Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies
Abstract
:1. Anemia: Classifications and Global Significance
- –
- children 6 months to 4 years: Hb < 11.0 g/dL,
- –
- children 5 to 11 years: Hb < 11.5 g/dL,
- –
- children 12–14 years: Hb < 12.0 g/dL,
- –
- adult > 15 years old: men Hb < 13.5 g/dL, women Hb < 12 g/dL
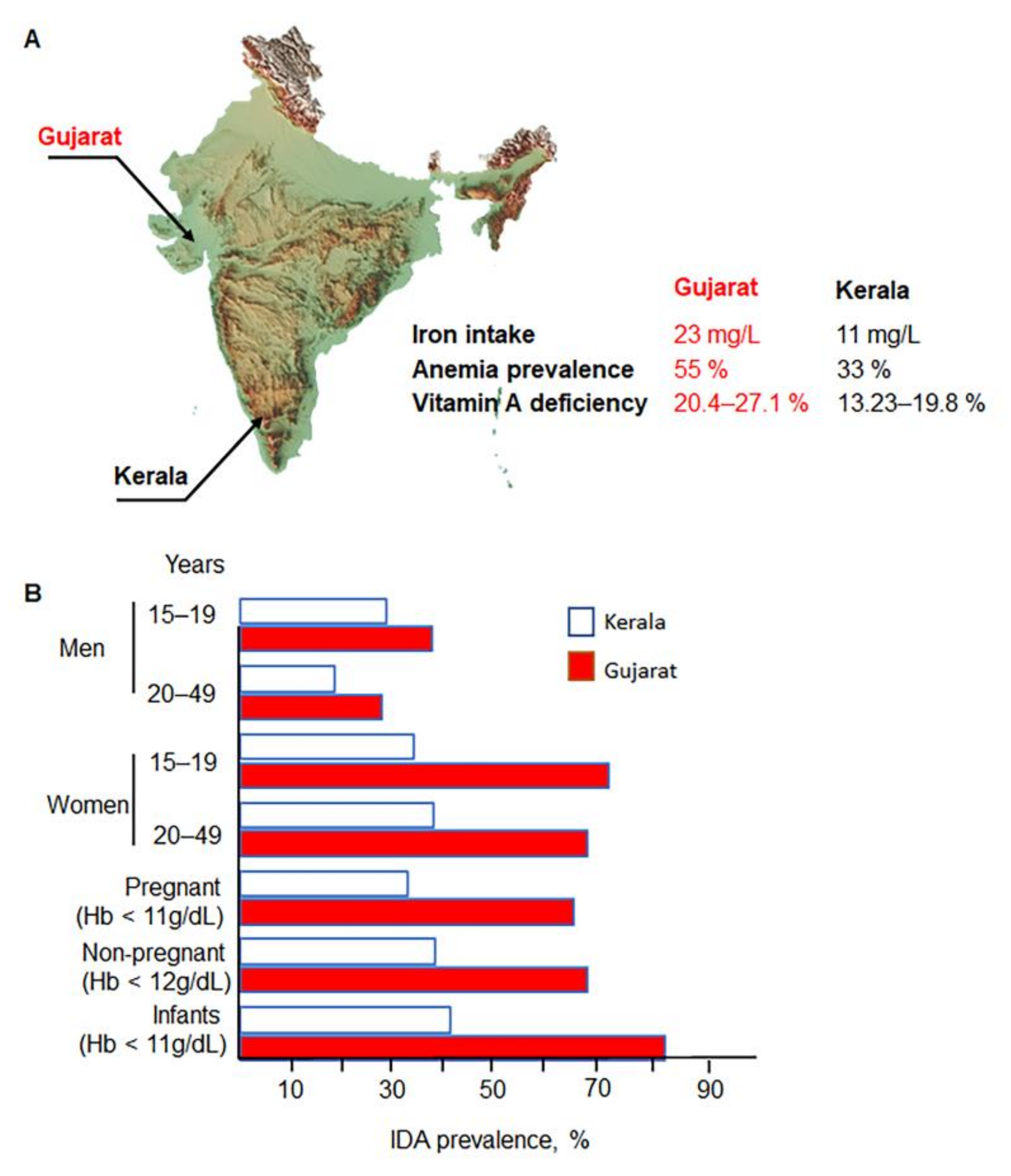
2. Disparities in India: Relevance of IDA Risk Factors
Risk Factors for IDA in Different Populations
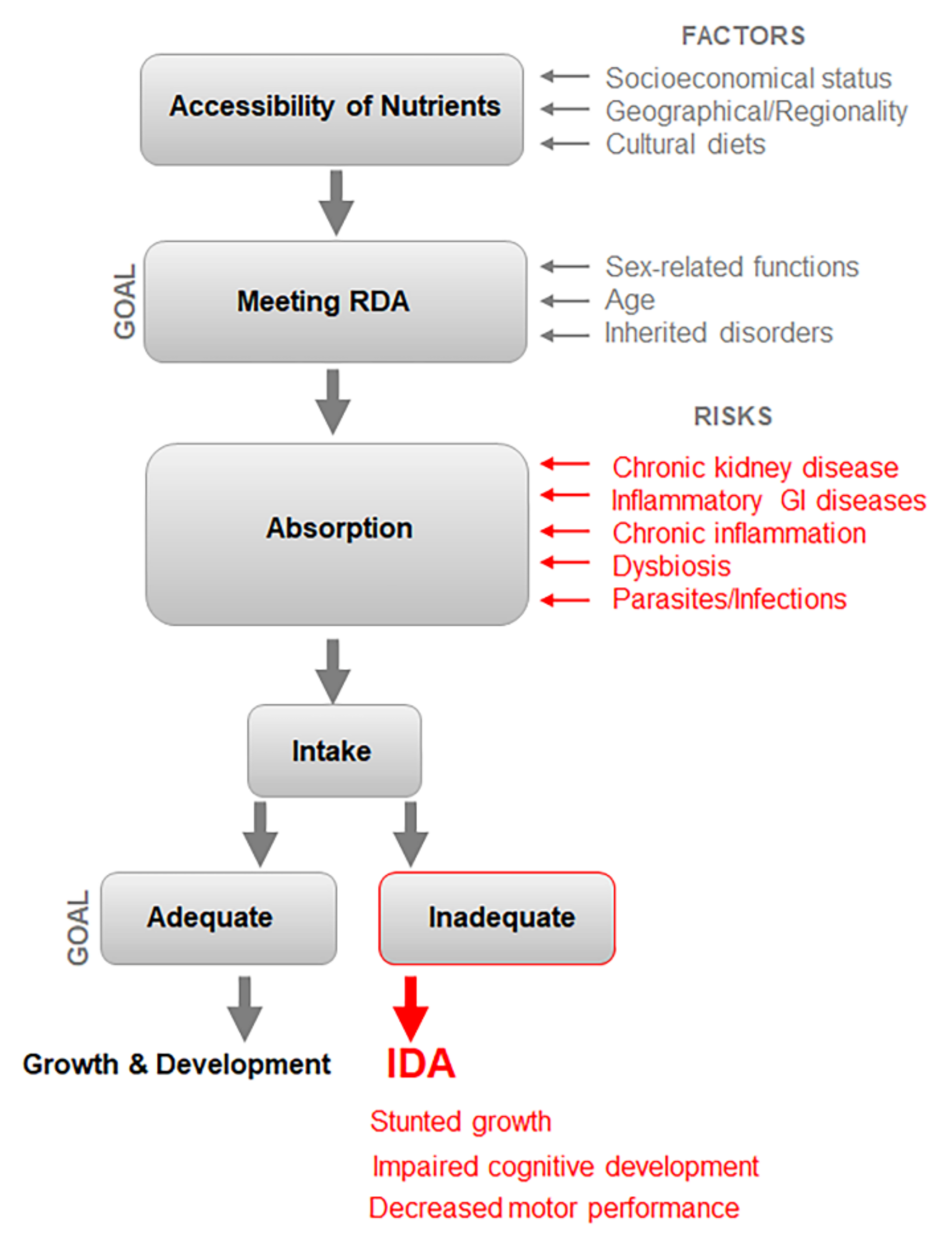
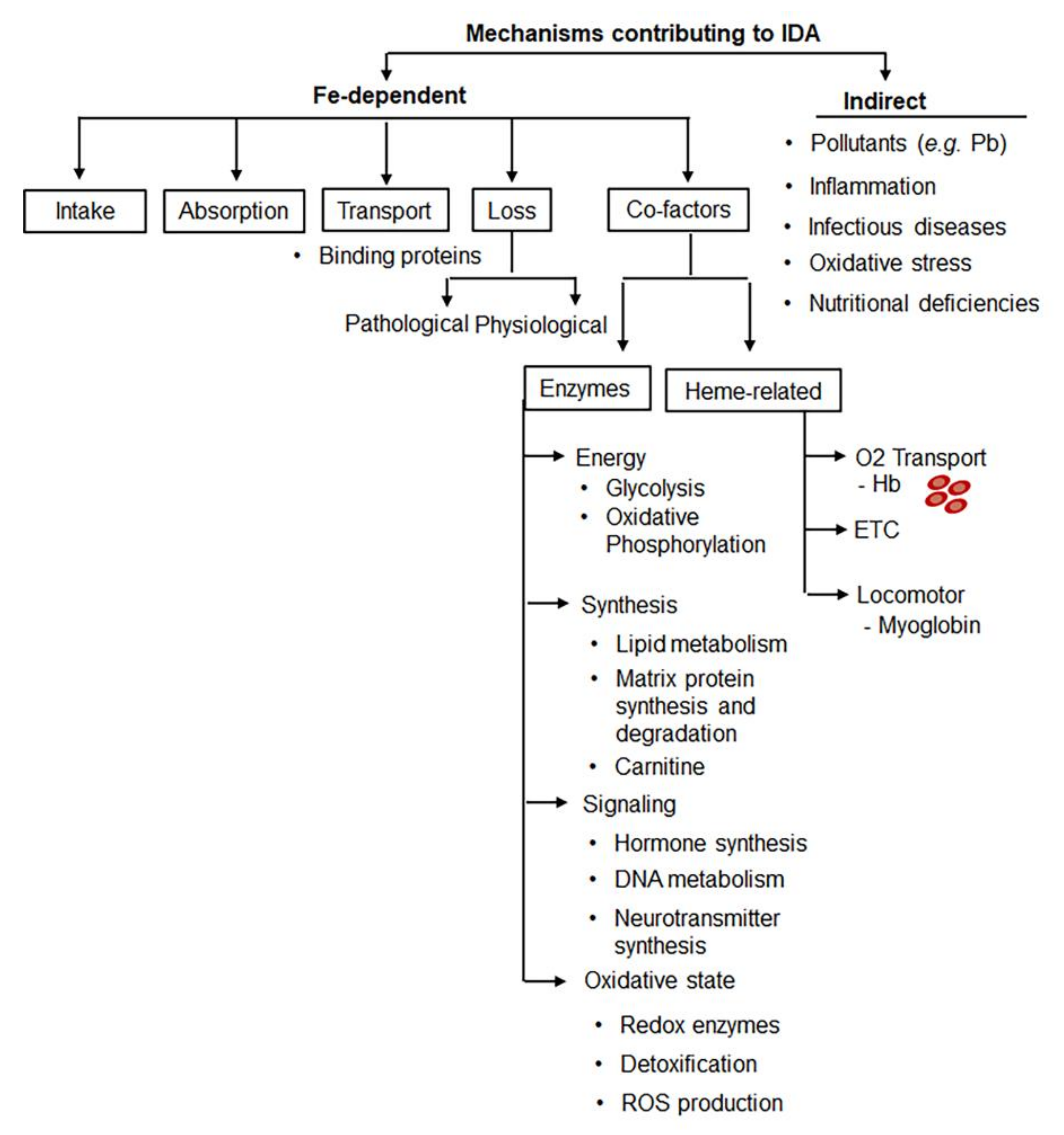
3. Multifactorial Etiology of IDA Poses Challenges for Diagnosis and RDA
4. Recommended RDA of Iron for Indians
5. Efficacy of IDA Prevention Programs in India
6. Current Prevention and Control Strategies against IDA in India
6.1. Programs
- Setting up an intersectoral coordination mechanism at the center, state, and district levels;
- Advocacy and sensitization of policymakers and program managers;
- Intensifying activities to address micronutrient malnutrition;
- Providing nutrition information to people;
- Establishing a monitoring system of nutrition and mapping at the community, district, and state levels;
- Establishing district-wide disaggregated data on nutrition.
- Early Childhood Care Education & Development (ECCED)
- Health Services
- Community Mobilization Awareness, Advocacy and Information, Education, and Communication
- Care and Nutrition Counselling
- (i)
- Assessment of asymptomatic pathologies affecting digestion of supplements: the hidden and silent diseases beyond diarrhea are not elucidated.
- (ii)
- Poor compliance with IFA supplementation often resulted from insufficient counseling regarding the benefits and possible minor side effects of IFA.
- (iii)
- Accessibility of IFA supplements to providers: IFA supplementation is commonly hindered by the transportation of IFA from district warehouses to the providers, such as community health centers.
- (iv)
- Procurement and monitoring by ASHA: Workers have been given the major responsibility to implement all the activities of the NIPI program. The Ministry of Health and Family (MoHFW) and other ministries allocate ASHA responsibilities for the execution of NIPI with newer other programs without additional personnel. The procurement and distribution of IFA are disrupted by these inadequate logistics.
- (v)
- Impaired coordination of programs by various ministries, such as the Ministries of Health and Family Welfare, Women and Child Human Resource, Tribal Affairs, Rural Development, and Urban Development: Therefore, the Government of India has emphasized and taken imperative steps to improve interdepartmental coordination, supervision, and monitoring milestones for the success of this program [110].
- Reducing stunting by 2% annually;
- Reducing under-nutrition by 2% annually;
- Reducing anemia by 3% annually;
- Reducing low birth weight by 2% annually.
6.2. Food Fortification
6.3. Current Efficiency of IDA Mitigation Programs
6.4. Strategical Directions to Improve Interventions to Combat IDA
- Poverty reduction;
- Ensuring food security;
- Improvement in antenatal services;
- Creating awareness program among mothers;
- Improvement of accessibility to diversified diets;
- Promotion of better care and feeding practices i.e., exclusive breastfeeding;
- Food fortification and food-based approaches;
- Prevention and treatment of infectious diseases such as malaria and tuberculosis, etc.;
- Promoting safe water, sanitation, and hygiene (WASH);
- Parasitic disease control programs, especially for manifestation of helminths;
- Integration with other micro-nutrient control programs;
- Monitoring and evaluation of programs;
- Implementing innovative programs;
- Intersectoral coordination;
- Advocacy and social communication;
- Strengthening the surveillance system;
- Continuous monitoring of new research and its incorporation into the program.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change With Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Peña-Rosas, J. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf (accessed on 25 April 2022).
- Chauhan, S.; Kumar, P.; Marbaniang, S.P.; Srivastava, S.; Patel, R. Prevalence and predictors of anaemia among adolescents in Bihar and Uttar Pradesh, India. Sci. Rep. 2022, 12, 8197. [Google Scholar] [CrossRef]
- Piva, E.; Brugnara, C.; Chiandetti, L.; Plebani, M. Automated reticulocyte counting: State of the art and clinical applications in the evaluation of erythropoiesis. Clin. Chem. Lab. Med. 2010, 48, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Silva, D.P.; Infante, J.; Ferro, J.M. Cerebrovascular Complications of Anemia. Curr. Neurol. Neurosci. Rep. 2021, 21, 51. [Google Scholar] [CrossRef]
- Turner, J.; Parsi, M.; Badireddy, M. Anemia. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499994/ (accessed on 25 April 2022).
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Da Cunha, M.S.B.; Campos Hankins, N.A.; Arruda, S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, F.; Garcia-Lopez, S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 2009, 15, 4638–4643. [Google Scholar] [CrossRef]
- Nair, K.M.; Fernandez-Rao, S.; Nagalla, B.; Kankipati, R.V.; Punjal, R.; Augustine, L.F.; Hurley, K.M.; Tilton, N.; Harding, K.B.; Reinhart, G.; et al. Characterisation of anaemia and associated factors among infants and pre-schoolers from rural India. Public Health Nutr. 2016, 19, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Thankachan, P.; Muthayya, S.; Walczyk, T.; Kurpad, A.V.; Hurrell, R.F. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr. Bull. 2007, 28, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Available online: https://apps.who.int/iris/handle/10665/43894 (accessed on 25 April 2022).
- Işık Balcı, Y.; Karabulut, A.; Gürses, D.; Ethem Çövüt, I. Prevalence and Risk Factors of Anemia among Adolescents in Denizli, Turkey. Iran. J. Pediatr. 2012, 22, 77–81. [Google Scholar] [PubMed]
- Nair, K.M.; Iyengar, V. Iron content, bioavailability & factors affecting iron status of Indians. Indian J. Med. Res. 2009, 130, 634–645. [Google Scholar]
- NFHS. National Family Health Survey V. 2021. Available online: http://rchiips.org/nfhs/factsheet_NFHS-5.shtml (accessed on 25 April 2022).
- Mehdad, A.; Siqueira, E.M.; Arruda, S.F. Effect of vitamin A deficiency on iron bioavailability. Ann. Nutr. Metab. 2010, 57, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Bloem, M.W. The anemia of vitamin A deficiency: Epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002, 56, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Arruda, S.F.; Siqueira, E.M.; de Valencia, F.F. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition 2009, 25, 472–478. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Gongora, V.; Salinas-Rodriguez, A.; Villalpando, S.; Flores-Aldana, M. Serum Retinol but Not 25(OH)D Status Is Associated With Serum Hepcidin Levels in Older Mexican Adults. Nutrients 2019, 11, 988. [Google Scholar] [CrossRef] [Green Version]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar] [CrossRef]
- Azizi-Soleiman, F.; Vafa, M.; Abiri, B.; Safavi, M. Effects of Iron on Vitamin D Metabolism: A Systematic Review. Int. J. Prev. Med. 2016, 7, 126. [Google Scholar] [CrossRef]
- Mohanty, D.; Gorakshakar, A.C.; Colah, R.B.; Patel, R.Z.; Master, D.C.; Mahanta, J.; Sharma, S.K.; Chaudhari, U.; Ghosh, M.; Das, S.; et al. Interaction of iron deficiency anemia and hemoglobinopathies among college students and pregnant women: A multi center evaluation in India. Hemoglobin 2014, 38, 252–257. [Google Scholar] [CrossRef]
- Ali, S.S. A brief review of risk-factors for growth and developmental delay among preschool children in developing countries. Adv. Biomed. Res. 2013, 2, 91. [Google Scholar] [CrossRef] [PubMed]
- Greig, A.J.; Patterson, A.J.; Collins, C.E.; Chalmers, K.A. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: A systematic review. J. Nutr. Sci. 2013, 2, e14. [Google Scholar] [CrossRef] [Green Version]
- Butensky, E.; Harmaz, P.; Lubin, B. Part V: Nutrional Aspects of Specific Disease Staes, Chapter 62: Nutritional Anemias. In Nutrition in Pediatrics, 4th ed.; BC Becker: Hamilton, ON, Canada, 2008. [Google Scholar]
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Daru, J.; Zamora, J.; Fernandez-Felix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tuncalp, O.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef] [Green Version]
- NFHS. National Family Health Survey III 2005–2006. Available online: https://dhsprogram.com/pubs/pdf/frind3/frind3-vol1andvol2.pdf (accessed on 25 April 2022).
- NFHS. National Family Health Survey IV 2005–2006. Available online: https://dhsprogram.com/pubs/pdf/FR339/FR339.pdf (accessed on 25 April 2022).
- National Nutrition Monitoring Bureau Report. 2003. Available online: https://ghdx.healthdata.org/organizations/national-nutrition-monitoring-bureau-india (accessed on 25 April 2022).
- Crichton, R.R. Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Nutrition Atlas. Available online: http://218.248.6.39/nutritionatlas/dashbord/anaemia.php (accessed on 25 April 2022).
- Moisidis-Tesch, C.M.; Shulman, L.P. Iron Deficiency in Women’s Health: New Insights into Diagnosis and Treatment. Adv. Ther. 2022, 39, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.S. Iron metabolism and iron deficiency in India. Am. J. Clin. Nutr. 1968, 21, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S. Current issues for the prevention and treatment of iron deficiency anemia. Indian Pediatr. 2002, 39, 125–129. [Google Scholar]
- Khaskheli, M.N.; Baloch, S.; Sheeba, A.; Baloch, S.; Khaskheli, F.K. Iron deficiency anaemia is still a major killer of pregnant women. Pak. J. Med. Sci. 2016, 32, 630–634. [Google Scholar] [CrossRef]
- Gilreath, J.A.; Stenehjem, D.D.; Rodgers, G.M. Diagnosis and treatment of cancer-related anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef]
- Fishbane, S.; Pollack, S.; Feldman, H.I.; Joffe, M.M. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin. J. Am. Soc. Nephrol. 2009, 4, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Bager, P.; Befrits, R.; Wikman, O.; Lindgren, S.; Moum, B.; Hjortswang, H.; Dahlerup, J.F. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand. J. Gastroenterol. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Mabry-Hernandez, I.R. Screening for iron deficiency anemia—Including iron supplementation for children and pregnant women. Am. Fam. Physician 2009, 79, 897–898. [Google Scholar] [PubMed]
- Piccin, A.; Murphy, C.; Eakins, E.; Rondinelli, M.B.; Daves, M.; Vecchiato, C.; Wolf, D.; Mc Mahon, C.; Smith, O.P. Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. Eur. J. Haematol. 2019, 102, 319–330. [Google Scholar] [CrossRef]
- Hyacinth, H.I.; Gee, B.E.; Hibbert, J.M. The Role of Nutrition in Sickle Cell Disease. Nutr. Metab. Insights 2010, 3, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahovic, S.; Vukobrat-Bijedic, Z.; Sahovic, V. Importance of sideropenic anemia in the diagnosis of gastrointestinal tract tumors. Mater. Sociomed. 2012, 24, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Kadla, S.A.; Shah, N.A.; Bindroo, M.A.; Khan, B.A.; Farooq, A.; Yousf, W.; Wani, B.A. Evaluation of iron deficiency anaemia for gastrointestinal causes in patients without GI symptoms in high prevalent GI malignancy zones. Arab. J. Gastroenterol. 2016, 17, 67–72. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, S.; Meena, L.P.; Meher, M.P.; Rai, M.; Kumar, S.; Bharti, A. Study to evaluate the etiology of iron deficiency anemia at a teaching hospital in northeastern part of India. J. Fam. Med. Prim. Care 2020, 9, 3076–3081. [Google Scholar] [CrossRef]
- Gunjan, D.; Sharma, V.; Rana, S.S.; Bhasin, D.K. Small bowel bleeding: A comprehensive review. Gastroenterol. Rep. 2014, 2, 262–275. [Google Scholar] [CrossRef] [Green Version]
- Katariya, D.; Swain, D.; Singh, S.; Satapathy, A. The Effect of Different Timings of Delayed Cord Clamping of Term Infants on Maternal and Newborn Outcomes in Normal Vaginal Deliveries. Cureus 2021, 13, e17169. [Google Scholar] [CrossRef]
- Berry, N.; Basha, J.; Varma, N.; Varma, S.; Prasad, K.K.; Vaiphei, K.; Dhaka, N.; Sinha, S.K.; Kochhar, R. Anemia in celiac disease is multifactorial in etiology: A prospective study from India. JGH Open 2018, 2, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, A.N.; Hamide, A.; Bobby, Z.; Krishnan, R. Effect of anti-Helicobacter pylori therapy on outcome of iron-deficiency anemia: A randomized, controlled study. Indian J. Gastroenterol. 2005, 24, 155–157. [Google Scholar] [PubMed]
- Zijp, I.M.; Korver, O.; Tijburg, L.B. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef]
- Advani, S.; Kochhar, G.; Chachra, S.; Dhawan, P. Eating everything except food (PICA): A rare case report and review. J. Int. Soc. Prev. Community Dent. 2014, 4, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, R.; Matsuda, T.; Chonan, A. Iron-deficiency anemia caused by a proton pump inhibitor. Intern. Med. 2014, 53, 2297–2299. [Google Scholar] [CrossRef] [Green Version]
- Sarzynski, E.; Puttarajappa, C.; Xie, Y.; Grover, M.; Laird-Fick, H. Association between proton pump inhibitor use and anemia: A retrospective cohort study. Dig. Dis. Sci. 2011, 56, 2349–2353. [Google Scholar] [CrossRef]
- Kanuri, G.; Sawhney, R.; Varghese, J.; Britto, M.; Shet, A. Iron Deficiency Anemia Coexists with Cancer Related Anemia and Adversely Impacts Quality of Life. PLoS ONE 2016, 11, e0163817. [Google Scholar] [CrossRef]
- Reddy, P.H.; Petrou, M.; Reddy, P.A.; Tiwary, R.S.; Modell, B. Hereditary anaemias and iron deficiency in a tribal population (the Baiga) of central India. Eur. J. Haematol. 1995, 55, 103–109. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 416–418. [Google Scholar] [CrossRef]
- Agustina, R.; Nadiya, K.; Andini, E.A.; Setianingsih, A.A.; Sadariskar, A.A.; Prafiantini, E.; Wirawan, F.; Karyadi, E.; Raut, M.K. Associations of meal patterning, dietary quality and diversity with anemia and overweight-obesity among Indonesian school-going adolescent girls in West Java. PLoS ONE 2020, 15, e0231519. [Google Scholar] [CrossRef]
- Adiwinata, R.; Livina, A.; Waleleng, B.J.; Haroen, H.; Rotty, L.; Gosal, F.; Rotty, L.; Hendratta, C.; Lasut, P.; Winarta, J.; et al. Anemia in Inflammatory Bowel Disease: A Neglected Issue in Comprehensive Inflammatory Bowel Disease Management. Acta Med. Indones. 2021, 53, 360–370. [Google Scholar] [CrossRef]
- Sood, A.; Ahuja, V.; Kedia, S.; Midha, V.; Mahajan, R.; Mehta, V.; Sudhakar, R.; Singh, A.; Kumar, A.; Puri, A.S.; et al. Diet and inflammatory bowel disease: The Asian Working Group guidelines. Indian J. Gastroenterol. 2019, 38, 220–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkata Surekha, M.; Sujatha, T.; Gadhiraju, S.; Uday Kumar, P.; Siva Prasad, M.; Sailaja, G.; Bhaskar, V.; Srinivas, T. Expression of iron transport protein Divalent metal transporter 1 (DMT1) increases in response to maternal iron deficiency anemia in near term to term placenta. J. Matern.-Fetal Neonatal Med. 2022, 35, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, D.C.; Lee, W.T.C.; Wild, S.; Jozwiakowski, S.K.; Rothenberg, E.; Gari, K. Cancer-associated mutations in the iron-sulfur domain of FANCJ affect G-quadruplex metabolism. PLoS Genet. 2020, 16, e1008740. [Google Scholar] [CrossRef] [PubMed]
- Warang, P.; Kedar, P.; Ghosh, K.; Colah, R. Molecular and clinical heterogeneity in pyruvate kinase deficiency in India. Blood Cells Mol. Dis. 2013, 51, 133–137. [Google Scholar] [CrossRef]
- Venugopal, A.; Chandran, M.; Eruppakotte, N.; Kizhakkillach, S.; Breezevilla, S.C.; Vellingiri, B. Monogenic diseases in India. Mutat. Res. Rev. Mutat. Res. 2018, 776, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Fleming, P.; Eakins, E.; McGovern, E.; Smith, O.P.; McMahon, C. Sickle cell disease and dental treatment. J. Ir. Dent. Assoc. 2008, 54, 75–79. [Google Scholar]
- Hershko, C.; Skikne, B. Pathogenesis and management of iron deficiency anemia: Emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin. Hematol. 2009, 46, 339–350. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Kapoor, D.; Singh, K.; Narayan, K.M.; Ali, M.K.; Kadir, M.M.; Mohan, V.; Tandon, N.; Prabhakaran, D. Vegetarianism and cardiometabolic disease risk factors: Differences between South Asian and US adults. Nutrition 2016, 32, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.K.; Gupta, P.S.; Chuttani, K.H. Gastric acid secretion in Indians with particular reference to the ratio of basal to maximal acid output. Gut 1966, 7, 619–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, V.P.; Taneja, D.K.; Sharma, N.; Gupta, V.K.; Ingle, G.K. Dietary aspects of pregnant women in rural areas of Northern India. Matern. Child Nutr. 2008, 4, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, A.; Paul, P.; Saha, J.; Barman, B.; Chouhan, P. Maternal determinants of low birth weight among Indian children: Evidence from the National Family Health Survey-4, 2015–2016. PLoS ONE 2020, 15, e0244562. [Google Scholar] [CrossRef] [PubMed]
- Gulati, B.K.; Sharma, S.; Vardhana Rao, M.V. Analyzing the Changes in Certain Infectious and Parasitic Diseases in Urban Population of India By Using Medical Certification of Cause of Death Data. Indian J. Community Med. 2021, 46, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, D.A.; Cook, J.D.; Finch, C.A. A clinical evaluation of serum ferritin as an index of iron stores. N. Engl. J. Med. 1974, 290, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta 2013, 1836, 245–254. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef] [Green Version]
- Leitch, H.A.; Fibach, E.; Rachmilewitz, E. Toxicity of iron overload and iron overload reduction in the setting of hematopoietic stem cell transplantation for hematologic malignancies. Crit. Rev. Oncol. Hematol. 2017, 113, 156–170. [Google Scholar] [CrossRef]
- DeLoughery, T.G. Iron Deficiency Anemia. Med. Clin. N. Am. 2017, 101, 319–332. [Google Scholar] [CrossRef]
- Serjeant, G.R.; Ghosh, K.; Patel, J. Sickle cell disease in India: A perspective. Indian J. Med. Res. 2016, 143, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.R.; Silveira, L., Jr.; Fernandes, A.B. Diagnosing sickle cell disease and iron deficiency anemia in human blood by Raman spectroscopy. Lasers Med. Sci. 2020, 35, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kabootarizadeh, L.; Jamshidnezhad, A.; Koohmareh, Z. Differential Diagnosis of Iron-Deficiency Anemia from beta-Thalassemia Trait Using an Intelligent Model in Comparison with Discriminant Indexes. Acta Inform. Med. 2019, 27, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.; Berube, C.; Lyell, D.J. Iron deficiency anemia in pregnancy. Curr. Opin. Obstet. Gynecol. 2022, 34, 69–76. [Google Scholar] [CrossRef]
- Hoenemann, C.; Ostendorf, N.; Zarbock, A.; Doll, D.; Hagemann, O.; Zimmermann, M.; Luedi, M. Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review. J. Clin. Med. 2021, 10, 4250. [Google Scholar] [CrossRef]
- ICMR. Nutrient Requirements and Recommended Dietary Allowances for Indians: A Report of the Expert Group of the ICMR. 2010. Available online: https://www.enacnetwork.com/files/pdf/ICMR_RDA_BOOK_2010.pdf (accessed on 3 May 2022).
- WHO. Global Nutrition Targets 2025: Anaemia Policy Brief. 2015. Available online: http://www.who.int/nutrition/topics/globaltargets_anaemia_policybrief.pdf (accessed on 9 April 2022).
- WHO. Guidelines and Recommendations on Micronutrients: Policy, Practice and Service Delivery Issues. 2015. Available online: https://apps.who.int/iris/handle/10665/160764 (accessed on 5 April 2022).
- NIHFW. National Health Programme. Available online: http://www.nihfw.org/ (accessed on 29 March 2022).
- Mutharayappa, R. A study of acceptors and non-acceptors of family planning methods among three tribal communities. Man India 1995, 75, 11–24. [Google Scholar]
- MoHFW. Adolescent Division, GoI. Guidelines for Control of Iron Deficiency Anemia: National Iron + Initiative; MoHFW: New Delhi, India, 2013; pp. 23–29. [Google Scholar]
- Kotecha, P.V. Nutritional anemia in young children with focus on Asia and India. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2011, 36, 8–16. [Google Scholar] [CrossRef]
- John, D.A.; Babu, G.R. Lessons From the Aftermaths of Green Revolution on Food System and Health. Front. Sustain. Food Syst. 2021, 5, 644559. [Google Scholar] [CrossRef]
- DeFries, R.; Chhatre, A.; Davis, K.F.; Dutta, A.; Fanzo, J.; Ghosh-Jerath, S.; Myers, S.; Rao, N.D.; Smith, M.R. Impact of Historical Changes in Coarse Cereals Consumption in India on Micronutrient Intake and Anemia Prevalence. Food Nutr. Bull. 2018, 39, 377–392. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Padilla-Zakour, O.I. Plant-Based Dietary Practices and Socioeconomic Factors That Influence Anemia in India. Nutrients 2021, 13, 3538. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Botha, R.; Givens, D.I.; Sulaiman, N.L.B.; Upadhyay, S.; Vetriventhan, M.; Tsusaka, T.W.; Parasannanavar, D.J.; Longvah, T.; et al. Millets Can Have a Major Impact on Improving Iron Status, Hemoglobin Level, and in Reducing Iron Deficiency Anemia—A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 725529. [Google Scholar] [CrossRef] [PubMed]
- National Nutrition Policy. New Delhi. 1993. Available online: http://wcd.nic.in/sites/default/files/nnp_0.pdf (accessed on 3 May 2022).
- Ministry of Human Resource Development, Government of India. National Plan of Action on Nutrition. New Delhi. 1995. Available online: http://14.139.60.153/bitstream/123456789/50/1/NATIONAL%20PLAN%20OF%20ACTION%20ON%20NUTRITION.pdf (accessed on 3 May 2022).
- ICDS. Integrated Child Development Services. 2010. Available online: http://wcd.nic.in/icds.htm (accessed on 3 May 2022).
- MDMS. Mid Day Meal Scheme. 2008. Available online: http://mdm.nic.in/mdm_website/ (accessed on 3 May 2022).
- Global Hunger Index. Available online: https://www.globalhungerindex.org/india.html (accessed on 3 May 2022).
- WHO India. Section 6. 2012. Available online: http://www.whoindia.org/en/Section6/Section324_1467.htm (accessed on 3 May 2022).
- 12by12 Initiative IIS. 2012. Available online: http://www.12by12initiative.com/iis.asp (accessed on 3 May 2022).
- Upadhyay, R.P.; Palanivel, C.; Kulkarni, V. Unrelenting burden of anaemia in India: Highlighting possible prevention strategies. Int. J. Med. Public Health 2012, 2, 1–6. [Google Scholar] [CrossRef]
- WCD. Rajiv Gandhi Scheme for Girls Empowerment of Adolescent (RGSEAG)-SABALA-the Scheme. 2010. Available online: http://wcd.nic.in/schemes/SABLAscheme.pdf (accessed on 3 May 2022).
- SAG. RRS. Available online: https://www.sag-rrs.nic.in/ (accessed on 3 May 2022).
- NIS. Nischay. 2007. Available online: http://nischay2007.tripod.com/id2.html (accessed on 3 May 2022).
- Kapil, U.; Kapil, R.; Gupta, A. National Iron Plus Initiative: Current status & future strategy. Indian J. Med. Res. 2019, 150, 239–247. [Google Scholar] [CrossRef] [PubMed]
- National Iron Plus Initiative. New Delhi: MoHFW. 2018. Available online: https://www.nhp.gov.in/national-iron-plus-initiative-for-anemia-control_pg (accessed on 3 May 2022).
- Fathima, F.N.; Raju, M.; Varadharajan, K.S.; Krishnamurthy, A.; Ananthkumar, S.R.; Mony, P.K. Assessment of ‘accredited social health activists’—A national community health volunteer scheme in Karnataka State, India. J. Health Popul. Nutr. 2015, 33, 137–145. [Google Scholar]
- Anemia. Mukt Bharat 6x6x6. Available online: https://anemiamuktbharat.info/home/6x6x6-strategy/ (accessed on 3 May 2022).
- MI. AnnualReport07-EN Nutrition International User_Files. 2014. Available online: https://www.nutritionintl.org (accessed on 3 May 2022).
- Naandi Foundation. Available online: https://www.naandi.org/ (accessed on 3 May 2022).
- Fisher, A.E.; Naughton, D.P. Iron supplements: The quick fix with long-term consequences. Nutr. J. 2004, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Hemery, Y.M.; Laillou, A.; Fontan, L.; Jallier, V.; Moench-Pfanner, R.; Berger, J.; Avallone, S. Storage conditions and packaging greatly affects the stability of fortified wheat flour: Influence on vitamin A, iron, zinc, and oxidation. Food Chem. 2018, 240, 43–50. [Google Scholar] [CrossRef]
- Gibson, R.S.; Anderson, V.P. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr. Bull. 2009, 30, S108–S143. [Google Scholar] [CrossRef]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.K.; Augustine, L.F.; Konapur, A. Food-Based Interventions to Modify Diet Quality and Diversity to Address Multiple Micronutrient Deficiency. Front. Public Health 2015, 3, 277. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, P. Food & nutrition security: Challenges in the new millennium. Indian J. Med. Res. 2013, 138, 373–382. [Google Scholar]
- Sanchez, P.A.; Swaminathan, M.S. Hunger in Africa: The link between unhealthy people and unhealthy soils. Lancet 2005, 365, 442–444. [Google Scholar] [CrossRef]
- Das, A.; Das, M.; Ghosh, S. Impact of nutritional status and anemia on COVID-19: Is it a public health concern? Evidence from National Family Health Survey-4 (2015–2016), India. Public Health 2020, 185, 93–94. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Borrelli de Andreis, F.; Aronico, N.; Lenti, M.V.; Barteselli, C.; Merli, S.; Pellegrino, I.; Coppola, L.; Cremonte, E.M.; Croce, G.; et al. Anemia in patients with COVID-19: Pathogenesis and clinical significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus. Apher. Sci. 2020, 59, 102926. [Google Scholar] [CrossRef]
- Hou, S.W.; Wang, Z.F.; Guan, S.H.; Yang, K.; Wang, Q.; Chen, L.W.; Zhang, H.; Wang, X.; Duan, Y.L.; Pan, Z.L. Alterations in the Iron Homeostasis Network of Hepatocytes Caused by Hepatitis B Virus. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron sequestration and anemia of inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr. Clin. Microbiol. Rep. 2020, 7, 13–19. [Google Scholar] [CrossRef]
- WHO. Iron Deficiency Anemia: Assessment, 405 Prevention and Control—A Guide for Programme Managers. 2001. Available online: https://www.who.int/publications/m/item/iron-children-6to23--archived-iron-deficiency-anaemia-assessment-prevention-and-control (accessed on 3 May 2022).
- Strategies to Prevent IDA. Recommendation from Expert Group Consultation. 2016. Available online: https://apps.who.int/iris/handle/10665/312109 (accessed on 3 May 2022).
- Thompson, B. Food Based Approaches for Combating Iron Deficiency. 2007. Available online: ftp://ftp.fao.org/ag/agn/nutrition/Kapitel_21_210207.pdf (accessed on 29 April 2022).

| Group | Age (Years old) | Hb Level (g/dL) | NFHS II (%) | NFHS III (%) | NFHS IV (%) |
|---|---|---|---|---|---|
| Children | 6–35 | <11 | 74.2 | 78.9 | 58.4 |
| Childbearing women | 15–49 | <12 | 51.8 | 56.2 | 53.0 |
| Pregnant women | 15–49 | <11 | 49.7 | 57.9 | 50.3 |
| Men | 15–49 | <13 | -- | 24.3 | 22.7 |
| Blood Loss-Related Conditions | Malabsorption | Anemia of Chronic Diseases | Genetic Disorders |
|---|---|---|---|
| Digestive tract Colorectal cancer [46] Gastric carcer [47] IBD [37] Peptic ulcers [48] Angiodysplasia [49] Parasites [49] Entamoeba histolytica Giardia intestinalis Ascaris lumbricoides Plasmodium falciparum Gynecological causes [50] Menstruation Labor Delayed umbilical cord clamping Surgeries Urinary tract (hematuria): UTI Bladder cancer Renal cancer Hemodialysis Respiratory tract: Epistaxis Hemoptysis Drugs: Aspirin and other NSAID [11] Vitamin E toxicity | Celiac disease [51] Gastrectomy Helicobacter pylori [52] Bowel resection Atrophic gastritis Bypass gastric surgery Bacterial overgrowth Interaction with food elements [53]: Tea, coffee, Calcium Flavonoids, Oxalates, Phytates Millet Wheat Pica syndrome [54] Pagophagia Drugs [55,56]: PPI H2 blockers | Congestive heart failure [49] Cancer [57] Chronic kidney disease [58] Rheumatoid arthritis [28] Obesity [59,60] IBD [61,62] | Iron-refractory iron deficiency anemia [58] Divalent metal transporter deficiency anemia [63] Fanconi anemia [64] Pyruvate kinase deficiency [65] Thalassemia [66] Sickle cell anemia [44,67] |
| Group | Age Range | Body Weight (kg) | Requirement (mg/kg/d) | Absorption (Assumed %) | RDA (mg/d) | |
|---|---|---|---|---|---|---|
| Man | 15-lifelong | 60 | 14 | 5 | 17 | |
| Woman | Overall | 15-lifelong | 55 | 30 | 8 | 21 |
| Pregnant | 15–49 y | 55 | 51 | 8 | 35 | |
| Lactating | 15–49 y | 55 | 23 | 8 | 25 | |
| Infants | 0–6 m | 5.4 | 46 | -- | -- | |
| 6–12 m | 8.4 | 87 | 15 | 15 | ||
| Children | 1–3 y | 12.9 | 35 | 5 | 9 | |
| 4–6 y | 18.0 | 35 | 5 | 13 | ||
| 7–9 y | 25.1 | 31 | 5 | 16 | ||
| Adolescents | ||||||
| Boy | 10–12 y | 34.3 | 31 | 5 | 27 | |
| 13–15 y | 47.6 | 34 | 5 | 32 | ||
| 16–17 y | 55.4 | 25 | 5 | 28 | ||
| Girl | 10–12 y | 35.0 | 38 | 5 | 27 | |
| 13–15 y | 46.6 | 29 | 5 | 27 | ||
| 16–17 y | 52.1 | 25 | 5 | 26 | ||
| Age Group | Intervention/Dose | Regime | Service Delivery |
|---|---|---|---|
| 6–60 m old | IFA syrup | IFA biweekly from 6 to 60 m old Deworming, children >12 m old | ASHA/ANM: inclusion in MCP card |
| 5–10 y old | Tablet (45 mg iron/400 mg folic acid) | Weekly from 5 to 10 y old Deworming, biannually | Teacher for aged-school children AWC: children out of the school |
| 10–19 y old | Tablet (100 mg iron/500 µg folic acid | Weekly from 10 to 19 y old Deworming, biannually | Teacher: school children AWC: children out of the school |
| Pregnant/lactating women | Tablet (100 mg iron/ 500 mg of folic acid) | Daily from 14 to 16 weeks of gestation. Repeated for 100 days during post-partum | ANC/ANM/ASHA: inclusion in MCP card |
| Women in reproductive age (WRA) | Tablet (100 mg iron/500 mg of folic acid) | Weekly throughout the reproductive period | FHW during a home visit for contraceptive distribution |
| Percentage (%) Prevalence of Anemia | ||
|---|---|---|
| Group | NFHS IV | Target * |
| Children (6–59 months-old) | 58 | 40 |
| Adolescent girls (15–19 years-old) | 54 | 36 |
| Adolescent boys (15–19 years) | 29 | 11 |
| WRA | 53 | 35 |
| Pregnant women | 50 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.B.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. https://doi.org/10.3390/nu14142976
Kumar SB, Arnipalli SR, Mehta P, Carrau S, Ziouzenkova O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients. 2022; 14(14):2976. https://doi.org/10.3390/nu14142976
Chicago/Turabian StyleKumar, Shashi Bhushan, Shanvanth R. Arnipalli, Priyanka Mehta, Silvia Carrau, and Ouliana Ziouzenkova. 2022. "Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies" Nutrients 14, no. 14: 2976. https://doi.org/10.3390/nu14142976
APA StyleKumar, S. B., Arnipalli, S. R., Mehta, P., Carrau, S., & Ziouzenkova, O. (2022). Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients, 14(14), 2976. https://doi.org/10.3390/nu14142976








