A New Potential Dietary Approach to Supply Micronutrients to Physically Active People through Consumption of Biofortified Vegetables
Abstract
:1. Introduction
2. Materials and Methods
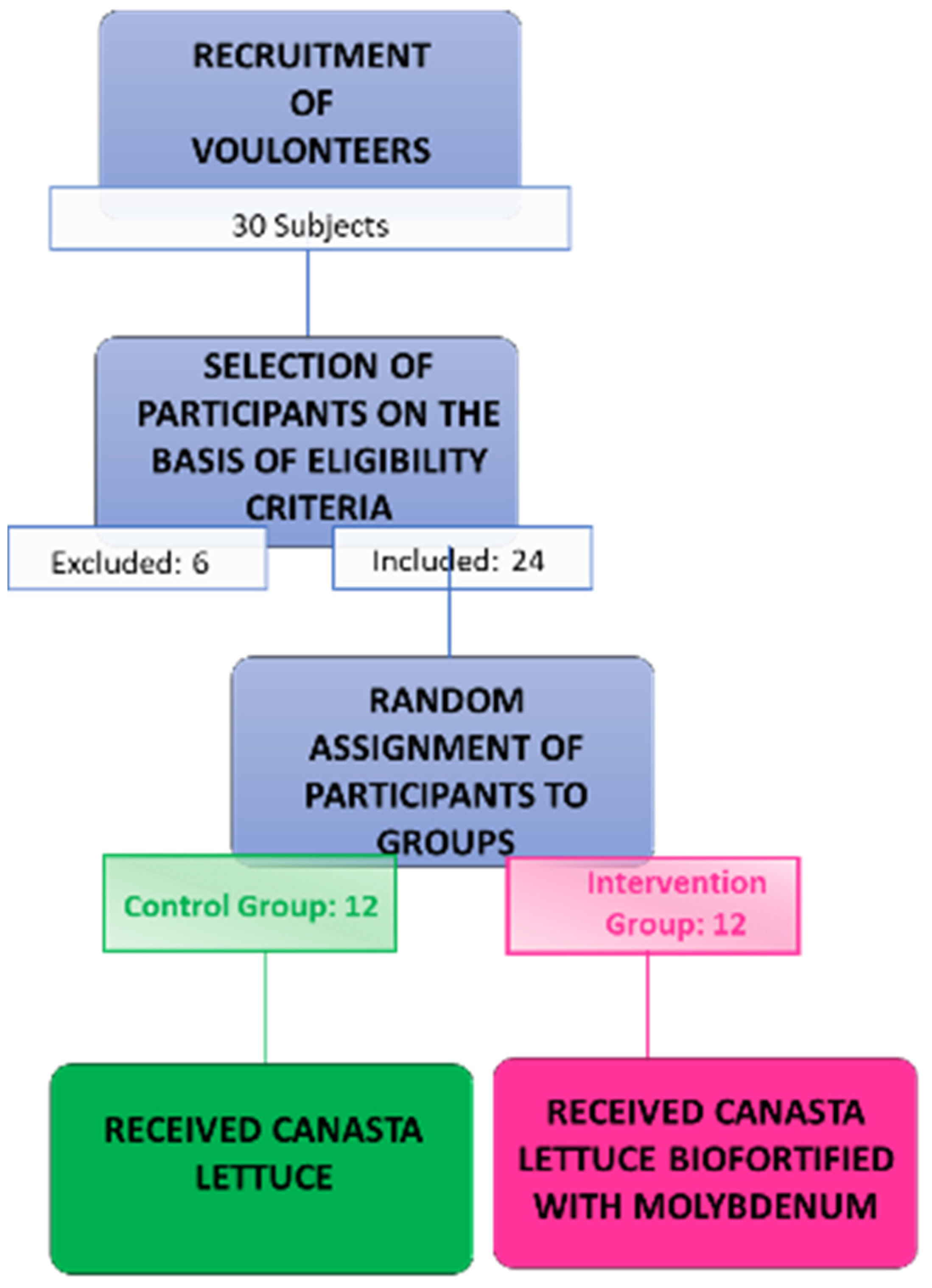
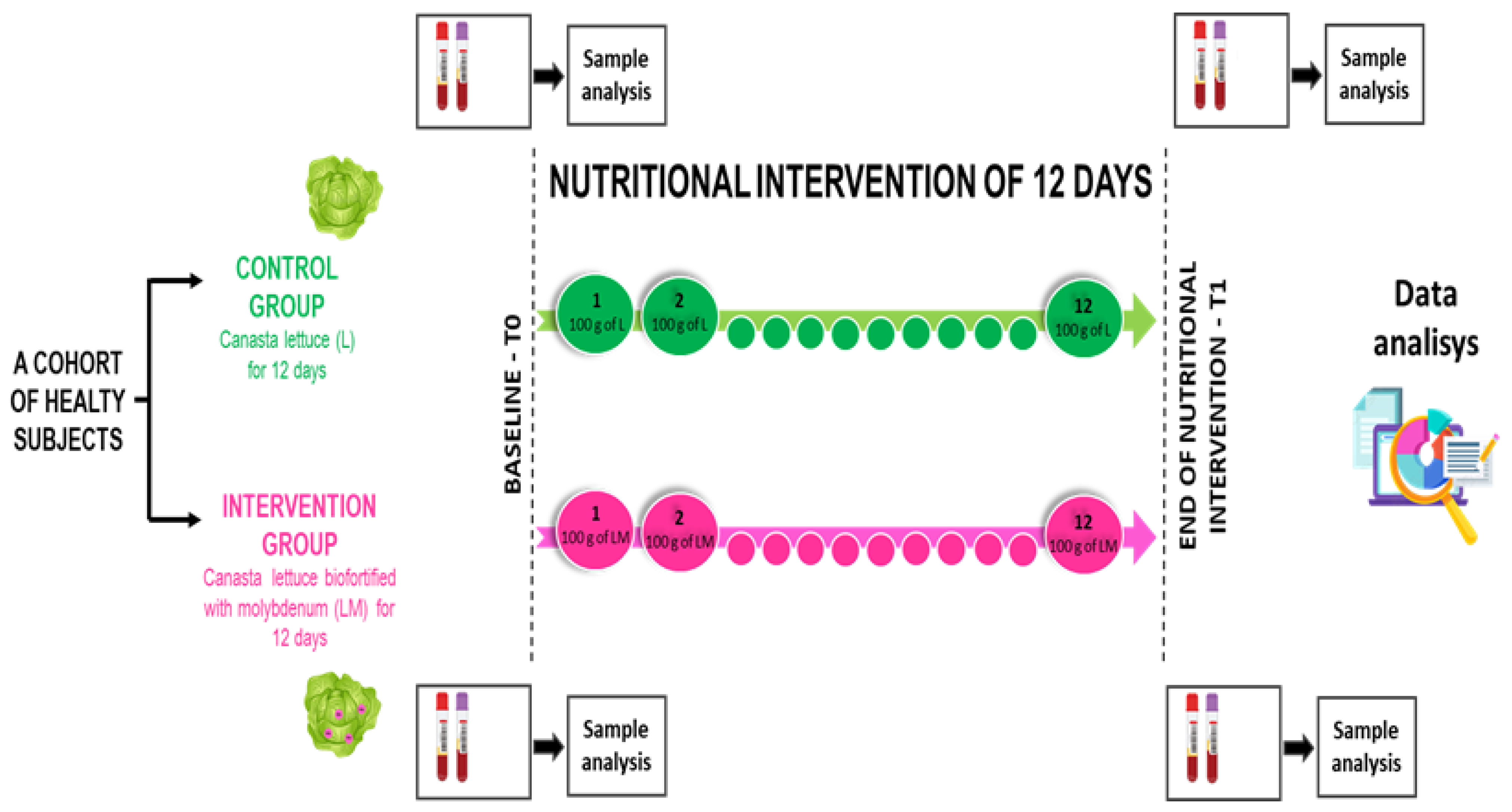
2.1. Experimental Design
2.2. Participants
2.3. Procedures of the Experimental Design
2.4. Analysis of Blood Samples
2.5. Analysis of Mo Concentration in Serum
2.6. Statistical Analyses
3. Results
3.1. Consumption of Mo-Biofortified Lettuce and Hematological Parameters
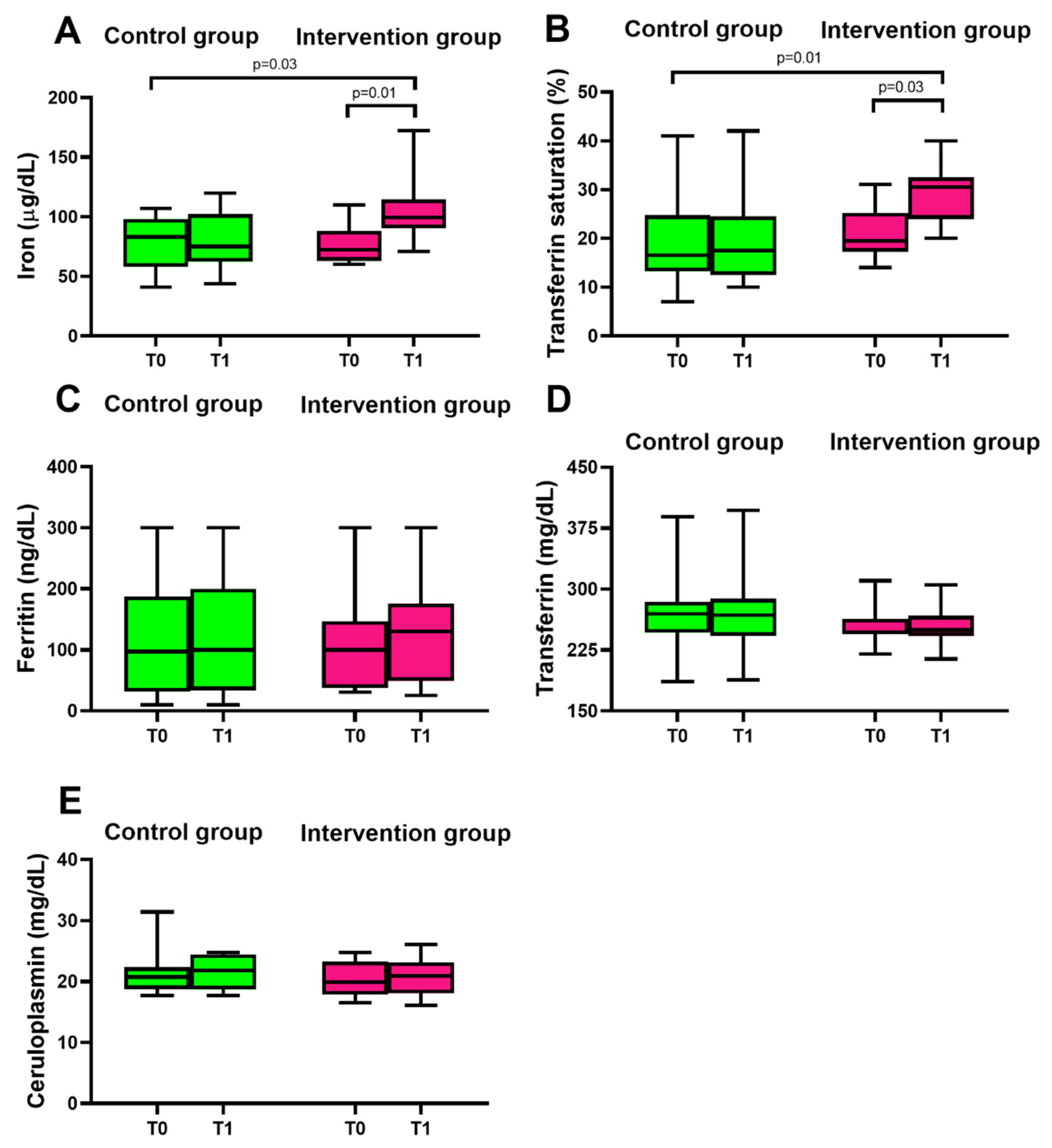
3.2. Consumption of Mo-Biofortified Lettuce and Iron Homeostasis
3.3. Consumption of Mo-Biofortified Lettuce: Liver Function and Lipid Homeostasis
3.4. Serum Mo Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maughan, R.J. Role of micronutrients in sport and physical activity. Br. Med. Bull. 1999, 55, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Amato, A.; D’Amico, G.; Baldassano, S.; Proia, P. Effects of protein supplementation in fitness world: A 12-week cross-over studio. J. Hum. Sport Exerc. 2019, 15, S308–S314. [Google Scholar] [CrossRef]
- Baldassano, S.; Accardi, G.; Vasto, S. Beta-glucans and cancer: The influence of inflammation and gut peptide. Eur. J. Med. Chem. 2017, 142, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Baldassano, S.; Cortis, C.; Cooper, J.; Proia, P. Physical activity, nutrition, and bone health. Hum. Mov. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Biesalski Hans, K.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Reiss, J.; Hahnewald, R. Molybdenum cofactor deficiency: Mutations in GPHN, MOCS1, and MOCS2. Hum. Mutat. 2011, 32, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A. Molybdenum Nutriture in Humans. J. Evid. Based Complementary Altern. Med. 2011, 16, 164–168. [Google Scholar] [CrossRef]
- Otten, J.; Hellwig, J.; Meyers, L. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2007; Volume 85, p. 924. [Google Scholar]
- Hays, S.M.; Macey, K.; Poddalgoda, D.; Lu, M.; Nong, A.; Aylward, L.L. Biomonitoring Equivalents for molybdenum. Regul. Toxicol. Pharm. 2016, 77, 223–229. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of Preharvest Gibberellic Acid Treatments on Postharvest Quality of Minimally Processed Leaf Lettuce and Rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphael, Y. Rootstock and Arbuscular Mycorrhiza Combinatorial Effects on Eggplant Crop Performance and Fruit Quality under Greenhouse Conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Mola, I.D.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; Placa, G.G.L.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; Bella, S.L.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Consentino, B.B.; Ntatsi, G.; La Bella, S.; Baldassano, S.; Rouphael, Y. Stand-Alone or Combinatorial Effects of Grafting and Microbial and Non-Microbial Derived Compounds on Vigour, Yield and Nutritive and Functional Quality of Greenhouse Eggplant. Plants 2022, 11, 1175. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive Effects of Genotype and Molybdenum Supply on Yield and Overall Fruit Quality of Tomato. Front. Plant Sci. 2018, 9, 1922. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; Di Gaudio, F.; Consentino, B.B.; Rouphael, Y.; El-Nakhel, C.; La Bella, S.; Vasto, S.; Mauro, R.P.; D’Anna, F.; Iapichino, G.; et al. Iodine Biofortification Counters Micronutrient Deficiency and Improve Functional Quality of Open Field Grown Curly Endive. Horticulturae 2021, 7, 58. [Google Scholar] [CrossRef]
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima Seaweed Extract and Mo Foliar Treatments on Biofortification, Spinach Yield, Quality and NUE. Plants 2021, 10, 1139. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.; Rouphael, Y.; Pasquale, C.D.; Iapichino, G.; D’Anna, F.; Bella, S.L. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- Sabatino, L.; Bella, S.L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; Pasquale, C.D.; Consentino, B.B.; Rouphael, Y. Selenium biofortification and grafting modulate plant performance and functional features of cherry tomato grown in a soilless system. Sci. Hortic. 2021, 285, 110095. [Google Scholar] [CrossRef]
- Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; Pasquale, C.D.; Iapichino, G.; D’Anna, F.; Bella, S.L.; Sabatino, L. Agronomic performance and fruit quality in greenhouse grown eggplant are interactively modulated by iodine dosage and grafting. Sci. Hortic. 2022, 295, 110891. [Google Scholar] [CrossRef]
- Malik, K.A.; Maqbool, A. Transgenic Crops for Biofortification. Front. Sustain. Food Syst. 2020, 4, 571402. [Google Scholar] [CrossRef]
- Vasto, S.; Di Gaudio, F.; Raso, M.; Sabatino, L.; Caldarella, R.; De Pasquale, C.; Di Rosa, L.; Baldassano, S. Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study. Nutrients 2022, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, S.; Di Gaudio, F.; Sabatino, L.; Caldarella, R.; De Pasquale, C.; Di Rosa, L.; Nuzzo, D.; Picone, P.; Vasto, S. Biofortification: Effect of Iodine Fortified Food in the Healthy Population, Double-Arm Nutritional Study. Front. Nutr. 2022, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, K.; Kulnigg-Dabsch, S.; Gasche, C. Management of Iron Deficiency Anemia. Gastroenterol. Hepatol. 2015, 11, 241–250. [Google Scholar]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Dieckmann, W.J.; Priddle, H.D.; King, J.A.; Forman, J.B. The Prophylactic Use of a Molybdenum-Iron Complex in Pregnant Patients. Am. J. Obstet. Gynecol. 1950, 59, 442–444. [Google Scholar] [CrossRef]
- POSNER, L.B.; WILSON, F. An Evaluation of Sustained-Release Molybdenized Ferrous Sulfate in Iron-Deficiency Anemia of Pregnancy. J. New Drugs 1963, 3, 155–160. [Google Scholar] [CrossRef]
- Dieckmann, W.J.; Priddle, H.D. Anemia of pregnancy treated with molybdenum-iron complex. J. Lab. Clin. Med. 1948, 33, 1641. [Google Scholar] [CrossRef]
- Vasto, S.; Amato, A.; Proia, P.; Baldassano, S. Is the Secret in the Gut? SuperJump Activity Improves Bone Remodeling and Glucose Homeostasis by GLP-1 and GIP Peptides in Eumenorrheic Women. Biology 2022, 11, 296. [Google Scholar] [CrossRef]
- Amato, A.; Proia, P.; Caldara, G.; Alongi, A.; Ferrantelli, V.; Baldassano, S. Analysis of Body Perception, Preworkout Meal Habits and Bone Resorption in Child Gymnasts. Int. J. Environ. Res. Public Health 2021, 18, 2184. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Amato, A.; Proia, P.; Caldarella, R.; Cortis, C.; Baldassano, S. Dare to Jump: The Effect of New High Impact Activity SuperJump on Bone Remodeling. A New Tool to Be Fit During COVID-19 Home Confinement. Biol. Sport 2022, 39, 1011–1019. [Google Scholar] [CrossRef]
- Baldassano, S.; Gasbjerg, L.S.; Kizilkaya, H.S.; Rosenkilde, M.M.; Holst, J.J.; Hartmann, B. Increased Body Weight and Fat Mass After Subchronic GIP Receptor Antagonist, but Not GLP-2 Receptor Antagonist, Administration in Rats. Front. Endocrinol. 2019, 10, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldassano, S.; Accardi, G.; Aiello, A.; Buscemi, S.; Di Miceli, G.; Galimberti, D.; Candore, G.; Ruisi, P.; Caruso, C.; Vasto, S. Fibres as functional foods and the effects on gut hormones: The example of β-glucans in a single arm pilot study. J. Funct. Foods 2018, 47, 264–269. [Google Scholar] [CrossRef]
- Cammilleri, G.; Calabrese, V.; Accordino, L.; Pantano, L.; Migliazzo, A.; Galluzzo, F.G.; Parrino, V.; Brunone, A.; Lo Dico, G.M.; Bacchi, E.; et al. Toxic metals and total lipids comparison between wild and farmed fish of South mediterranean. Nat. Prod. Res. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Lam, N.H.; Chow, S.; Botta, A.; Sweeney, G.; Pantopoulos, K. Regulatory Connections between Iron and Glucose Metabolism. Int. J. Mol. Sci. 2020, 21, 7773. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Green, S.; Saha, A.; Carleton, A. Mechanism of release of ferritin iron in vivo by xanthine oxidase. J. Clin. Investig. 1958, 37, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Frazer, D.M.; Anderson, G.J. Iron homeostasis: Transport, metabolism, and regulation. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 276–281. [Google Scholar] [CrossRef]
- Grech, B.J. Mechanistic insights into the treatment of iron-deficiency anemia and arthritis in humans with dietary molybdenum. Eur. J. Clin. Nutr. 2021, 75, 1170–1175. [Google Scholar] [CrossRef]
- Liu, N.; Guan, Y.; Li, B.; Yao, S. Biomonitorization of concentrations of 28 elements in serum and urine among workers exposed to indium compounds. PLoS ONE 2021, 16, e0246943. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Luo, C.; Peng, X.; Jiao, Y.; Zhou, L.; Yin, J.; Liu, L. Inverse Association of Plasma Molybdenum with Metabolic Syndrome in a Chinese Adult Population: A Case-Control Study. Nutrients 2021, 13, 4544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Liang, X.; Roberts, M.S. Chapter 30—Hepatic Metabolism in Liver Health and Disease. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 391–400. [Google Scholar]
| Parameters | Control Group Age Range: 23–57 (n = 12), Mean ± SD | Intervention Group Age Range: 27–53 (n = 12), Mean ± SD | p-Value |
|---|---|---|---|
| Age | 37.5 ± 13.5 | 40 ± 10 | >0.05 |
| Height(m) | 1.68 ± 10 | 1.72 ± 9 | >0.05 |
| Weight (Kg) | 69 ± 9.8 | 72 ± 13 | >0.05 |
| BMI (kg/m2) | 24.3 ± 2.5 | 24.2 ± 2.8 | >0.05 |
| Visceral fat (%) | 6.5 ± 3.3 | 7.2 ± 3.2 | >0.05 |
| Fat mass (%) | 28.6 ± 7.3 | 26.5 ± 7.1 | >0.05 |
| Lean mass (%) | 32.8 ± 5.8 | 33.2 ± 5.4 | >0.05 |
| Median age | 38.5 | 39 | >0.05 |
| Selection Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Absence of blood-related dysfunction; cardiac, gastrointestinal, and metabolic disorders; recent viral infection; and food allergies | Volunteers of Italian ethnicity | Presence of chronic disease |
| Not taking medications | Age range: 18–60 years | Use of medication |
| Absence of obesity | Clinically healthy | Use of dietary supplements |
| Not taking supplements | Body mass index: 18.5–28 kg/m2 | Breastfeeding, Pregnancy |
| Parameters | Control Group | Intervention Group | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | |||
| T0 | T1 | T0 | T1 | |||
| WBC (103/µL) | 7.3 ± 2.0 | 7.0 ± 2.2 | >0.05 | 7.1 ± 1.3 | 7.5 ± 1.8 | >0.05 |
| NEUT. (%) | 57 ± 6.1 | 54.3 ± 7.2 | >0.05 | 56.7 ± 6.5 | 58.7 ± 9.7 | >0.05 |
| LYMP. (%) | 30.7 ± 3.7 | 34.1 ± 5.1 | >0.05 | 31.7 ± 7.1 | 30.3 ± 9.6 | >0.05 |
| MON. (%) | 8.3 ± 1.8 | 7.9 ± 1.5 | >0.05 | 7.9 ± 0.9 | 7.7 ± 1.1 | >0.05 |
| EOS. (%) | 2.2 ± 1.4 | 2.0 ± 1.0 | >0.05 | 2.8 ± 1.9 | 2.3 ± 1.5 | >0.05 |
| BAS. (%) | 0.7 ± 0.3 | 0.7 ± 0.4 | >0.05 | 0.6 ± 0.3 | 0.7 ± 0.5 | >0.05 |
| NEUT. (103/µL) | 3.5 ± 1.3 | 3.6 ± 1.4 | >0.05 | 3.2 ± 1.0 | 3.1 ± 1.0 | >0.05 |
| LYM. (103/µL) | 2.3 ± 0.8 | 2.4 ± 1.0 | >0.05 | 2.2 ± 0.8 | 2.2 ± 0.8 | >0.05 |
| MON. (103/µL) | 0.6 ± 0.2 | 0.5 ± 0.1 | >0.05 | 0.5 ± 0.2 | 0.6 ± 0.1 | >0.05 |
| EOS. (103/µL) | 0.2 ± 0.2 | 0.2 ± 0.1 | >0.05 | 0.2 ± 0.1 | 0.2 ± 0.1 | >0.05 |
| BAS. (103/µL) | 0.0 ± 0.0 | 0.0 ± 0.0 | >0.05 | 0.0 ± 0.0 | 0.0 ± 0.0 | >0.05 |
| RBC (106/µL) | 4.8 ± 0.6 | 4.8 ± 0.6 | >0.05 | 4.8 ± 1.0 | 4.9 ± 0.6 | >0.05 |
| HGB (g/dL) | 13.9 ± 1.1 | 13.2 ± 1.4 | >0.05 | 14.4 ± 1.8 | 14.0 ± 1.6 | >0.05 |
| HCT % | 39.1 ± 5.3 | 38.7 ± 4.6 | >0.05 | 41.4 ± 6.4 | 40.5 ± 4.5 | >0.05 |
| MCV (fL) | 83.2 ± 6.4 | 81.7 ± 6.7 | >0.05 | 84.5 ± 7.3 | 82.9 ± 7.0 | >0.05 |
| MCH (pg) | 27.6 ± 2.7 | 27.8 ± 2.7 | >0.05 | 28.6 ± 3.2 | 28.7± 2.9 | >0.05 |
| MCHC (g/dL) | 33.1 ± 1.4 | 33.6 ± 1.9 | >0.05 | 33.2 ± 1.8 | 34.1 ± 1.7 | >0.05 |
| RDW (%) | 13.5 ± 1.3 | 13.6 ± 1.3 | >0.05 | 13.5 ± 1.3 | 13.5 ± 1.3 | >0.05 |
| RDW (fL) | 40.0 ± 2.4 | 39.8 ± 2.8 | >0.05 | 40.6 ± 2.9 | 40.2 ± 2.8 | >0.05 |
| PLT (103/µL) | 245.2 ± 42.8 | 232.7 ± 43.3 | >0.05 | 262.0 ± 72.0 | 273.2 ± 47.4 | >0.05 |
| Parameters | Control Group | Intervention Group | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | |||
| T0 | T1 | T0 | T1 | |||
| AST (U/L) | 22.0 ± 4.5 | 19.0 ± 5.0 | >0.05 | 20.7 ± 4.4 | 18.6 ± 6.9 | >0.05 |
| ALT (U/L) | 19.9 ± 11.3 | 20.6 ± 9.1 | >0.05 | 19.3 ± 8.5 | 12.7 ± 4.9 | >0.05 |
| ALP (U/L) | 63.0 ± 10.0 | 58.5 ± 10.9 | >0.05 | 65.5 ± 11.6 | 59.4 ± 8.8 | >0.05 |
| GGT (U/L) | 15.2 ± 6.2 | 14.7 ± 5.1 | >0.05 | 15.7 ± 6.4 | 15.7 ± 6.4 | >0.05 |
| TP (g/L) | 71.1 ± 3.0 | 70.8 ± 3.9 | >0.05 | 71.4 ± 2.9 | 70.7 ± 3.7 | >0.05 |
| ALB (g/L) | 45.5 ± 3.5 | 46.5 ± 2.7 | >0.05 | 44.1 ± 2.3 | 46.7 ± 2.7 | >0.05 |
| Parameters | Control Group | Intervention Group | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | |||
| T0 | T1 | T0 | T1 | |||
| TG (mg/dL) | 84.7± 45.6 | 81.5 ± 28.6 | >0.05 | 89.0 ± 36.8 | 87.0 ± 40.9 | >0.05 |
| CHOL TOT (mg/dL) | 167.9 ± 23.6 | 166.9 ± 27.8 | >0.05 | 174.2 ± 23.0 | 173.7 ± 23.0 | >0.05 |
| CHOL LDL (mg/dL) | 96.9 ± 22.4 | 97.2 ± 28.0 | >0.05 | 102.1 ± 17.7 | 104.2 ± 28.8 | >0.05 |
| CHOL HDL (mg/dL) | 49.1 ± 7.00 | 47.2 ± 7.5 | >0.05 | 55.3 ± 15.6 | 56.1 ± 16 | >0.05 |
| Control Group | Intervention Group | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| T0 | T1 | T0 | T1 |
| 4.9 ± 1.6 μg/L | 5.1 ± 1.7 μg/L | 5.0 ± 1.7 μg/L | 7.1* ± 1.5 μg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassano, S.; Polizzi, M.R.; Sabatino, L.; Caldarella, R.; Macaluso, A.; Alongi, A.; Caldara, G.F.; Ferrantelli, V.; Vasto, S. A New Potential Dietary Approach to Supply Micronutrients to Physically Active People through Consumption of Biofortified Vegetables. Nutrients 2022, 14, 2971. https://doi.org/10.3390/nu14142971
Baldassano S, Polizzi MR, Sabatino L, Caldarella R, Macaluso A, Alongi A, Caldara GF, Ferrantelli V, Vasto S. A New Potential Dietary Approach to Supply Micronutrients to Physically Active People through Consumption of Biofortified Vegetables. Nutrients. 2022; 14(14):2971. https://doi.org/10.3390/nu14142971
Chicago/Turabian StyleBaldassano, Sara, Maria Rita Polizzi, Leo Sabatino, Rosalia Caldarella, Andrea Macaluso, Angelina Alongi, Gaetano Felice Caldara, Vincenzo Ferrantelli, and Sonya Vasto. 2022. "A New Potential Dietary Approach to Supply Micronutrients to Physically Active People through Consumption of Biofortified Vegetables" Nutrients 14, no. 14: 2971. https://doi.org/10.3390/nu14142971
APA StyleBaldassano, S., Polizzi, M. R., Sabatino, L., Caldarella, R., Macaluso, A., Alongi, A., Caldara, G. F., Ferrantelli, V., & Vasto, S. (2022). A New Potential Dietary Approach to Supply Micronutrients to Physically Active People through Consumption of Biofortified Vegetables. Nutrients, 14(14), 2971. https://doi.org/10.3390/nu14142971









