Abstract
Background: Clinical data on the correlation of dyslipidaemia with the long-term outcomes of ulcerative colitis (UC) are limited. This study aimed to evaluate the impact of lipid levels on disease activity and prognosis in UC. Methods: The retrospective data of UC patients who had detailed lipid profiles were collected from January 2003 to September 2020. All patients were followed-up to 30 September 2021. The long-term outcomes were UC-related surgery and tumorigenesis. Results: In total, 497 patients were included in the analysis. Compared to patients with normal lipid levels, those with dyslipidaemia commonly presented with more serious disease activity. Low high-density lipoprotein cholesterol (p < 0.05) levels were associated with higher risks of severe disease activity in UC. Regarding the long-term outcomes, patients with persistent dyslipidaemia were at higher risks of UC-related surgery (HR: 3.27, 95% CI: 1.86–5.75, p < 0.001) and tumorigenesis (HR: 7.92, 95% CI: 3.97–15.78, p < 0.001) and had shorter surgery- and tumour-free survival (p < 0.001) than patients with transient dyslipidaemia and normal lipid levels. Low levels of high-density lipoprotein cholesterol (p < 0.001) and apolipoprotein A1 (p < 0.05) were associated with higher risks of surgery and tumorigenesis. Conclusion: Persistent dyslipidaemia was associated with a higher risk of serious disease activity and worse long-term outcomes among patients with UC. Lipid patterns should be assessed to improve the management of high-risk patients with UC in the early phase.
1. Introduction
Ulcerative colitis (UC) is a subtype of inflammatory bowel disease (IBD) characterized by inflammation of the gastrointestinal tract with a relapsing-remitting pattern, and surgery and tumorigenesis are two of its most serious long-term outcomes [1]. Serious disease activity presented with increased levels of inflammatory biomarkers, and aggravated clinical manifestations were commonly attributed to the acute inflammation status. Notably, recurrent inflammatory attacks that cannot be alleviated for a long time, leading to a prolonged active disease phase, are associated with an increased risk of a poor prognosis, including abdominal surgery and tumorigenesis [2]. Dyslipidaemia is a common pathophysiological characteristic that leads to metabolic disorders, altered steroid hormone secretion, and chronic subclinical inflammation [3,4,5]. Indeed, due to alterations in the tumour cell microenvironment and systemic immune status, abnormal lipid metabolism not only affects the inflammatory condition but also affects tumour growth and invasion [3,5,6].
It has been reported that serum lipid profiles differ not only in Eastern and Western populations [7,8,9,10] but also in patients with UC and the general population [11,12]. Remarkably, previous studies have demonstrated that serum lipid abnormalities not only aggravate disease activity in UC [2,13] but are also associated with an increased likelihood of surgery [14] and tumours [15] among patients with IBD, revealing a potential role of dyslipidaemia in inflammation and disease progression. However, only a few studies have assessed the association between lipid profiles and morbidity [16] and disease activity [13] of UC, and they had small sample sizes and inconsistent results. Due to low mortality, long-term outcomes, including tumorigenesis, have rarely been evaluated.
In this study, we comprehensively estimated the correlation between lipid patterns and disease activity as well as long-term prognosis, as represented by surgery-free survival (SFS) and tumour-free survival (TFS), to better distinguish high-risk subgroups and optimize management along with the individualized treatment of UC patients.
2. Materials and Methods
2.1. Patients
Consecutive patients diagnosed with UC at Peking Union Medical College Hospital (PUMCH) from January 2003 to September 2020 were enrolled in this study. The study enrolment flowchart is shown in Figure 1. The inclusion criteria were as follows: (1) patients diagnosed with UC according to the European Crohn’s and Colitis Organization consensus guidelines [17] combined with data about the clinical presentation, endoscopic features, and imaging or pathological characteristics and (2) those followed-up for at least 6 months. The exclusion criteria were as follows: (1) patients diagnosed with tumours before UC diagnosis; (2) those with missing details about lipid levels, use of medications, and surgery; (3) those with previous autoimmune diseases and those receiving treatment with biologics, immunomodulators, or corticosteroids at baseline; and (4) those who changed the primary diagnosis from UC to other disease or were lost to follow-up. All participants with UC were followed-up (last visit record accessed via the hospital information system (HIS)/telephone follow-up).
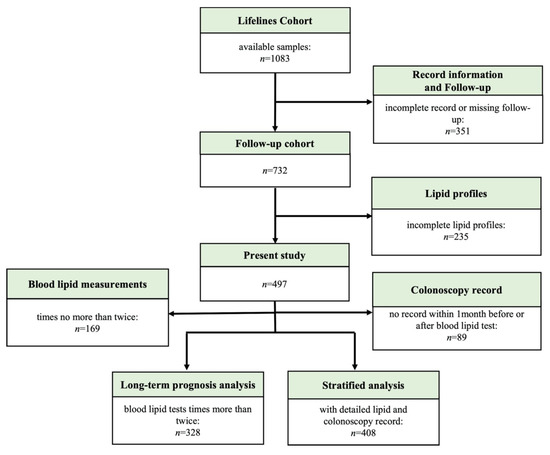
Figure 1.
Flowchart of cohort participant inclusion.
2.2. Outcome Measures
The long-term outcomes were UC-related surgery and tumorigenesis. Surgery-free survival (SFS) was defined as the period from UC diagnosis to UC-related surgery, and tumour-free survival (TFS) was defined as the period from UC diagnosis to the first tumour occurrence. The end-point events were initial tumour diagnosis, death, date of the last visit, and follow-up cut-off date (30 September 2021), whichever occurred first.
2.3. Study Variables
The following data were collected: demographic characteristics, age at diagnosis, the extent of lesions at diagnosis and maximal lesions, medications, the performance of UC-related surgery, complications, extraintestinal manifestations, intestinal infections, colonoscopy record, and lipid profiles. A history of concomitant use of medications was included in the analysis. The development of tumours (including intestinal and other locations) and deaths were reported during follow-up.
2.4. Definitions
According to Asian standards, overweight was defined as a body mass index (BMI) of ≥23 kg/m2. In line with previous national studies in China, dyslipidaemia was defined as either a total cholesterol (TC) level of ≥6.2 mmol/L, triglyceride (TG) level of ≥2.3 mmol/L, high-density lipoprotein cholesterol (HDL-C) level of <1.0 mmol/L, low-density lipoprotein cholesterol (LDL-C) level of ≥4.1 mmol/L, or self-reported use of lipid-lowering medication, according to the Guidelines for the Prevention and Treatment of Dyslipidaemia in Chinese Adults (2016).
Clinical symptoms and endoscopic findings were selected within one month before or after the lipid measurement. The point lipid measurement was used to evaluate the short-term lipid profiles. The average of multiple lipid measurements (which was used for assessment in grouping based on the severity of dyslipidaemia) was used to evaluate the long-term lipid profiles.
The extent of disease was characterized according to the Montreal classification [18]. According to the 2019 American College of Gastroenterology (ACG) Clinical Guidelines [19], the endoscopic disease activity index (DAI) scoring system includes the Mayo Endoscopic Score (MES) and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [20]. The full Mayo Score (MS) containing the endoscopic subscore was used to characterize the clinical DAI.
Biological remission was defined as normal hypersensitive C-reactive protein (hsCRP) level (<3 mg/L) [21], and endoscopic remission was defined as MES ≤ 1 [22]. UC-related surgery was defined as intestinal surgery (mainly including partial or total bowel resection and enterostomy) performed after diagnosis with UC. The severe complications during the disease course were UC-related bowel obstruction/stenosis, bowel perforation, toxic megacolon, abdominal infection, massive gastrointestinal bleeding, and infectious shock.
2.5. Statistical Analysis
The Statistical Package for the Social Sciences software version 26 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software version 9.0 (GraphPad Software, San Diego, CA, USA) were used for data analysis and statistical display. Continuous variables are presented as the mean ± standard deviation or median (interquartile range, IQR), and categorical variables are presented as numbers and proportions. Univariate analyses of qualitative/quantitative differences between groups were conducted using the chi-square test or Fisher’s exact test. Moreover, Student’s t-test or the Mann–Whitney U test was used to evaluate continuous variables. Of the patients included, 328 (66.0%) with multipoint lipid measurements (that are ≥3 times higher before the end-point events) were selected for substudy analysis. Patients were classified into the normal group (without abnormal lipid test results), transient dyslipidaemia group (with 1–2 abnormal lipid test results), and persistent dyslipidaemia group (with ≥3 abnormal lipid test results) according to the frequency of abnormal lipid test results before surgery and tumorigenesis. Significant factors in the univariate analyses were included in the multivariate Cox regression analysis and logistic regression analysis. Then, hazard ratios (HRs), odds ratios (ORs), and 95% confidence intervals (CIs) were calculated to determine factors associated with long-term outcomes. Survival analysis was performed using Kaplan–Meier curves. A two-sided p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Clinical Characteristics of Patients
In this study, 497 UC patients who had detailed lipid profiles were eligible, with a male-to-female ratio of 1.23:1 and four (0.8%) deaths. The average age at UC diagnosis was 35.4 (IQR 27.3–46.4) years, and the follow-up duration was 7.5 (IQR 3.8–13.3) years. There were 278 (55.9%) patients diagnosed with dyslipidaemia and 72 (14.5%) patients who experienced severe complications. The patients’ demographic features are shown in Table 1.

Table 1.
Demographic and clinical characteristics of patients with dyslipidaemia and normal lipid levels.
We also analysed the impact of lipid levels on baseline clinical characteristics (Table 1). Male sex (62.2% vs. 46.1%, p < 0.001), BMI ≥ 23 kg/m2 (57.9% vs. 30.6%, p < 0.001), a history of smoking (31.3% vs. 19.2%, p = 0.002), a maximum E3 classification (88.9% vs. 82.2%, p = 0.03), and exposure to aspirin (4.7% vs. 2.7%, p < 0.001) were associated with a higher proportion of dyslipidaemia. At disease onset, patients with dyslipidaemia were more likely to report a history of hypertension (16.2% vs. 6.8%, p = 0.002), hyperuricaemia (26.3% vs. 18.3%, p = 0.035), diabetes (15.8% vs. 5.0%, p < 0.001), non-alcoholic fatty liver (9.0% vs. 4.1%, p = 0.032), and appendectomy (6.1% vs. 4.1%, p = 0.004).
3.2. Correlation between Lipid Levels and Disease Activity
Of 497 patients with UC, excluding those receiving lipid-lowering drugs, 408 (82.1%) had complete and detailed data about colonoscopy records, lipid profiles, and hsCRP levels. When the severity of the disease was analysed, patients with dyslipidaemia had greater chances of experiencing both a severe clinical (Mayo score, MS) and endoscopic DAI (not only in the MES but also in the UCEIS) than those with normal lipid levels (all p < 0.001, Table 1). Patients in the moderate/severe disease activity phase had a higher proportion of dyslipidaemia, compared to those in the remission/mild disease activity phase (all p < 0.001, Figure 2A). Compared with the patients with normal lipid levels, patients with dyslipidaemia had significantly higher levels of hypersensitivity C-reactive protein (hsCRP) (2.28 (0.70–6.43) mg/L vs. 14.05 (3.75–48.88) mg/L, p < 0.001, Table 1).
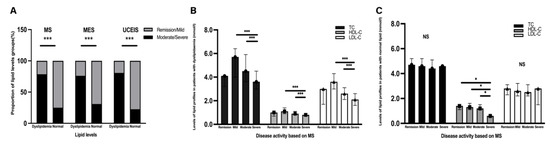
Figure 2.
Correlation between lipid levels and disease activity. (A) The proportion of patients with dyslipidaemia and normal lipid levels is presented by bar plots and stratified according to disease activity based on MS, MES, and UCEIS. (B,C) Correlation between lipid profiles and disease activity based on MS in patients with dyslipidaemia and normal lipid levels. (B) Bar plots of levels of TC, HDL-C, and LDL-C in different groups based on disease activity in the patients with dyslipidaemia. (C) Bar graphs of the levels of TC, HDL-C, and LDL-C in different groups based on disease activity in patients with normal lipid levels. The proportion of lipid levels in patients with dyslipidaemia and normal serum lipid levels is presented by bar plots and stratified according to disease activity. The chi-square test was performed to analyse statistical significance. MS, Mayo score; MES, Mayo Endoscopic Score; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; NS, no significance. Significant differences were adjusted for multiple tests by Bonferroni correction. Significant p-value < 0.05. * p < 0.05, *** p < 0.001.
To further analyse the relationship between the lipid patterns and disease severity, participants were grouped according to the full Mayo score (MS), which includes the endoscopic subscore, and they were stratified concurrently based on serum lipid category. Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (ApoA1), apolipoprotein B, lipoprotein alpha (Lp α), and free fatty acid (FFA) levels were included in the serum lipid profiles.
Based on MS, low TC (p < 0.001), HDL-C (p < 0.001), and LDL-C (p < 0.001) levels were associated with higher risks of severe instead of remission disease activity in the patients with dyslipidaemia (Table 2 and Figure 2B). In the patients with normal serum lipids, only the HDL-C levels showed a significant decreasing trend from remission to severe activity (p = 0.02, Figure 2C).

Table 2.
Comparison of lipid profiles between different groups of disease activity based on MS in patients with dyslipidaemia and normal lipid levels.
3.3. Correlation between Lipid Levels and Prognosis
In the 328 patients with long-term lipid levels, we found that a total of 270 (82.3%) and 164 (50.0%) patients had achieved biological remission and mucosal healing, respectively. Furthermore, the relationship between long-term treatment response and medications as well as long-term lipids levels of patients was also analysed. As shown in the univariate analysis (Supplementary Table S1) and the logistic regression analysis (Supplementary Table S2), no significant difference was found in the incidence of biologic remission between the patients with persistent dyslipidaemia and non-persistent dyslipidaemia (odds ratio (OR): 0.69, 95% CI: 0.30–1.62, p = 0.72). However, the incidence of endoscopic remission decreased in the patients with persistent dyslipidaemia (OR: 0.34, 95%CI: 0.18–0.64, p = 0.001). In addition, the exposure of glucocorticoids decreased both the incidence of biologic remission (OR: 0.33, 95%CI: 0.11–0.98, p < 0.05) and endoscopic remission (OR: 0.52, 95%CI: 0.28–0.96, p = 0.039). There was no significant difference in the incidence of biologic remission and mucosa healing in other drug treatments, including aspirin, 5-ASA, IM, and biologic therapy.
According to the univariate analysis (Table 3), there were 94 (18.9%) patients with UC-related surgery and 53 (10.7%) with tumorigenesis in the cohort of 497 UC patients. Dyslipidaemia was associated with an increased likelihood of experiencing UC-related surgery (23.0% vs. 13.7%, p = 0.008) and tumorigenesis (13.3% vs. 7.3%, p = 0.031), suggesting a relationship with a worse prognosis in patients with UC.

Table 3.
Correlation between dyslipidaemia and poor prognosis in UC patients.
Of 328 patients who had long-term lipid levels, 83 patients with UC-related surgery and 40 patients with tumours were enrolled in the subanalysis for further assessment of the association between average lipid levels and long-term outcomes. As shown in Supplementary Table S3, univariate and multivariate Cox regression analyses demonstrated that persistent dyslipidaemia was significantly identified as an independent risk factor for both surgery (HR (hazard ratio): 3.27, 95% CI (confidence interval): 1.86–5.75, p < 0.001) and tumorigenesis (HR: 7.92, 95% CI: 3.97–15.78, p < 0.001) among the patients with UC. In the Kaplan–Meier survival analysis, patients with persistent dyslipidaemia had statistically shorter SFS (HR: 3.15, p < 0.001, Figure 3A) and TFS (HR: 7.46, p < 0.001, Figure 3B) than the transient dyslipidaemia group and normal lipid group.
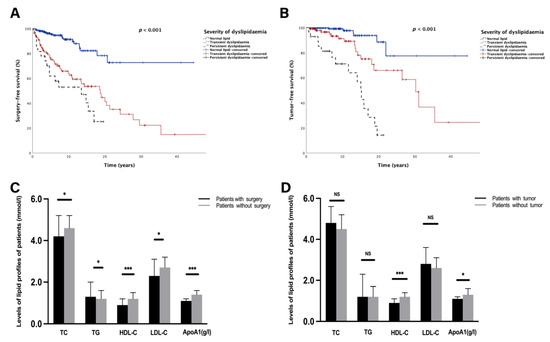
Figure 3.
Comparison of lipid levels and lipid profiles in patients with different long-term outcomes. (A,B) Kaplan–Meier survival curve presenting the surgery-free survival (A) and tumour-free survival (B) in different groups based on the severity of dyslipidaemia. (C) Bar graphs of long-term levels of TC, TG, HDL-C, LDL-C, and ApoA1 between patients who were surgery-free and those who underwent surgery. (D) Bar graphs of long-term levels of TC, TG, HDL-C, LDL-C, and ApoA1 between tumour-free and tumour-bearing patients. NS, no significance. Significant p-value < 0.05. * p < 0.05, *** p < 0.001. Surgery-free survival was defined as the period from diagnosis to UC-related surgery. Tumour-free survival was defined as the period from diagnosis to tumorigenesis. Transient dyslipidaemia was defined as occurrence of dyslipidaemia not more than twice during the whole following period. Persistent dyslipidaemia was defined as times of dyslipidaemia occurring not less than three times during the whole following period.
Patients were also stratified according to lipid compositions to analyse the relationship between lipid patterns and long-term outcomes. As shown in Supplementary Table S4 and Figure 3C, patients with UC-related surgery had lower TC (4.2 (3.1–5.2) mmol/L vs. 4.6 (4.0–5.2) mmol/L, p = 0.01); HDL-C (0.9 (0.7–1.2) mmol/L vs. 1.2 (1.1–1.5) mmol/L, p < 0.001); LDL-C (2.3 (1.8–3.1) mmol/L vs. 2.7 (2.2–3.2) mmol/L, p = 0.04); and ApoA1 (1.1 (1.0–1.2) g/L vs. 1.4 (1.2–1.6) g/L, p < 0.001) levels than those who were surgery-free. In contrast, patients experiencing surgery had a higher TG level than the surgery-free group (1.3 (1.0–2.0) mmol/L vs. 1.2 (0.9–1.6) mmol/L, p = 0.012, Figure 3C). Moreover, stratified analysis suggested that patients with tumours presented significantly lower HDL-C (0.9 (0.8–1.1) mmol/L vs. 1.2 (1.0–1.4) mmol/L, p < 0.001) and ApoA1 (1.1 (1.0–1.2) g/L vs. 1.3 (1.2–1.6) g/L, p < 0.05) levels than those without tumours (Figure 3D).In addition, patients who underwent surgery had higher hsCRP levels than those without surgery (18.0 (3.4–58.5) mg/L vs. 3.0 (0.8–11.2) mg/L, p < 0.001, Supplementary Table S4). A consistent trend was found in the tumour-bearing group when compared to tumour-free patients (7.0 (2.9–52.0) mg/L vs. 3.6 (0.9–19.0) mg/L, p < 0.05, Supplementary Table S4). Interestingly, when lipid profiles were analysed, levels of HDL-C and ApoA1 were inversely related to hsCRP levels (all p < 0.01, Supplementary Table S5).
4. Discussion
Our long-term cohort study demonstrated an association between dyslipidaemia and severe disease activity as well as early surgery and tumorigenesis among patients with UC in China. Dyslipidaemia may potentially aggravate acute inflammation and promote disease progression by exacerbating chronic inflammation in UC. Remarkably, severe disease activity and poor long-term outcomes were associated with lower HDL-C levels, suggesting a latent pathogenesis of disease development related to lipid profiles.
In our study, the severity of dyslipidaemia was shown to be positively correlated with not only higher hsCRP levels but also severe disease activity in UC, suggesting a relationship between abnormal lipid metabolism and acute inflammation. A previous study reported a similar result, which focused on the mechanism of the downregulation of the lipolytic enzyme activity of inflammatory cytokines, such as tumour necrosis factor, interleukin-6, and interferon-γ [2,14].
More importantly, changes in lipid profiles were correlated with disease activity in the stratified substudy, and the association remained significant in both the patients with dyslipidaemia and those with normal lipid levels. The findings indicated a potential role played by the specific lipid patterns in the acute inflammatory condition in UC. In fact, studies have reported alterations in lipid compositions with a pattern of low TC and high TG levels among patients with IBD [11,23], and this change was more evident during the active disease phase. Inconsistent with the studies above, a decreased level of HDL-C was found to correlate with higher Mayo scores (MS) in our work. The disparities in lipid patterns could be attributed to the differences in dietary habits and incidence of obesity between China and Western countries and revealed the potential disparities in mechanisms associated with inflammation. It has been reported that acute inflammatory phase disorders of lipoprotein metabolism cause changes in lipid and lipoprotein levels among patients with IBD [24]. The study identified an increased serum TC level in mucosal healing status compared to acute activity in UC, reflecting an improvement in general condition, including nutritional status, because of the resolution of acute inflammation [25]. Moreover, levels of TC and LDL-C were lower in the active IBD than in the healthy participants and were correlated with the systemic inflammatory status [26]. Indeed, low levels of HDL-C are associated with inflammatory and immune diseases because of the anti-inflammatory effects of HDL-C as a part of the innate immune system [27]. These studies have validated the close relationship between abnormal lipid compositions and the acute inflammatory state of IBD. Our work implicated a relationship between decreased levels of HDL-C and acute inflammation in UC, and physiological mechanisms should be explored. Notably, the results suggested that HDL-C could be a predictor of a serious acute inflammatory condition in UC when patients were trapped in a phase of severe disease activity. Since HDL-C levels change more slowly than CRP, HDL-C has a more lasting effect as a surrogate marker of disease severity. Moreover, due to the importance of endoscopy for disease activity assessment, HDL-C level testing might be a substitute in UC patients who do not undergo colonoscopy in clinical work.
Another significant finding of the present study was that persistent dyslipidaemia was independently associated with higher risks of surgery and systemic tumorigenesis in UC, implying a worse prognosis. Furthermore, the lipid patterns were comparable between different groups divided according to prognostic events and levels of hsCRP, suggesting a correlation between specific lipid patterns, long-term outcomes, and chronic inflammation.
There is no denying that abdominal operation is regarded as an important sign of disease progression. Although surgeries in IBD patients are considered to have an impact on lipid metabolism [28], Romanato et al. demonstrated that lipid profiles after intestinal resection were stable, and CD recurrence but not the extent of intestinal resection was the main predictor of changes in lipid profiles [29]. Another study implied that effective prevention of relapse for CD patients might be achieved through moderate dietary fat intake, particularly when the disease condition is unstable [30]. In general, existing studies support that changes in blood lipid levels influence disease development. Studies have reported a lipid pattern of lower TC and higher TG levels in IBD patients with intestinal resections [14,28]. In this work, persistent dyslipidaemia was confirmed as a risk factor for early abdominal surgery in UC, and the level of HDL-C but not the level of TC or TG, as previously described, could be a predictor of bowel resection following diagnosis. Disparities in lipid patterns may have resulted from the location of the operative intestinal segment, inflammatory state, nutritional status, and dietary structure. However, some studies also show that dyslipidaemia aggravates chronic inflammation of the intestine [2], suggesting a potential pathophysiological mechanism between alterations in lipid profiles and disease progression.
Consistent with a previous study [15], our work also showed that dyslipidaemia played a role in the incidence of tumorigenesis associated with colitis. Regarding the mechanism, dyslipidaemia has been validated to prolong the disease course and increase cumulative genetic mutations in the intestinal mucosa, leading to an increased risk of tumorigenesis [2]. Furthermore, dyslipidaemia alters the tumour microenvironment and immunosuppressive function via systemic inflammation, which accelerates tumour progression [15]. However, the reprogramming of lipid metabolism by tumour cells affects serum lipid levels in the body, which are inversely associated with C-reactive protein (CRP) levels in IBD [26,31]. Another study indicated that high TG and LDL-C levels are risk factors for tumorigenesis [32], and applying abnormal lipid patterns is associated with the long-term outcomes of IBD. Similar to our results, studies have reported that serum HDL-C and ApoA1 levels were inversely associated with CRP in IBD patients [26], suggesting a relationship with systemic inflammation. Clearly, low HDL-C levels were not only associated with the incidence of IBD, being [33] inversely related to acute inflammatory conditions [23], but also correlated with chronic inflammation, which may promote tumorigenesis [34,35]. Remarkably, HDL-C participates in both innate and adaptive immune responses [36] and is found to have pleiotropic properties in cell growth, which revealed underlying mechanisms of occurrence and development of tumours [37]. In our study, patients with shorter SFS and TFS were more likely to have lower HDL-C and ApoA1 levels, reflecting a specific lipid pattern related to a worse prognosis. Considering the significantly lower levels in the severe activity phase in our study, low HDL-C levels may be a predictive marker of disease severity and poor prognosis. Consequently, in addition to controlling LDL-C levels emphasized in the existing guidelines, our study demonstrated that the management of HDL-C levels cannot be disregarded in preventing severe disease activity and unfavourable prognosis in UC.
To our knowledge, our cohort is one of the largest cohorts of UC, clarifying the correlation between dyslipidaemia and disease activity in China. Remarkably, this is the first study validating the disparities in lipid profiles of long-term prognosis in UC. A variety of disease activity scoring methods, including clinical DAI, two endoscopic scores, and laboratory indicators (selection of hsCRP), were used to evaluate the inflammatory status of the disease, showing reliability. Importantly, instead of point measurements, the average lipid levels from multiple tests provided a more realistic image of long-term lipid profiles to assess the long-term outcomes. However, this was a retrospective, single-centre observational study. Thus, the continuity of clinical data was compromised and might still be biased despite correction using statistical methods. Finally, we have no data on enteral and parenteral nutrition, diet, or other pharmacological factors, which usually have potential effects on serum lipid levels.
5. Conclusions
Our findings revealed a potential pathophysiological mechanism between lipid metabolism and the development of UC. Good management of dyslipidaemia, particularly HDL-C levels, is needed for the early identification of high-risk groups for surgery and prevention of poor outcomes. Therefore, future prospective cohort and laboratory studies should be performed to validate our findings and explore the molecular mechanisms of lipid metabolism and UC progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14153040/s1, Table S1: Comparison of clinical features in the patients with biological remission and endoscopic remission; Table S2: Logistic regression analysis in the patients with biological remission and endoscopic remission; Table S3: Univariate and multivariate Cox regression analysis of risk factors for surgery and tumorigenesis; Table S4: Comparison of lipid profiles in the patients with surgery or tumorigenesis; Table S5: Correlation analysis between lipid profiles and hsCRP levels.
Author Contributions
Conceptualization, J.Q., H.Y., and H.W.; methodology, Z.L. and X.B.; software, Z.L.; validation, Z.L., H.T., H.L., H.Z., and L.W.; formal analysis, Z.L. and H.T.; investigation, H.T., H.L., H.Z., and X.B.; resources, J.Q.; data curation, Z.L. and J.Q.; writing—original draft preparation, Z.L.; writing—review and editing, J.Q. and H.Y.; visualization, Z.L.; supervision, J.Q.; project administration, J.Q.; funding acquisition, J.Q. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Health Research & Special Projects Grant of China (No.201002020 and No.201502005), National Natural Science Foundation of China (No.81570505 and No.81970495), Natural Science Foundation of Beijing (No.7202161), and CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-3-001 and No.2019-I2M-2-007).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Peking Union Medical College Hospital (PUMCH) (protocol code: S-K1889, date of approval: 1 September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this study are available from the corresponding author upon reasonable request.
Acknowledgments
We express our appreciation to all patients with UC for their collaboration in the current study. All individuals have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and cancer: Local and systemic mechanisms. Annu. Rev. Med. 2015, 66, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, H.; Lu, J.; Ding, Q.; Li, X.; Wang, X.; Sun, D.; Tan, L.; Mu, L.; Liu, J.; et al. Prevalence of Dyslipidemia and Availability of Lipid-Lowering Medications Among Primary Health Care Settings in China. JAMA Netw. Open. 2021, 4, e2127573. [Google Scholar] [CrossRef]
- Primatesta, P.; Poulter, N.R. Levels of dyslipidaemia and improvement in its management in England: Results from the Health Survey for England 2003. Clin. Endocrinol. 2006, 64, 292–298. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Gil-Montero, M.; León-Muñoz, L.M.; Graciani, A.; Bayán-Bravo, A.; Taboada, J.M.; Banegas, J.R.; Rodrigues-Artalejo, F. Magnitude and management of hypercholesterolemia in the adult population of Spain, 2008–2010: The ENRICA Study. Rev. Esp. Cardiol. 2012, 65, 551–558. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, P.; Zhou, T.; Lu, J.; Spatz, E.S.; Nasir, K.; Jiang, L.; Krumholz, H.M. Comparison of Prevalence, Awareness, Treatment, and Control of Cardiovascular Risk Factors in China and the United States. J. Am. Heart Assoc. 2018, 7, e007462. [Google Scholar] [CrossRef] [Green Version]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Im, J.P.; Han, K.; Park, S.; Hong, S.W.; Moon, J.M.; Kang, E.A.; Chun, J.; Lee, H.J.; Kim, J.S. Crohn’s disease and ulcerative colitis are associated with different lipid profile disorders: A nationwide population-based study. Aliment. Pharmacol. Ther. 2020, 51, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Iijima, H.; Otake, Y.; Amano, T.; Tani, M.; Yoshihara, T.; Tashiro, T.; Tsujii, Y.; Inoue, T.; Hayashi, Y.; et al. Novel mass spectrometry-based comprehensive lipidomic analysis of plasma from patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2020, 35, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Ramos-Rivers, C.; Regueiro, M.; Hashash, J.G.; Barrie, A.; Swoger, J.; Baidoo, L.; Schwartz, M.; Dunn, M.A.; Koutroubakis, I.E.; et al. Association Between Long-Term Lipid Profiles and Disease Severity in a Large Cohort of Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guo, J.; Zhang, T.; Gu, J.; Li, H.; Wang, J. The Role of Dyslipidemia in Colitis-Associated Colorectal Cancer. J. Oncol. 2021, 2021, 6640384. [Google Scholar] [CrossRef] [PubMed]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Sokic-Milutinovic, A.; Milovanovic, T.; Lukic, S.; Milosavljevic, T.; Srzentic Drazilov, S.; Klaassen, K.; Pavlovic, S.; et al. Metabolic Syndrome in Inflammatory Bowel Disease: Association with Genetic Markers of Obesity and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 31–38. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2021, 16, 2–17. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Travis, S.P.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lichtenstein, G.R.; Marteau, P.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Verstockt, B.; Mertens, E.; Dreesen, E.; Outtier, A.; Noman, M.; Tops, S.; Schops, G.; Van Assche, G.; Vermeire, S.; Gils, A.; et al. Influence of Drug Exposure on Vedolizumab-Induced Endoscopic Remission in Anti-Tumour Necrosis Factor [TNF] Naïve and Anti-TNF Exposed IBD Patients. J. Crohns Colitis 2020, 14, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripollés Piquer, B.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.-M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: Consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Rizwan, Y.; Thibault, L.; Lepage, G.; Brunet, S.; Bouthillier, L.; Seidman, E. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am. J. Clin. Nutr. 2000, 71, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motobayashi, M.; Matsuoka, K.; Takenaka, K.; Fujii, T.; Nagahori, M.; Ohtsuka, K.; Iwamoto, F.; Tsuchiya, K.; Negi, M.; Eishi, Y.; et al. Predictors of mucosal healing during induction therapy in patients with acute moderate-to-severe ulcerative colitis. J. Gastroenterol. Hepatol. 2019, 34, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Yu, B.L.; Wang, S.H.; Peng, D.Q.; Zhao, S.P. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010, 88, 285–290. [Google Scholar] [CrossRef]
- Becker, S.A.; McClave, S.A. Lipid profiles in Crohn’s disease patients with and without ileal resection. Am. J. Gastroenterol. 1996, 91, 2452. [Google Scholar]
- Romanato, G.; Scarpa, M.; Ruffolo, C.; Marin, R.; Zambon, S.; Zanoni, S.; Basato, S.; Filosa, T.; Pilon, F.; Angriman, I.; et al. Lipid and phospholipid profile after bowel resection for Crohn’s disease. Int. J. Colorectal Dis. 2008, 23, 931–938. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwao, Y.; Sasaki, S.; Okamoto, S.; Ogata, H.; Hibi, T.; Kazuma, K. Moderate dietary temperance effectively prevents relapse of Crohn disease: A prospective study of patients in remission. Gastroenterol. Nurs. 2007, 30, 202–210. [Google Scholar] [CrossRef]
- Li, T.; Qian, Y.; Li, H.; Deng, J. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018, 17, 261. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lei, X.; Pan, X.; Zeng, X.; Li, W. Association between serum lipids and breast cancer risk in premenopausal women: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211061033. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, M.; Hutri-Kähönen, N.; Tossavainen, P.; Sipponen, T.; Pitkänen, N.; Laitinen, T.; Jokinen, E.; Viikari, J.S.A.; Raitakari, O.T.; Juonala, M.; et al. Low childhood high density lipoprotein cholesterol levels and subsequent risk for chronic inflammatory bowel disease. Dig. Liver Dis. 2018, 50, 348–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, D.; Dayer, J.M. High-density lipoprotein-associated apolipoprotein A-I: The missing link between infection and chronic inflammation? Autoimmun. Rev. 2002, 1, 111–117. [Google Scholar] [CrossRef]
- Ossoli, A.; Wolska, A.; Remaley, A.T.; Gomaraschi, M. High-density lipoproteins: A promising tool against cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159068. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Berberich, A.J.; Hegele, R.A. A modern approach to dyslipidemia. Endocr. Rev. 2021, 43, 611–653. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).