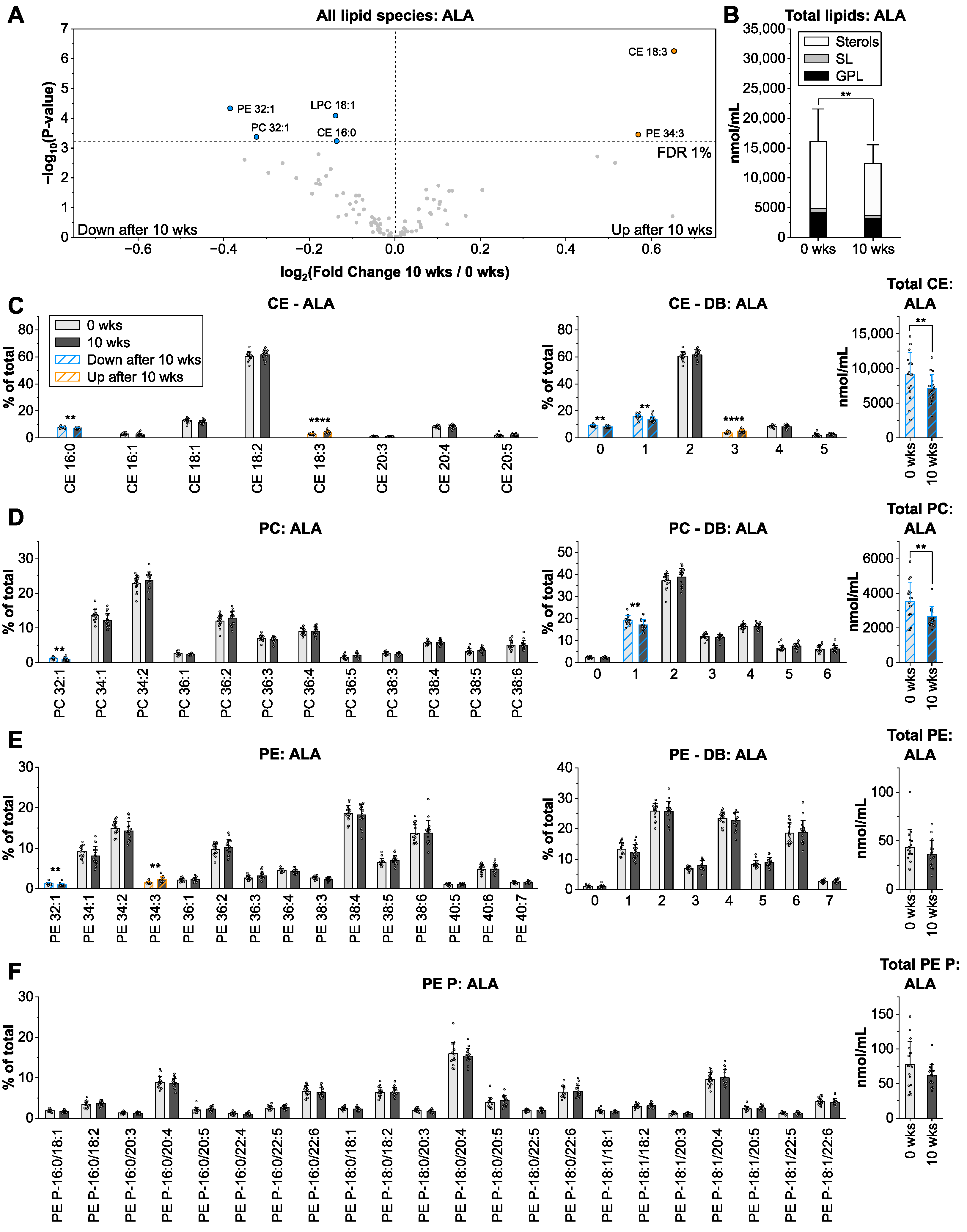
3.3. Alpha-Linolenic Acid from Linseed Oil Lowers Total Lipid Levels
3.4. Stearidonic Acid from Echium Oil Enriches in Glycerophospholipids with 4–5 Double Bonds
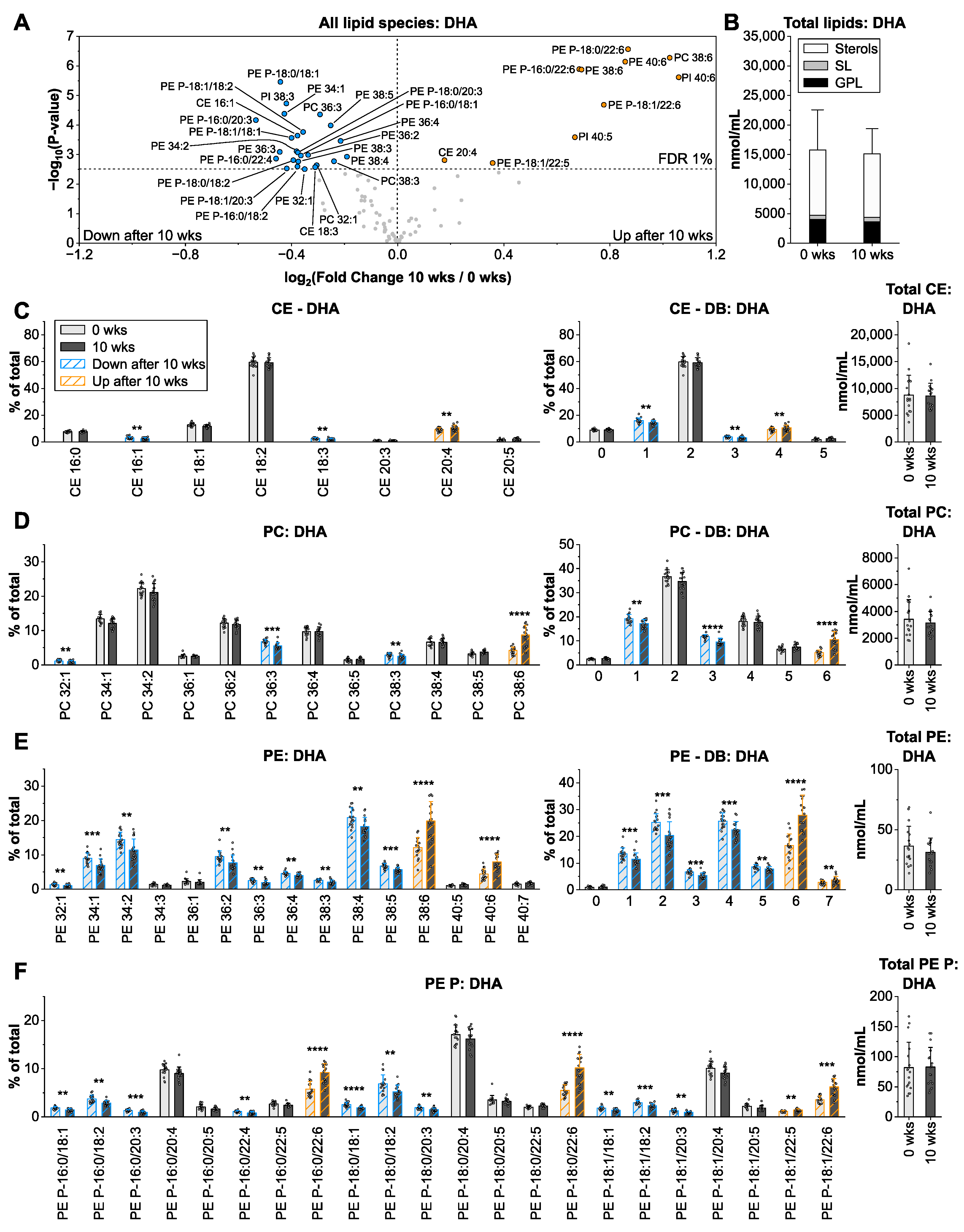
3.5. Docosahexaenoic Acid from Microalgae Oil Changes the Species Profiles of Ethanolamine-Containing Glycerophospholipids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Blom, T.; Somerharju, P.; Ikonen, E. Synthesis and Biosynthetic Trafficking of Membrane Lipids. Cold Spring Harb. Perspect. Biol. 2011, 3, a004713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.-A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular Anatomy of a Trafficking Organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-J.; Sugiura, Y.; Ikegami, K.; Konishi, Y.; Setou, M. Axonal Gradient of Arachidonic Acid-containing Phosphatidylcholine and Its Dependence on Actin Dynamics. J. Biol. Chem. 2012, 287, 5290–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, O.; Innis, S.M. Dietary Polyunsaturated Fatty Acids in Gestation Alter Fetal Cortical Phospholipids, Fatty Acids and Phosphatidylserine Synthesis. Dev. Neurosci. 2006, 28, 222–229. [Google Scholar] [CrossRef]
- Yabuuchi, H.; O’Brien, J.S. Positional distribution of fatty acids in glycerophosphatides of bovine gray matter. J. Lipid Res. 1968, 9, 65–67. [Google Scholar] [CrossRef]
- Manni, M.M.; Tiberti, M.L.; Pagnotta, S.; Barelli, H.; Gautier, R.; Antonny, B. Acyl chain asymmetry and polyunsaturation of brain phospholipids facilitate membrane vesiculation without leakage. eLife 2018, 7, e34394. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Liebisch, G.; Englmaier, M.; Grandl, M.; Robenek, H.; Schmitz, G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 7817–7822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, J.; Liebisch, G.; Grandl, M.; Schmitz, G. Lower SCD expression in dendritic cells compared to macrophages leads to membrane lipids with less mono-unsaturated fatty acids. Immunobiology 2010, 215, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 2011, 50, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Liebisch, G.; Oeckl, J.; Hoering, M.; Seeliger, C.; Schiebel, C.; Klingenspor, M.; Ecker, J. The lipidome of primary murine white, brite, and brown adipocytes—Impact of beta-adrenergic stimulation. PLoS Biol. 2019, 17, e3000412. [Google Scholar] [CrossRef] [Green Version]
- Ecker, J.; Benedetti, E.; Kindt, A.S.; Höring, M.; Perl, M.; Machmüller, A.C.; Sichler, A.; Plagge, J.; Wang, Y.; Zeissig, S.; et al. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology 2021, 161, 910–923.e19. [Google Scholar] [CrossRef]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.-Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Blüher, M.; Dawczynski, C. Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Lieser, B.; Rathenberg, J.; Drobnik, W.; Schmitz, G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta 2004, 1686, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Binder, M.; Schifferer, R.; Langmann, T.; Schulz, B.; Schmitz, G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS). Biochim. Biophys. Acta 2006, 1761, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Drobnik, W.; Lieser, B.; Schmitz, G. High-Throughput Quantification of Lysophosphatidylcholine by Electrospray Ionization Tandem Mass Spectrometry. Clin. Chem. 2002, 48, 2217–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, K.A.Z.; Murphy, R.C. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass Spectrom. 2004, 15, 1499–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebisch, G.; Drobnik, W.; Reil, M.; Trumbach, B.; Arnecke, R.; Olgemoller, B.; Roscher, A.; Schmitz, G. Quantitative meas-urement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J. Lipid Res. 1999, 40, 1539–1546. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Liebisch, G.; Janssen, K.-P. Reply. Gastroenterology 2022, 162, 658–659. [Google Scholar] [CrossRef]
- Ecker, J.; Liebisch, G.; Scherer, M.; Schmitz, G. Differential effects of conjugated linoleic acid isomers on macrophage glycer-ophospholipid metabolism. J. Lipid Res. 2010, 51, 2686–2694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, L.; McQuaid, S.E.; Karpe, F.; Frayn, K.N.; Fielding, B.A. Differences in partitioning of meal fatty acids into blood lipid fractions: A comparison of linoleate, oleate, and palmitate. Am. J. Physiol. Metab. 2009, 296, E64–E71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C]linoleic acid and α-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Hassani, S.; Borge, G.I.A.; Kohler, A.; Vogt, G.; Hyötyläinen, T.; Oresic, M.; Brønner, K.W.; Holven, K.B.; Ulven, S.M.; et al. Fish Oil Supplementation Alters the Plasma Lipidomic Profile and Increases Long-Chain PUFAs of Phospholipids and Triglycerides in Healthy Subjects. PLoS ONE 2012, 7, e42550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Sinclair, A.J.; Su, X.Q. Enrichment of n-3 containing ether phospholipids in plasma after 30 days of krill oil compared with fish oil supplementation. Lipids 2022, 57, 115–124. [Google Scholar] [CrossRef]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Han, L.; Sayanova, O.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br. J. Nutr. 2019, 121, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar] [CrossRef]
- Holub, B. Differential utilization of 1-palmitoyl and 1-stearoyl homologues of various unsaturated 1,2-diacyl-sn-glycerols for phosphatidylcholine and phosphatidylethanolamine synthesis in rat liver microsomes. J. Biol. Chem. 1978, 253, 691–696. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Fuhrmann, C.; Köhler, M.; Kiehntopf, M.; Jahreis, G. Dietary Echium Oil Increases Long-Chain n–3 PUFAs, Including Docosapentaenoic Acid, in Blood Fractions and Alters Biochemical Markers for Cardiovascular Disease Independently of Age, Sex, and Metabolic Syndrome. J. Nutr. 2014, 144, 447–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| n = 59 (39 f, 20 m) | Mean ± SD | Min | Max | |
|---|---|---|---|---|
| Age | years | 54 ± 12 | 27 | 74 |
| Body mass index | kg/m2 | 28.6 ± 5.3 | 20.0 | 43.8 |
| Systolic blood pressure | mmHg | 146.3 ± 20.0 | 105.0 | 192.0 |
| Diastolic blood pressure | mmHg | 89.7 ± 10.9 | 60.0 | 117.0 |
| Pulse | beats per minute | 71.4 ± 13.0 | 53.0 | 120.0 |
| Triacylglycerol | mmol/L | 2.18 ± 1.40 | 0.70 | 7.36 |
| Total cholesterol | mmol/L | 5.74 ± 1.06 | 3.13 | 8.29 |
| LDL cholesterol | mmol/L | 3.61 ± 0.96 | 1.64 | 6.00 |
| HDL cholesterol | mmol/L | 1.09 ± 0.37 | 0.54 | 2.32 |
| LDL/HDL | 3.60 ± 1.18 | 0.70 | 7.71 | |
| hs-CRP | mg/L | 3.64 ± 3.74 | 0.50 | 14.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawczynski, C.; Plagge, J.; Jahreis, G.; Liebisch, G.; Höring, M.; Seeliger, C.; Ecker, J. Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome. Nutrients 2022, 14, 3055. https://doi.org/10.3390/nu14153055
Dawczynski C, Plagge J, Jahreis G, Liebisch G, Höring M, Seeliger C, Ecker J. Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome. Nutrients. 2022; 14(15):3055. https://doi.org/10.3390/nu14153055
Chicago/Turabian StyleDawczynski, Christine, Johannes Plagge, Gerhard Jahreis, Gerhard Liebisch, Marcus Höring, Claudine Seeliger, and Josef Ecker. 2022. "Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome" Nutrients 14, no. 15: 3055. https://doi.org/10.3390/nu14153055
APA StyleDawczynski, C., Plagge, J., Jahreis, G., Liebisch, G., Höring, M., Seeliger, C., & Ecker, J. (2022). Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome. Nutrients, 14(15), 3055. https://doi.org/10.3390/nu14153055







