Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of L. tetragonum in a Smart-Farming System
2.2. Preparation of LTE
2.3. Analysis of LTE Using Liquid Chromatography–Mass Spectrometry (LC–MS)
2.4. Animals and LTE Treatment
2.5. Moderate Intensity Treadmill Running Capacity
2.6. Histology
2.7. Indirect Calorimetry
2.8. Biochemical Analysis
2.9. Cell Culture
2.10. Western Blotting
2.11. RNA Isolation and Real-Time Quantitative RT-PCR (qPCR)
2.12. Statistical Analysis
3. Results
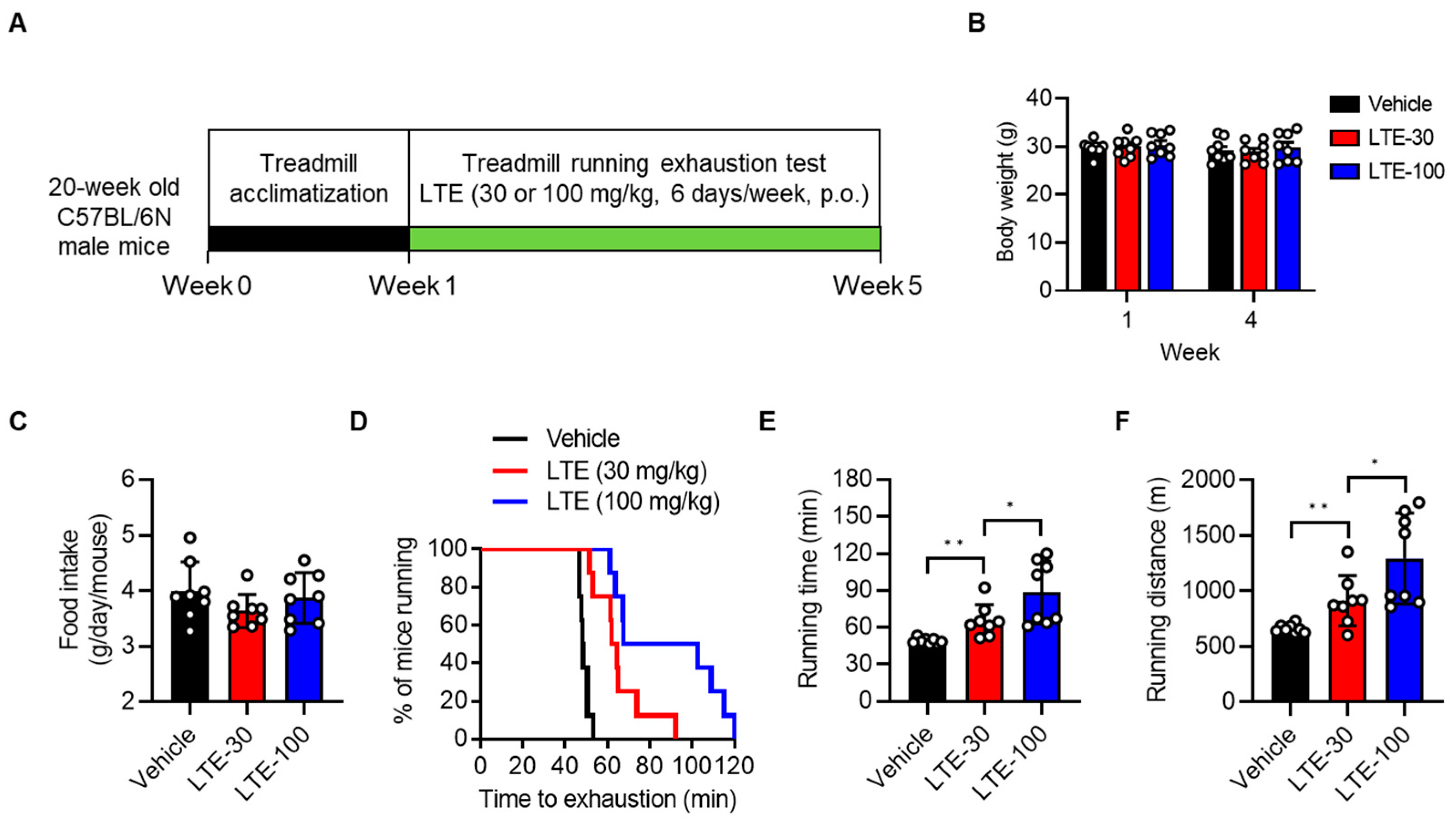
3.1. LTE Supplementation Enhances Endurance Exercise Performance
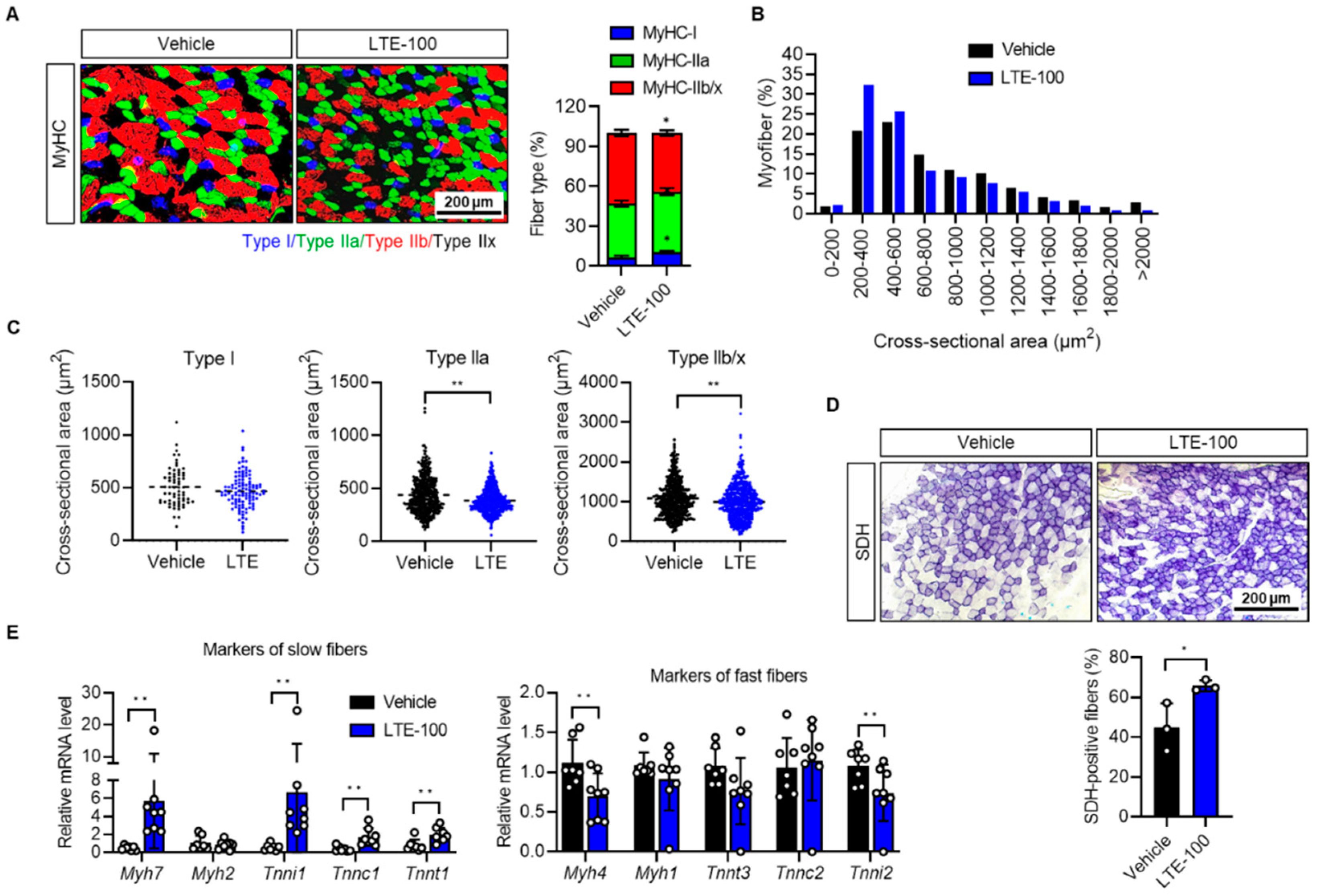
3.2. LTE Supplementation Increases the Proportion of Oxidative Fibers
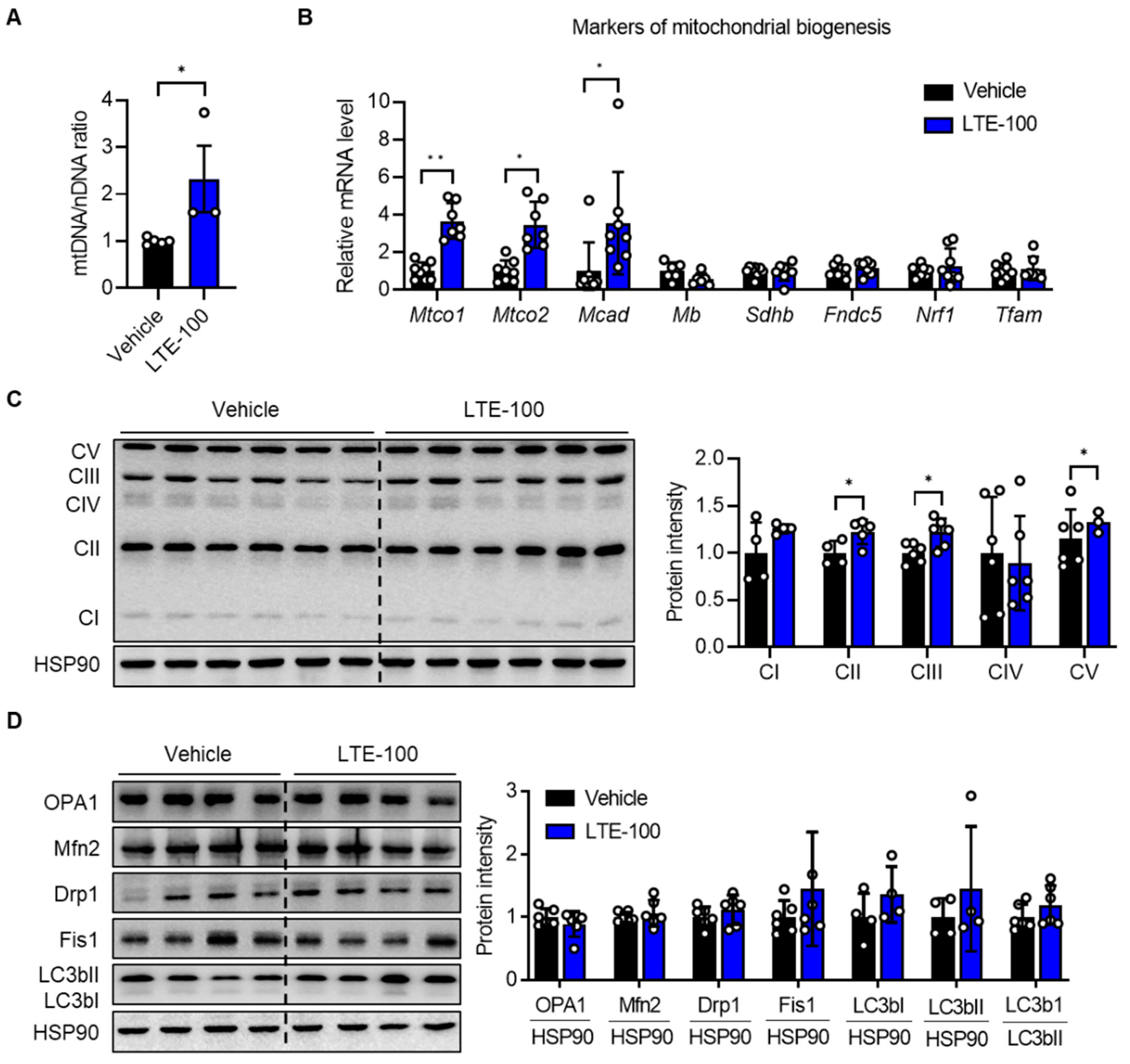
3.3. LTE Supplementation Increases Mitochondrial Content and Oxidative Capacity
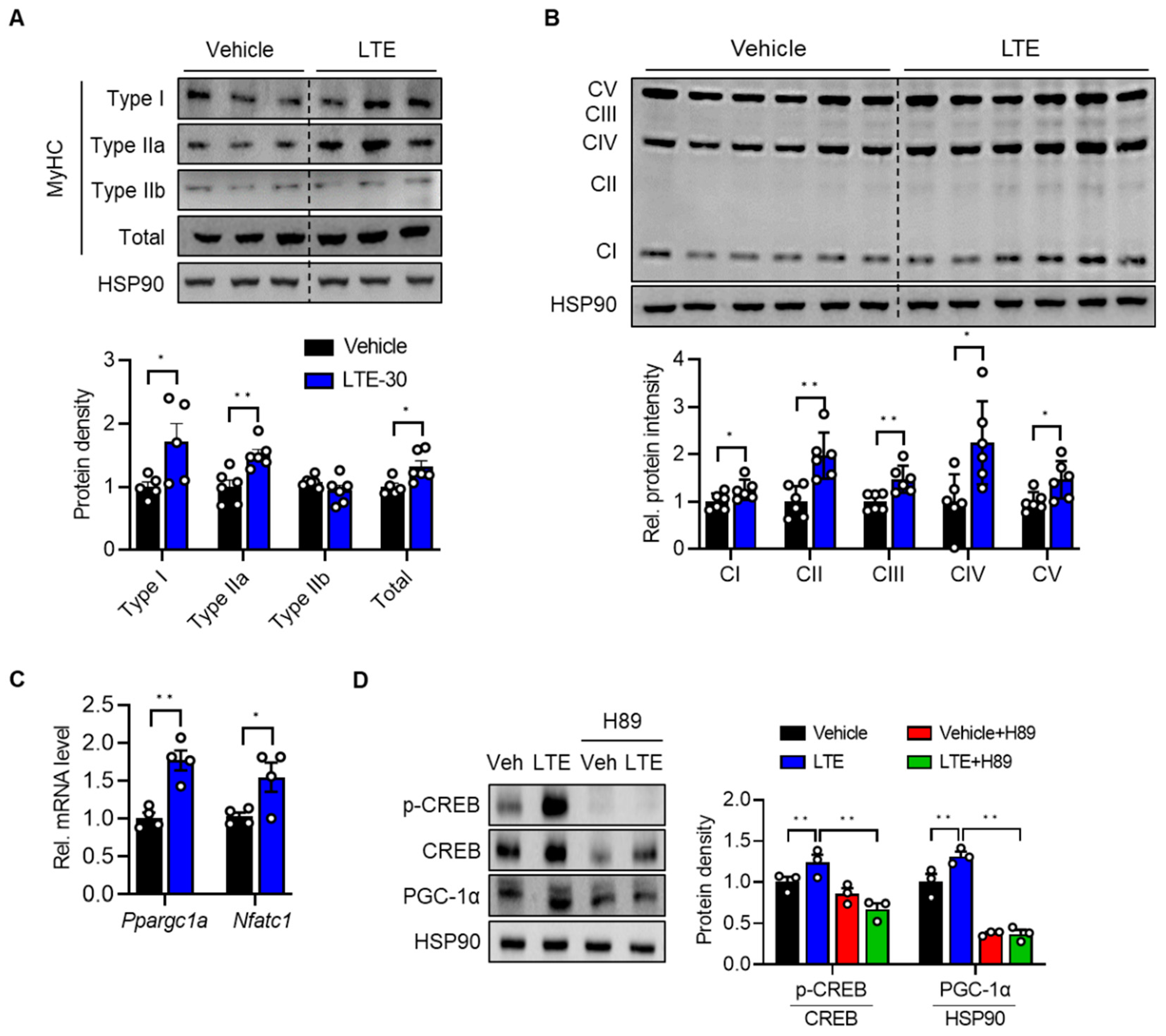
3.4. LTE Supplementation Activates PKA–CREB–PGC1α Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Dogra, S.; Carter, S.E.; Owen, N. Sit less and move more for cardiovascular health: Emerging insights and opportunities. Nat. Rev. Cardiol. 2021, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.E.; Mistry, D.; Alleyne, S.; Atherton, N.; Brown, D.; Copsey, B.; Dosanjh, S.; Finnegan, S.; Fordham, B.; Griffiths, F.; et al. Aerobic and strength training exercise programme for cognitive impairment in people with mild to moderate dementia: The DAPA RCT. Health Technol. Assess. 2018, 22, 1–202. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.R.; Sandler, C.X.; Newton, R.U.; Galvao, D.A.; Hayes, S.C. Physical activity and exercise guidelines for people with cancer: Why are they needed, who should use them, and when? Semin. Oncol. Nurs. 2020, 36, 151075. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.R.; Staines, K.A.; Kelley, G.A.; Kelley, K.S.; Kohrt, W.M.; Pitsiladis, Y.; Guppy, F.M. The effects of exercise on bone mineral density in men: A systematic review and meta-analysis of randomised controlled trials. Calcif. Tissue Int. 2022, 110, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Simpson, R.J.; Ramirez, M.; Lucia, A.; Berger, N.A. Physical function and quality of life in patients with chronic GvHD: A summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transplant. 2016, 51, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Chahla, E.; Alkaade, S. Antiaging, longevity and calorie restriction. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 40–45. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Agbulut, O.; Noirez, P.; Beaumont, F.; Butler-Browne, G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell 2003, 95, 399–406. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Fitzsimons, D.P.; Diffee, G.M.; Herrick, R.E.; Baldwin, K.M. Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J. Appl. Physiol. 1990, 68, 1950–1955. [Google Scholar] [CrossRef]
- Mogensen, M.; Sahlin, K.; Fernstrom, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Hojlund, K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef]
- Yao, L.; Cui, X.; Chen, Q.; Yang, X.; Fang, F.; Zhang, J.; Liu, G.; Jin, W.; Chang, Y. Cold-inducible SIRT6 regulates thermogenesis of brown and beige fat. Cell Rep. 2017, 20, 641–654. [Google Scholar] [CrossRef]
- Nakae, J.; Cao, Y.; Oki, M.; Orba, Y.; Sawa, H.; Kiyonari, H.; Iskandar, K.; Suga, K.; Lombes, M.; Hayashi, Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes 2008, 57, 563–576. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Czubryt, M.P.; McAnally, J.; Fishman, G.I.; Olson, E.N. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc. Natl. Acad. Sci. USA 2003, 100, 1711–1716. [Google Scholar] [CrossRef]
- Vega, R.B.; Huss, J.M.; Kelly, D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000, 20, 1868–1876. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Huss, J.M.; Kopp, R.P.; Kelly, D.P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J. Biol. Chem. 2002, 277, 40265–40274. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Jager, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Kim, N.H.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Anti-obesity effect of halophyte crop, Limonium tetragonum in high-fat diet-induced obese mice and 3T3-L1 adipocytes. Biol. Pharm. Bull. 2017, 40, 1856–1865. [Google Scholar] [CrossRef]
- Kim, N.H.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. The standardized extract of Limonium tetragonum alleviates chronic alcoholic liver injury in C57Bl/6J mice. Pharmacogn. Mag. 2018, 14, 58–63. [Google Scholar] [CrossRef]
- Kim, N.H.; Heo, J.D.; Kim, T.B.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Protective effects of ethyl acetate soluble fraction of Limonium tetragonum on diethylnitrosamine-induced liver fibrosis in rats. Biol. Pharm. Bull. 2016, 39, 1022–1028. [Google Scholar] [CrossRef][Green Version]
- Lee, S.G.; Karadeniz, F.; Seo, Y.; Kong, C.S. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (Thunb.) bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Jang, M.S.; Nam, K.H.; Seo, Y.; Kong, C.S. MMP-inhibitory effects of flavonoid glycosides from edible medicinal halophyte Limonium tetragonum. Evid. Based Complementary Altern. Med. 2017, 2017, 6750274. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.N.; Kim, N.H.; Heo, J.D.; Yang, M.H.; Rho, J.R.; Jeong, E.J. Identification of hepatoprotective constituents in Limonium tetragonum and development of simultaneous analysis method using high-performance liquid chromatography. Pharmacogn. Mag. 2017, 13, 535–541. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Amaral, I.M.; Canas, P.M.; Cunha, R.A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci. Rep. 2018, 8, 10742. [Google Scholar] [CrossRef]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial dynamics and tts involvement in disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Henique, C.; Mansouri, A.; Vavrova, E.; Lenoir, V.; Ferry, A.; Esnous, C.; Ramond, E.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; et al. Increasing mitochondrial muscle fatty acid oxidation induces skeletal muscle remodeling toward an oxidative phenotype. FASEB J. 2015, 29, 2473–2483. [Google Scholar] [CrossRef]
- Gehlert, S.; Weber, S.; Weidmann, B.; Gutsche, K.; Platen, P.; Graf, C.; Kappes-Horn, K.; Bloch, W. Cycling exercise-induced myofiber transitions in skeletal muscle depend on basal fiber type distribution. Eur. J. Appl. Physiol. 2012, 112, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle. In press. [CrossRef] [PubMed]
- Huttemann, M.; Lee, I.; Malek, M.H. (-)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J. 2012, 26, 1413–1422. [Google Scholar] [CrossRef]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.; et al. (-)-Epicatechin induces mitochondrial biogenesis and markers of muscle regeneration in adults with Becker muscular dystrophy. Muscle Nerve 2021, 63, 239–249. [Google Scholar] [CrossRef]
- Dean, S.; Braakhuis, A.; Paton, C. The effects of EGCG on fat oxidation and endurance performance in male cyclists. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 624–644. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Nagasawa, A.; Tokimitsu, I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R708–R715. [Google Scholar] [CrossRef]
- Teng, Y.S.; Wu, D. Anti-Fatigue Effect of Green Tea Polyphenols (-)-epigallocatechin-3-gallate (EGCG). Pharmacogn. Mag. 2017, 13, 326–331. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Xi, Q.; Zhang, Y.; Jiang, Q. Epigallocatechin gallate reduces slow-twitch muscle fiber formation and mitochondrial biosynthesis in C2C12 cells by repressing AMPK activity and PGC-1α expression. J. Agric. Food Chem. 2016, 64, 6517–6523. [Google Scholar] [CrossRef]
- Wu, L.; Ran, L.; Lang, H.; Zhou, M.; Yu, L.; Yi, L.; Zhu, J.; Liu, L.; Mi, M. Myricetin improves endurance capacity by inducing muscle fiber type conversion via miR-499. Nutr. Metab. 2019, 16, 27. [Google Scholar] [CrossRef]
- Zou, D.; Liu, P.; Chen, K.; Xie, Q.; Liang, X.; Bai, Q.; Zhou, Q.; Liu, K.; Zhang, T.; Zhu, J.; et al. Protective effects of myricetin on acute hypoxia-induced exercise intolerance and mitochondrial impairments in rats. PLoS ONE 2015, 10, e0124727. [Google Scholar] [CrossRef]
- Quiat, D.; Voelker, K.A.; Pei, J.; Grishin, N.V.; Grange, R.W.; Bassel-Duby, R.; Olson, E.N. Concerted regulation of myofiber-specific gene expression and muscle performance by the transcriptional repressor Sox6. Proc. Natl. Acad. Sci. USA 2011, 108, 10196–10201. [Google Scholar] [CrossRef]
- Pang, B.P.S.; Chan, W.S.; Chan, C.B. Mitochondria homeostasis and oxidant/antioxidant balance in skeletal muscle-do myokines play a role? Antioxidants 2021, 10, 179. [Google Scholar] [CrossRef]
- Russell, A.P.; Feilchenfeldt, J.; Schreiber, S.; Praz, M.; Crettenand, A.; Gobelet, C.; Meier, C.A.; Bell, D.R.; Kralli, A.; Giacobino, J.P.; et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes 2003, 52, 2874–2881. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef]
- Berdeaux, R.; Hutchins, C. Anabolic and pro-metabolic functions of CREB-CRTC in skeletal muscle: Advantages and obstacles for type 2 diabetes and cancer cachexia. Front. Endocrinol. 2019, 10, 535. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Li, X.; Monks, B.; Ge, Q.; Birnbaum, M.J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 2007, 447, 1012–1016. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.G.; Song, M.-Y.; Cho, H.; Jin, J.S.; Park, B.-H.; Bae, E.J. Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation. Nutrients 2022, 14, 3904. https://doi.org/10.3390/nu14193904
Lee YG, Song M-Y, Cho H, Jin JS, Park B-H, Bae EJ. Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation. Nutrients. 2022; 14(19):3904. https://doi.org/10.3390/nu14193904
Chicago/Turabian StyleLee, Yong Gyun, Mi-Young Song, Hwangeui Cho, Jong Sik Jin, Byung-Hyun Park, and Eun Ju Bae. 2022. "Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation" Nutrients 14, no. 19: 3904. https://doi.org/10.3390/nu14193904
APA StyleLee, Y. G., Song, M.-Y., Cho, H., Jin, J. S., Park, B.-H., & Bae, E. J. (2022). Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation. Nutrients, 14(19), 3904. https://doi.org/10.3390/nu14193904






