Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Assessment of Insulin Resistance
- (1)
- METS-IR = ln [2 × FPG (mg/dL) + fasting serum TG (mg/dL)] × BMI (kg/m2)/ln [HDL-C (mg/dL)].
- (2)
- TyG index = ln [fasting serum TG (mg/dL) × FPG (mg/dL)/2].
- (3)
- HOMA-IR = [fasting serum insulin (μU/mL) × FPG (mg/dL)/405].
2.4. Assessment of NAFLD
2.5. Assessment of ALF
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Comparison of Predictive Power for Incident ALF of METS-IR, TyG Index, and HOMA-IR at Baseline
3.3. Longitudinal Relationships between the METS-IR, TyG Index, and HOMA-IR and Incident ALF in Patients with NAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Heyens, L.J.M.; Busschots, D.; Koek, G.H.; Robaeys, G.; Francque, S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front. Med. 2021, 8, 615978. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tuo, B. Current and Emerging Approaches for Hepatic Fibrosis Treatment. Gastroenterol. Res. Pract. 2021, 2021, 6612892. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N.; Japan Study Group Of Nafld, J.-N. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Buey, G.; Núñez-Córdoba, J.M.; Llavero-Valero, M.; Gargallo, J.; Salvador, J.; Escalada, J. Is HOMA-IR a potential screening test for non-alcoholic fatty liver disease in adults with type 2 diabetes? Eur. J. Intern. Med. 2017, 41, 74–78. [Google Scholar] [CrossRef]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Gao, J.; Hu, J.; Wen, W.; Zhu, Q.; Wang, M.; Liu, S.; Hong, J.; Wu, T.; Yang, S.; et al. Dose-Response Associations of Metabolic Score for Insulin Resistance Index with Nonalcoholic Fatty Liver Disease among a Nonobese Chinese Population: Retrospective Evidence from a Population-Based Cohort Study. Dis. Markers 2022, 2022, 4930355. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.C.; Beste, L.A.; Utzschneider, K.M. The Impact of Insulin Resistance on Hepatic Fibrosis among United States Adults with Non-Alcoholic Fatty Liver Disease: NHANES 2017 to 2018. Endocrinol. Metab. 2022, 37, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010, 10, 285–288. [Google Scholar] [PubMed]
- Arvind, A.; Osganian, S.A.; Cohen, D.E.; Corey, K.E. Lipid and Lipoprotein Metabolism in Liver Disease; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Jiang, Z.G.; Tsugawa, Y.; Tapper, E.B.; Lai, M.; Afdhal, N.; Robson, S.C.; Mukamal, K.J. Low-fasting triglyceride levels are associated with non-invasive markers of advanced liver fibrosis among adults in the United States. Aliment. Pharmacol. Ther. 2015, 42, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.J.; Lee, M.Y.; Jung, I.; Moon, S.J.; Kwon, H.; Park, S.E.; Rhee, E.J.; Lee, W.Y. Changes in Insulin Resistance Index and the Risk of Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease without Diabetes: Kangbuk Samsung Health Study. Endocrinol. Metab. 2021, 36, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.G.; the KoGES group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sanchez-Lazaro, D.; Meza-Oviedo, D.; Vargas-Vazquez, A.; Campos, O.A.; et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Drolz, A.; Wolter, S.; Wehmeyer, M.H.; Piecha, F.; Horvatits, T.; Schulze Zur Wiesch, J.; Lohse, A.W.; Mann, O.; Kluwe, J. Performance of non-invasive fibrosis scores in non-alcoholic fatty liver disease with and without morbid obesity. Int. J. Obes. 2021, 45, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research, N. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.J.; Lee, M.Y.; Jung, I.; Moon, S.J.; Kwon, H.; Park, S.E.; Rhee, E.J.; Lee, W.Y. Baseline homeostasis model assessment of insulin resistance associated with fibrosis progression in patients with nonalcoholic fatty liver disease without diabetes: A cohort study. PLoS ONE 2021, 16, e0255535. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.R.; Wang, J.L.; Ren, H.Z.; Shi, X.L. Lipometabolism and Glycometabolism in Liver Diseases. Biomed. Res. Int. 2018, 2018, 1287127. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef]
- Privitera, G.; Spadaro, L.; Marchisello, S.; Fede, G.; Purrello, F. Abnormalities of Lipoprotein Levels in Liver Cirrhosis: Clinical Relevance. Dig. Dis. Sci. 2018, 63, 16–26. [Google Scholar] [CrossRef]
- Koruk, M.; Savaş, M.C.; Yilmaz, O.; Tayşi, S.; Karakok, M.; Gündoğdu, C.; Yilmaz, A. Serum lipids, lipoproteins and apolipoproteins levels in patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2003, 37, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
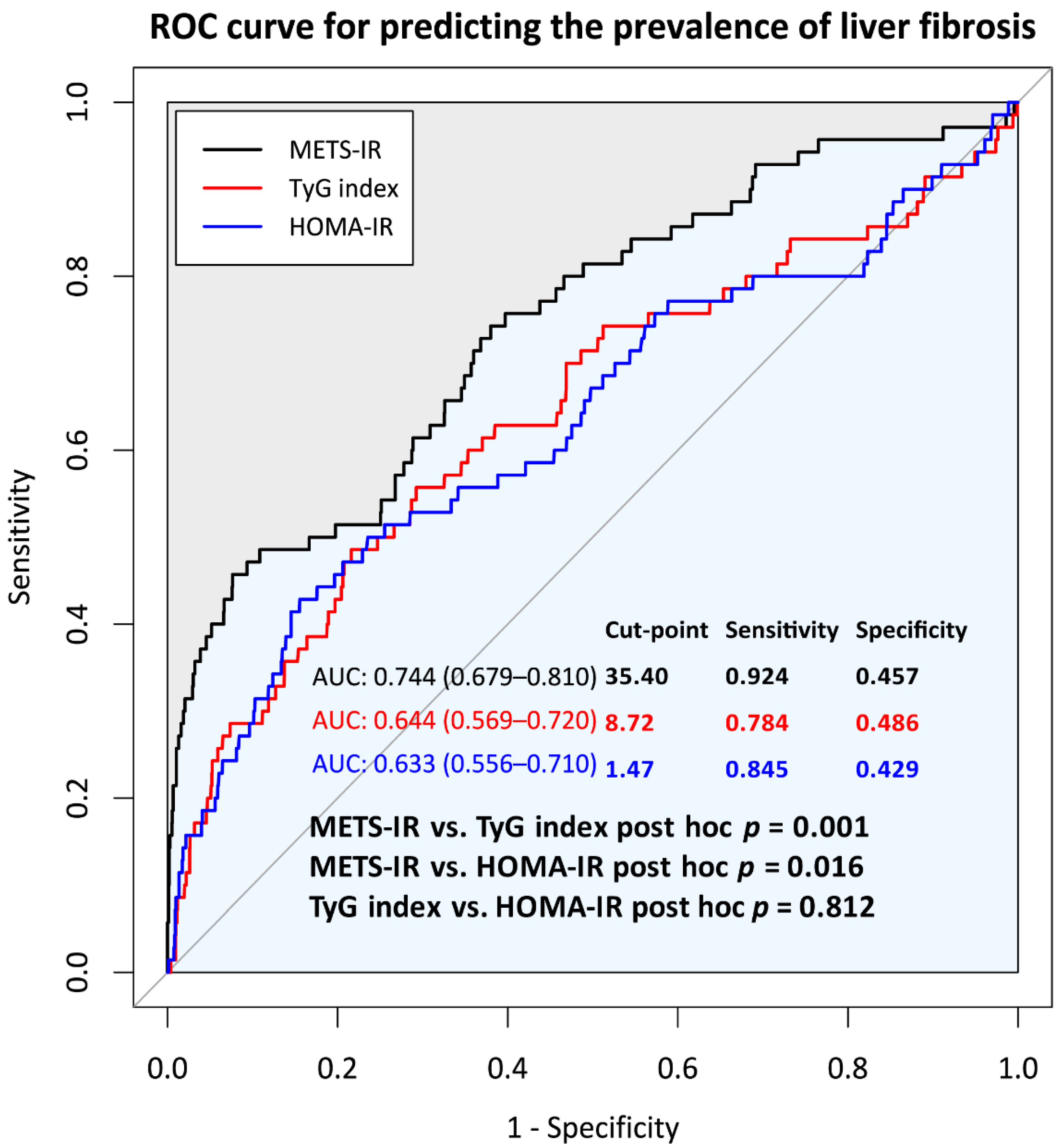
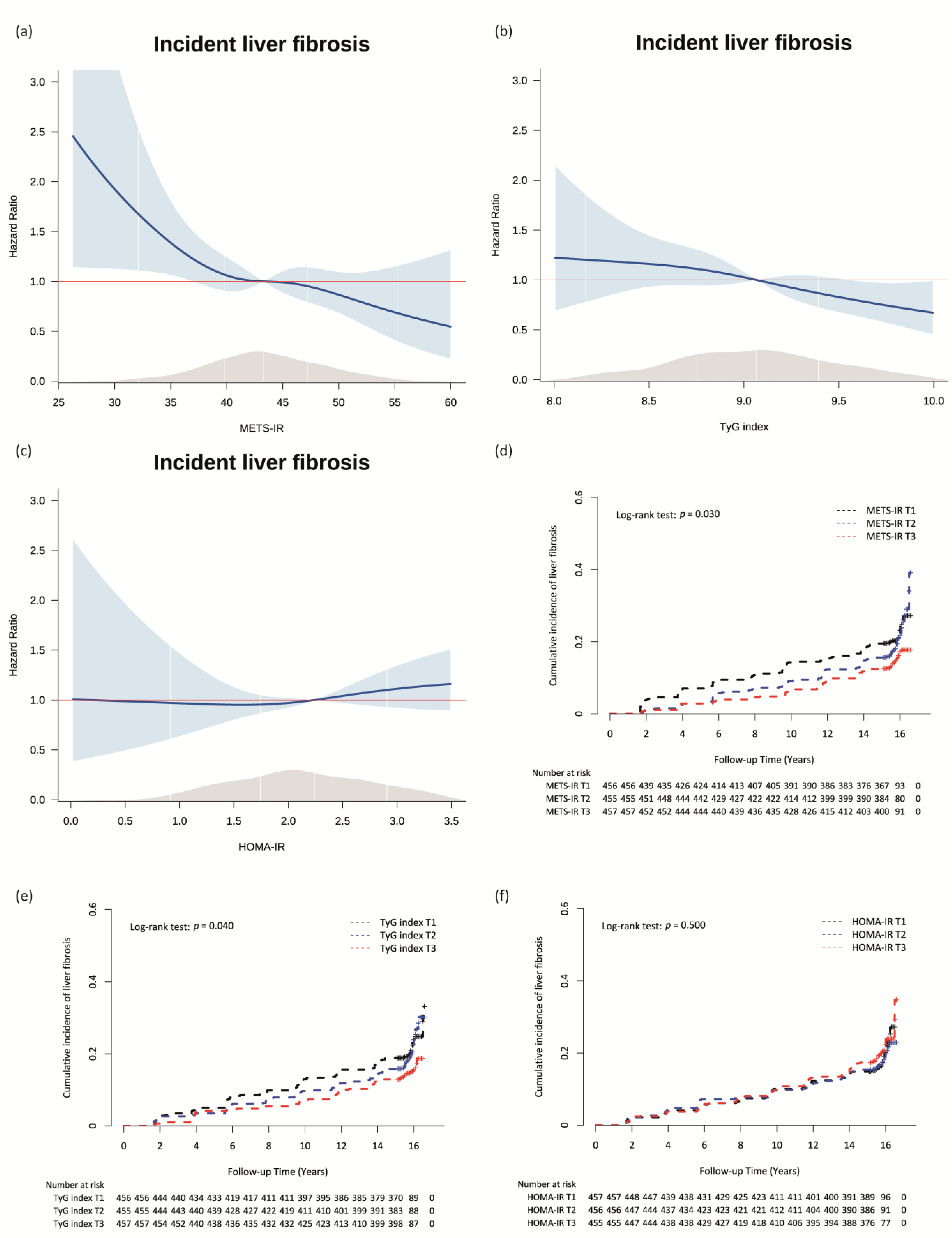
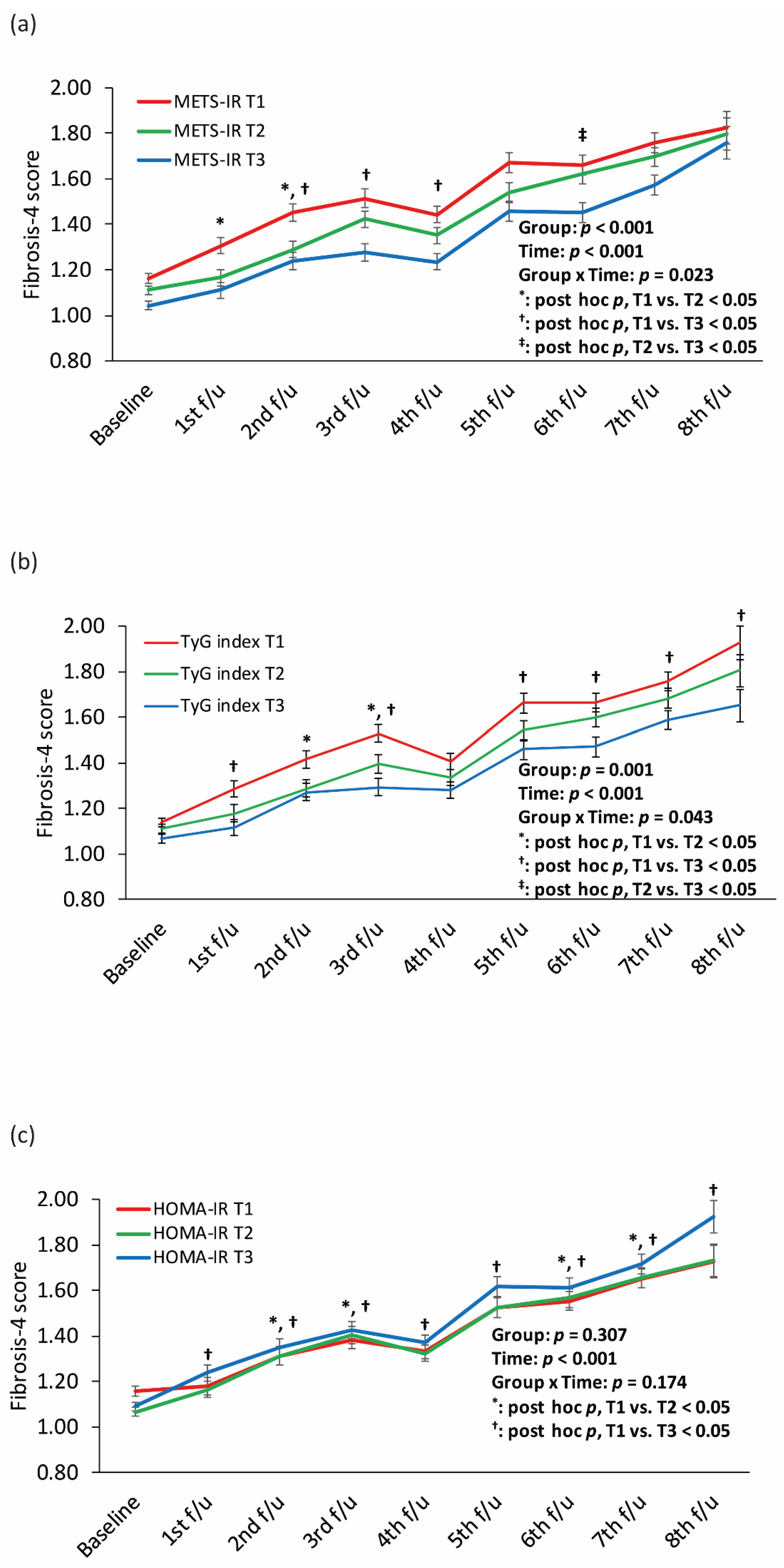
| Variables. | Did Not Develop ALF | Newly Developed ALF | Total | p * |
|---|---|---|---|---|
| Number of participants, n | 1108 | 260 | 1368 | |
| Male sex, n (%) | 460 (41.5%) | 126 (48.5%) | 586 (42.8%) | 0.049 |
| Age, years | 52.3 ± 8.0 | 59.3 ± 7.7 | 53.6 ± 8.4 | <0.001 |
| Waist circumference, cm | 88.9 ± 7.3 | 90.2 ± 7.8 | 89.1 ± 7.4 | 0.012 |
| Body mass index, kg/m2 | 26.7 ± 2.8 | 26.5 ± 3.2 | 26.7 ± 2.9 | 0.192 |
| MBP, mmHg | 102.5 ± 12.3 | 104.5 ± 11.5 | 102.9 ± 12.2 | 0.017 |
| Current drinker, n (%) | 430 (39.2%) | 102 (40.0%) | 532 (39.3%) | 0.869 |
| Smoking status, n (%) | 0.003 | |||
| Never smoker | 719 (65.8%) | 146 (57.3%) | 865 (64.2%) | |
| Former smoker | 155 (14.2%) | 55 (21.6%) | 210 (15.6%) | |
| Some days smoker | 19 (1.7%) | 10 (3.9%) | 29 (2.2%) | |
| Everyday smoker | 200 (18.3%) | 44 (17.3%) | 244 (18.1%) | |
| Physical activity | 0.031 | |||
| <7.5 METs-h/week | 719 (65.8%) | 146 (57.3%) | 865 (64.2%) | |
| 7.5–30 METs-h/week | 155 (14.2%) | 55 (21.6%) | 210 (15.6%) | |
| ≥30 METs-h/week | 19 (1.7%) | 10 (3.9%) | 29 (2.2%) | |
| Platelets,/mm3 | 287.3 ± 60.5 | 232.1 ± 52.2 | 276.8 ± 62.8 | <0.001 |
| Glucose, mg/dL | 94.9 ± 29.1 | 93.9 ± 28.0 | 94.8 ± 28.9 | 0.617 |
| Insulin, µIU/mL | 10.2 (7.8; 12.5) | 10.4 (8.2; 13.3) | 10.2 (7.8; 12.6) | 0.147 |
| Total cholesterol, mg/dL | 201.3 ± 34.0 | 192.4 ± 36.0 | 199.6 ± 34.6 | <0.001 |
| Triglyceride, mg/dL | 192.0 (146.5; 260.5) | 178.0 (138.0; 225.5) | 188.0 (145.0; 253.5) | 0.005 |
| HDL cholesterol, mg/dL | 39.7 ± 7.9 | 40.8 ± 9.2 | 39.9 ± 8.2 | 0.071 |
| LDL cholesterol, mg/dL | 120.3 ± 31.2 | 113.8 ± 32.1 | 119.1 ± 31.5 | 0.004 |
| AST, U/L | 31.1 ± 14.5 | 37.8 ± 17.6 | 32.4 ± 15.4 | <0.001 |
| ALT, U/L | 37.2 ± 27.1 | 40.6 ± 23.8 | 37.8 ± 26.6 | 0.047 |
| Gamma-GTP, U/L | 26.0 (16.0; 44.0) | 30.5 (18.0; 62.0) | 27.0 (16.0; 47.5) | 0.001 |
| Total bilirubin, mg/dL | 0.5 (0.4; 0.7) | 0.5 (0.4; 0.7) | 0.5 (0.4; 0.7) | 0.195 |
| Albumin, g/L | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | <0.001 |
| CRP, mg/dL | 0.18 (0.10; 0.29) | 0.19 (0.10; 0.31) | 0.18 (0.10; 0.29) | 0.429 |
| Total energy intake, kcal/day | 1997.1 ± 713.3 | 1949.0 ± 737.0 | 1988.0 ± 717.8 | 0.340 |
| CHO intake, g/day | 358.3 ± 129.5 | 354.2 ± 133.8 | 357.5 ± 130.3 | 0.653 |
| Protein intake, g/day | 66.3 ± 27.0 | 63.2 ± 27.4 | 65.7 ± 27.1 | 0.109 |
| Fat intake, g/day | 31.1 ± 19.0 | 28.8 ± 19.3 | 30.7 ± 19.1 | 0.096 |
| Vitamin E intake, mg/day | 9.7 ± 5.8 | 9.1 ± 5.0 | 6 ± 5.7 | 0.112 |
| Diabetes mellitus, n (%) | 310 (28.0%) | 79 (30.4%) | 389 (28.4%) | 0.485 |
| Hypertension, n (%) | 662 (59.7%) | 178 (68.5%) | 840 (61.4%) | 0.012 |
| Dyslipidemia, n (%) | 825 (74.5%) | 181 (69.6%) | 1006 (73.5%) | 0.130 |
| Fibrosis-4 score | 1.00 ± 0.34 | 1.60 ± 0.49 | 1.11 ± 0.44 | <0.001 |
| METS-IR | 43.76 ± 5.78 | 42.65 ± 5.82 | 43.55 ± 5.80 | 0.005 |
| TyG index | 9.11 ± 0.53 | 9.01 ± 0.48 | 9.09 ± 0.52 | 0.006 |
| HOMA-IR | 2.62 ± 2.26 | 2.78 ± 2.21 | 2.65 ± 2.25 | 0.284 |
| Year Range | Follow-Up | Total (n) | Incidence Cases (n) | Incidence Rate Per 2 Years |
|---|---|---|---|---|
| 2001–2002 | Baseline | 1368 | ||
| 2003–2004 | 2 years | 1368 | 33 | 2.41 |
| 2005–2006 | 4 years | 1368 | 25 | 1.83 |
| 2007–2008 | 6 years | 1368 | 31 | 2.27 |
| 2009–2010 | 8 years | 1368 | 17 | 1.24 |
| 2011–2012 | 10 years | 1368 | 34 | 2.49 |
| 2013–2014 | 12 years | 1368 | 34 | 2.49 |
| 2015–2016 | 14 years | 1368 | 43 | 3.14 |
| 2017–2018 | 16 years | 1368 | 43 | 3.14 |
| Total Cases, n | New Onset ALF Cases, n | Person-Years of Follow-Up | Incidence Rate Per 1000 Person-Years | Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |||||
| 1368 | 260 | 19,939.4 | 13.0 | |||||||||
| METS-IR | ||||||||||||
| T1 (<41.15) | 456 | 99 | 6443.1 | 15.4 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| T2 (41.15–45.70) | 455 | 91 | 6670.2 | 13.6 | 0.89 (0.67–1.19) | 0.429 | 0.82 (0.58–1.15) | 0.250 | 0.81 (0.58–1.14) | 0.235 | 0.82 (0.58–1.15) | 0.250 |
| T3 (≥45.71) | 457 | 70 | 6826.1 | 10.3 | 0.67 (0.49–0.90) | 0.009 | 0.63 (0.40–0.99) | 0.047 | 0.60 (0.38–0.95) | 0.030 | 0.59 (0.37–0.94) | 0.026 |
| TyG index | ||||||||||||
| T1 (<8.84) | 456 | 98 | 6514.4 | 15.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| T2 (8.84–9.26) | 455 | 93 | 6645.0 | 14.0 | 0.93 (0.70–1.24) | 0.620 | 0.88 (0.65–1.20) | 0.410 | 0.92 (0.68–1.26) | 0.616 | 0.96 (0.70–1.31) | 0.774 |
| T3 (≥9.27) | 457 | 69 | 6780.0 | 10.2 | 0.68 (0.50–0.92) | 0.014 | 0.66 (0.47–0.92) | 0.013 | 0.73 (0.52–1.03) | 0.071 | 0.74 (0.53–1.04) | 0.087 |
| HOMA-IR | ||||||||||||
| T1 (<1.91) | 457 | 84 | 6685.6 | 12.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| T2 (1.91–2.65) | 456 | 82 | 6648.3 | 12.3 | 0.98 (0.73–1.33) | 0.919 | 1.08 (0.78–1.48) | 0.654 | 1.07 (0.77–1.47) | 0.697 | 1.16 (0.81–1.54) | 0.505 |
| T3 (≥2.65) | 455 | 94 | 6605.5 | 14.2 | 1.15 (0.86–1.55) | 0.347 | 1.04 (0.75–1.44) | 0.811 | 1.02 (0.74–1.40) | 0.920 | 1.04 (0.75–1.44) | 0.826 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kwon, Y.-J.; Park, K.; Lee, H.S.; Park, H.-K.; Han, J.H.; Ahn, S.B. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3039. https://doi.org/10.3390/nu14153039
Lee J-H, Kwon Y-J, Park K, Lee HS, Park H-K, Han JH, Ahn SB. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2022; 14(15):3039. https://doi.org/10.3390/nu14153039
Chicago/Turabian StyleLee, Jun-Hyuk, Yu-Jin Kwon, Kyongmin Park, Hye Sun Lee, Hoon-Ki Park, Jee Hye Han, and Sang Bong Ahn. 2022. "Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease" Nutrients 14, no. 15: 3039. https://doi.org/10.3390/nu14153039
APA StyleLee, J.-H., Kwon, Y.-J., Park, K., Lee, H. S., Park, H.-K., Han, J. H., & Ahn, S. B. (2022). Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients, 14(15), 3039. https://doi.org/10.3390/nu14153039






