Gut Microbiota in Psoriasis
Abstract
:1. Introduction
2. Gut Microbiota—An Overview
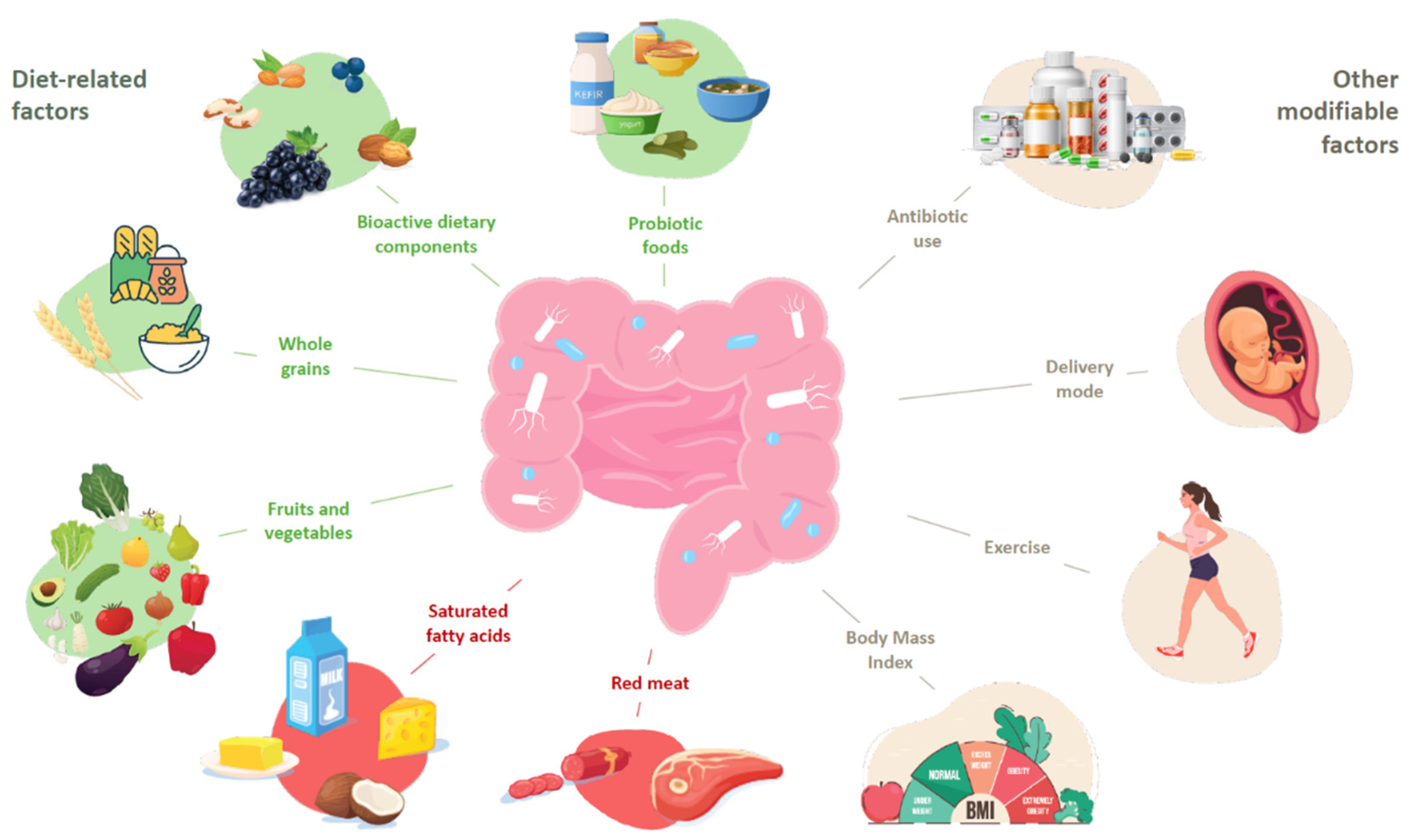
The Implications of Diet and External Factors on the Composition of the Gut Microbiota
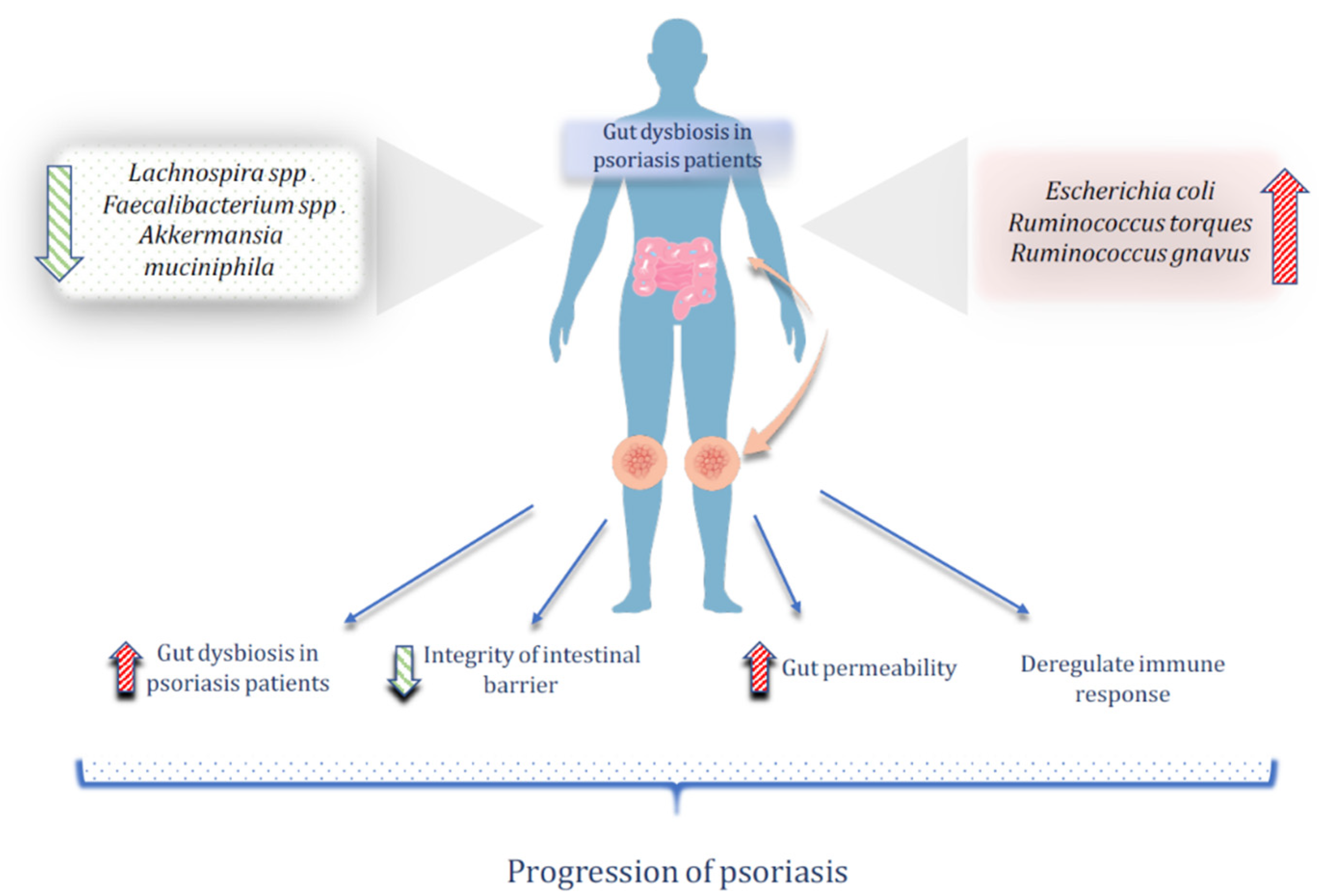
3. Gut microbiome Alterations in Psoriasis
3.1. The Role of the Gut Microbiota in the Pathogenesis of Psoriasis
3.2. Changes in Gut Microbiota after Antipsoriatic Treatment
3.3. TNF-α Inhibitor
3.4. IL-17 and IL-12/23 Blockers
4. Gut Microbiome-Targeted Therapies for Psoriasis
4.1. Dietary Approaches
4.1.1. Mediterranean Diet
4.1.2. Gluten-Free and Low-FODMAP Diet
4.2. Probiotics/Prebiotics/Synbiotics
4.3. Bioactive Dietary Components
4.3.1. Curcumin
4.3.2. Omega-3 Fatty Acids
4.3.3. Resveratrol
4.3.4. Quercetin
4.4. Fecal Microbiota Transplantation
5. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis Pathogenesis and the Development of Novel Targeted Immune Therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, W.; Wang, C.; Wang, L.; He, T.; Hu, H.; Song, J.; Cui, C.; Qiao, J.; Qing, L.; et al. Enterotype Bacteroides Is Associated with a High Risk in Patients with Diabetes: A Pilot Study. J. Diabetes Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, A.; Fernandes, G.D.R.; da Silva, I.T.; Pititto, B.D.A.; Gomes, E.P.; Pereira, A.D.C.; Ferreira, S.R.G. Enterotype May Drive the Dietary-Associated Cardiometabolic Risk Factors. Front. Cell. Infect. Microbiol. 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Mobeen, F.; Sharma, V.; Prakash, T. Enterotype Variations of the Healthy Human Gut Microbiome in Different Geographical Regions. Bioinformation 2018, 14, 560–573. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Tonon, K.M.; Morais, T.B.; Taddei, C.R.; Araújo-Filho, H.B.; Abrão, A.C.F.V.; Miranda, A.; de Morais, M.B. Gut Microbiota Comparison of Vaginally and Cesarean Born Infants Exclusively Breastfed by Mothers Secreting α1–2 Fucosylated Oligosaccharides in Breast Milk. PLoS ONE 2021, 16, e0246839. [Google Scholar] [CrossRef]
- Liou, J.-M.; Chen, C.-C.; Chang, C.-M.; Fang, Y.-J.; Bair, M.-J.; Chen, P.-Y.; Chang, C.-Y.; Hsu, Y.-C.; Chen, M.-J.; Chen, C.-C.; et al. Long-Term Changes of Gut Microbiota, Antibiotic Resistance, and Metabolic Parameters after Helicobacter Pylori Eradication: A Multicentre, Open-Label, Randomised Trial. Lancet Infect. Dis. 2019, 19, 1109–1120. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.I.; Bajić, S.S.; Milutinović, D.V.; Tomić, M.; Golić, N.; Tolinački, M. Gut Microbiota Dysbiosis Associated with Altered Production of Short Chain Fatty Acids in Children With Neurodevelopmental Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Eslick, S.; Williams, E.J.; Berthon, B.S.; Wright, T.; Karihaloo, C.; Gately, M.; Wood, L.G. Weight Loss and Short-Chain Fatty Acids Reduce Systemic Inflammation in Monocytes and Adipose Tissue Macrophages from Obese Subjects. Nutrients 2022, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, J.; García-Carpintero, S.; Jimenez-Lucena, R.; Haro, C.; Rangel-Zúñiga, O.A.; Blanco-Rojo, R.; Yubero-Serrano, E.M.; Tinahones, F.J.; Delgado-Lista, J.; Pérez-Martínez, P.; et al. Effect of Dietary Lipids on Endotoxemia Influences Postprandial Inflammatory Response. J. Agric. Food Chem. 2017, 65, 7756–7763. [Google Scholar] [CrossRef] [PubMed]
- Rorato, R.; de Borges, B.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Elias, C.F.; Kagohara Elias, L.L. LPS-Induced Low-Grade Inflammation Increases Hypothalamic JNK Expression and Causes Central Insulin Resistance Irrespective of Body Weight Changes. Int. J. Mol. Sci. 2017, 18, 1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-Y.; Chang, Y.-T.; Juan, C.-K.; Shieh, J.-J.; Lin, Y.-P.; Liu, H.-N.; Lin, J.-T.; Chen, Y.-J. Risk of Inflammatory Bowel Disease in Patients with Rosacea: Results from a Nationwide Cohort Study in Taiwan. J. Am. Acad. Dermatol. 2017, 76, 911–917. [Google Scholar] [CrossRef]
- Kim, M.; Choi, K.H.; Hwang, S.W.; Lee, Y.B.; Park, H.J.; Bae, J.M. Inflammatory Bowel Disease Is Associated with an Increased Risk of Inflammatory Skin Diseases: A Population-Based Cross-Sectional Study. J. Am. Acad. Dermatol. 2017, 76, 40–48. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Li, N.; Liang, S.; Chen, Q.; Zhao, L.; Li, B.; Huo, G. Distinct Gut Microbiota and Metabolite Profiles Induced by Delivery Mode in Healthy Chinese Infants. J. Proteom. 2021, 232, 104071. [Google Scholar] [CrossRef]
- Kuang, Y.-S.; Li, S.-H.; Guo, Y.; Lu, J.-H.; He, J.-R.; Luo, B.-J.; Jiang, F.-J.; Shen, H.; Papasian, C.J.; Pang, H.; et al. Composition of Gut Microbiota in Infants in China and Global Comparison. Sci. Rep. 2016, 6, 36666. [Google Scholar] [CrossRef] [Green Version]
- Forsgren, M.; Isolauri, E.; Salminen, S.; Rautava, S. Late Preterm Birth Has Direct and Indirect Effects on Infant Gut Microbiota Development during the First Six Months of Life. Acta Paediatr. 2017, 106, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-Analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients With Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliai, G.; Russo, E.; Niccolai, E.; Dinu, M.; Di Pilato, V.; Magrini, A.; Bartolucci, G.; Baldi, S.; Menicatti, M.; Giusti, B.; et al. Influence of a 3-Month Low-Calorie Mediterranean Diet Compared to the Vegetarian Diet on Human Gut Microbiota and SCFA: The CARDIVEG Study. Eur. J. Nutr. 2020, 59, 2011–2024. [Google Scholar] [CrossRef]
- Fouesnard, M.; Zoppi, J.; Petera, M.; le Gleau, L.; Migné, C.; Devime, F.; Durand, S.; Benani, A.; Chaffron, S.; Douard, V.; et al. Dietary Switch to Western Diet Induces Hypothalamic Adaptation Associated with Gut Microbiota Dysbiosis in Rats. Int. J. Obes. 2021, 45, 1271–1283. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Laudisi, F.; di Fusco, D.; Dinallo, V.; Stolfi, C.; di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress-Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.T.; Differding, M.K.; Østbye, T.; Hoyo, C.; Benjamin-Neelon, S.E. Association of Birth Mode of Delivery with Infant Faecal Microbiota, Potential Pathobionts, and Short Chain Fatty Acids: A Longitudinal Study over the First Year of Life. BJOG: Int. J. Obstet. 2021, 128, 1293–1303. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Hickman, B.; Kunz, C.; Sprenger, N.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; de Vos, W.M. Fucosylated Oligosaccharides in Mother’s Milk Alleviate the Effects of Caesarean Birth on Infant Gut Microbiota. Sci. Rep. 2018, 8, 13757. [Google Scholar] [CrossRef] [Green Version]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial Resistance in Clostridioides (Clostridium) Difficile Derived from Humans: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zuñiga, O.A.; Alcala-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on Gut Microbiota of a 1-y Lifestyle Intervention with Mediterranean Diet Compared with Energy-Reduced Mediterranean Diet and Physical Activity Promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.-I.; Riethoven, J.-J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of Whole Grains versus Fruits and Vegetables in Reducing Subclinical Inflammation and Promoting Gastrointestinal Health in Individuals Affected by Overweight and Obesity: A Randomized Controlled Trial. Nutr. J. 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierasińska, M.; Donskow-Łysoniewska, K. Both the Microbiome and the Macrobiome Can Influence Immune Responsiveness in Psoriasis. Central Eur. J. Immunol. 2021, 46, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut Microbiota Dysbiosis in a Cohort of Patients with Psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Cohen, N.A.; Shalev, V.; Uzan, A.; Koren, O.; Maharshak, N. Psoriatic Patients Have a Distinct Structural and Functional Fecal Microbiota Compared with Controls. J. Dermatol. 2019, 46, 595–603. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of Gut Microbiota and Its Correlation with Dysregulation of Cytokines in Psoriasis Patients. BMC Microbiol. 2021, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Eppinga, H.; Weiland, C.J.S.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium Prausnitzii in Psoriasis and Inflammatory Bowel Disease, but Not in Hidradenitis Suppurativa. J. Crohn’s Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Schade, L.; Mesa, D.; Faria, A.R.; Santamaria, J.R.; Xavier, C.A.; Ribeiro, D.; Hajar, F.N.; Azevedo, V.F. The Gut Microbiota Profile in Psoriasis: A Brazilian Case-Control Study. Lett. Appl. Microbiol. 2021, 74, 498–504. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia Muciniphila Is a Gut Microbiota Signature in Psoriasis. Exp. Dermatol. 2017, 27, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Guo, X.; Wei, W.; Li, R.; Hu, K.; Liu, X.; Jiang, W.; Liu, S.; Wang, W.; Sun, H.; et al. The Association of Fried Meat Consumption With the Gut Microbiota and Fecal Metabolites and Its Impact on Glucose Homoeostasis, Intestinal Endotoxin Levels, and Systemic Inflammation: A Randomized Controlled-Feeding Trial. Diabetes Care 2021, 44, 1970–1979. [Google Scholar] [CrossRef]
- Todberg, T.; Egeberg, A.; Zachariae, C.; Sørensen, N.; Pedersen, O.; Skov, L. Patients with Psoriasis Have a Dysbiotic Taxonomic and Functional Gut Microbiota*. Br. J. Dermatol. 2022, 187, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, G.; Jiang, C.; Liu, X.; Wang, X.; Li, Y.; Cheng, M.; Lv, H.; Xian, F.; Guo, X.; et al. Deciphering Gut Microbiota Dysbiosis and Corresponding Genetic and Metabolic Dysregulation in Psoriasis Patients Using Metagenomics Sequencing. Front. Cell. Infect. Microbiol. 2021, 11, 605825. [Google Scholar] [CrossRef]
- Witte, E.; Kokolakis, G.; Witte, K.; Philipp, S.; Doecke, W.-D.; Babel, N.; Wittig, B.M.; Warszawska, K.; Kurek, A.; Erdmann-Keding, M.; et al. IL-19 Is a Component of the Pathogenetic IL-23/IL-17 Cascade in Psoriasis. J. Investig. Dermatol. 2014, 134, 2757–2767. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, M.; Wang, C.; Wang, G.; Sun, K.; Xiong, S.; Cheng, L.; Yang, D.; Lin, X.; Zhao, X. Intrinsic Abnormalities of Keratinocytes Initiate Skin Inflammation through the IL-23/T17 Axis in a MALT1-Dependent Manner. J. Immunol. 2021, 206, 839–848. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.; Prinz, J. The Role of IL-23 and the IL-23/TH17 Immune Axis in the Pathogenesis and Treatment of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Tsianakas, A.; Brunner, P.M.; Ghoreschi, K.; Berger, C.; Loser, K.; Röcken, M.; Stingl, G.; Luger, T.; Jung, T. The Single-Chain Anti-TNF-α Antibody DLX105 Induces Clinical and Biomarker Responses upon Local Administration in Patients with Chronic Plaque-Type Psoriasis. Exp. Dermatol. 2016, 25, 428–433. [Google Scholar] [CrossRef]
- Sbidian, E.; Chaimani, A.; Garcia-Doval, I.; Do, G.; Hua, C.; Mazaud, C.; Droitcourt, C.; Hughes, C.; Ingram, J.R.; Naldi, L.; et al. Systemic Pharmacological Treatments for Chronic Plaque Psoriasis: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2017, 12, CD011535. [Google Scholar] [CrossRef] [PubMed]
- Furiati, S.C.; Catarino, J.S.; Silva, M.V.; Silva, R.F.; Estevam, R.B.; Teodoro, R.B.; Pereira, S.L.; Ataide, M.; Rodrigues, V.; Rodrigues, D.B.R. Th1, Th17, and Treg Responses Are Differently Modulated by TNF-α Inhibitors and Methotrexate in Psoriasis Patients. Sci. Rep. 2019, 9, 7526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Zhu, Z.; Gao, J.; Yang, C.; Wen, L.; Liu, L.; Zuo, X.; Zheng, X.; Shi, Y.; Zhu, C.; et al. NFKB1 Mediates Th1/Th17 Activation in the Pathogenesis of Psoriasis. Cell. Immunol. 2018, 331, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zákostelská, Z.; Málková, J.; Klimešová, K.; Rossmann, P.; Hornová, M.; Novosádová, I.; Stehlíková, Z.; Kostovčík, M.; Hudcovic, T.; Štepánková, R.; et al. Intestinal Microbiota Promotes Psoriasis-Like Skin Inflammation by Enhancing Th17 Response. PLoS ONE 2016, 11, e0159539. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.; Nolan, S.; Alphonse, M.; Dikeman, D.; Youn, C.; Wang, Y.; Orlando, N.; Patrick, G.; Lee, S.; Ortines, R.V.; et al. IL-6R/Signal Transducer and Activator of Transcription 3 Signaling in Keratinocytes Rather than in T Cells Induces Psoriasis-Like Dermatitis in Mice. J. Investig. Dermatol. 2021, 142, 1126–1135.e4. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Ruszkowski, J.; Daca, A.; Szewczyk, A.; Dębska-Ślizień, A.; Witkowski, J.M. The Influence of Biologics on the Microbiome in Immune-Mediated Inflammatory Diseases: A Systematic Review. Biomed. Pharmacother. 2021, 141, 111904. [Google Scholar] [CrossRef]
- Manasson, J.; Wallach, D.S.; Guggino, G.; Stapylton, M.; Badri, M.H.; Solomon, G.; Reddy, S.M.; Coras, R.; Aksenov, A.A.; Jones, D.R.; et al. Interleukin-17 Inhibition in Spondyloarthritis Is Associated With Subclinical Gut Microbiome Perturbations and a Distinctive Interleukin-25-Driven Intestinal Inflammation. Arthritis Rheumatol. 2019, 72, 645–657. [Google Scholar] [CrossRef]
- Sator, P. Safety and Tolerability of Adalimumab for the Treatment of Psoriasis: A Review Summarizing 15 Years of Real-Life Experience. Ther. Adv. Chronic Dis. 2018, 9, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, X.; Zhu, L.; Geng, S.; Guo, K. Effectiveness and Safety of Adalimumab in Psoriasis and Its Influence on Gut Microbiome. Microb. Pathog. 2021, 162, 105308. [Google Scholar] [CrossRef]
- Jeon, C.; Sekhon, S.; Yan, D.; Afifi, L.; Nakamura, M.; Bhutani, T. Monoclonal Antibodies Inhibiting IL-12, -23, and -17 for the Treatment of Psoriasis. Hum. Vaccines Immunother. 2017, 13, 2247–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, N.-L.; Hsu, C.-Y.; Tsai, T.-F.; Chiu, H.-Y. Gut Microbiome in Psoriasis Is Perturbed Differently During Secukinumab and Ustekinumab Therapy and Associated with Response to Treatment. Clin. Drug Investig. 2019, 39, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Silvestri, V.; Marraffa, F.; Greco, G.; Bucalo, A.; Grassi, S.; Gagliardi, A.; Mazzotta, A.; Ottini, L.; Richetta, A.G. Gut Microbiome Profile in Psoriatic Patients Treated and Untreated with Biologic Therapy. J. Dermatol. 2021, 48, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Cox, L.; Tjon, E.; Kivisakk, P.; Vanande, I.P.; Cook, S.; Gandhi, R.; Glanz, B.; et al. Investigation of Probiotics in Multiple Sclerosis. Mult. Scler. J. 2018, 24, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håkansson, Å.; Aronsson, C.A.; Brundin, C.; Oscarsson, E.; Molin, G.; Agardh, D. Effects of Lactobacillus Plantarum and Lactobacillus Paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 1925. [Google Scholar] [CrossRef] [Green Version]
- Moludi, J.; Khedmatgozar, H.; Saiedi, S.; Razmi, H.; Alizadeh, M.; Ebrahimi, B. Probiotic Supplementation Improves Clinical Outcomes and Quality of Life Indicators in Patients with Plaque Psoriasis: A Randomized Double-Blind Clinical Trial. Clin. Nutr. ESPEN 2021, 46, 33–39. [Google Scholar] [CrossRef]
- Fostering Healthier and More Sustainable Diets—Learning from the Mediterranean and New Nordic Experience. Available online: https://www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/news/news/2018/5/fostering-healthier-and-more-sustainable-diets-learning-from-the-mediterranean-and-new-nordic-experience (accessed on 10 May 2022).
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Barrea, L.; Fabbrocini, G.; Annunziata, G.; Muscogiuri, G.; Donnarumma, M.; Marasca, C.; Colao, A.; Savastano, S. Role of Nutrition and Adherence to the Mediterranean Diet in the Multidisciplinary Approach of Hidradenitis Suppurativa: Evaluation of Nutritional Status and Its Association with Severity of Disease. Nutrients 2018, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Donnarumma, M.; Cacciapuoti, S.; Muscogiuri, G.; de Gregorio, L.; Blasio, C.; Savastano, S.; Colao, A.; Fabbrocini, G. Phase Angle and Mediterranean Diet in Patients with Acne: Two Easy Tools for Assessing the Clinical Severity of Disease. J. Transl. Med. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Lorite-Fuentes, I.; Montero-Vilchez, T.; Arias-Santiago, S.; Molina-Leyva, A. Potential Benefits of the Mediterranean Diet and Physical Activity in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study in a Spanish Population. Nutrients 2022, 14, 551. [Google Scholar] [CrossRef]
- Ah-Thiane, L.; Nguyen, J.M.; Khammari, A.; Dréno, B. Lifestyle Habits and Impact of the Mediterranean Diet on Facial Acne Severity in French Women: A Case-Control Study. Int. J. Women’s Dermatol. 2022, 8, e017. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Napoleone, A.; Trio, R.; Nastasi, A.; Balato, N.; Laccetti, R.; Cataldi, M. Energy-Restricted, n-3 Polyunsaturated Fatty Acids-Rich Diet Improves the Clinical Response to Immuno-Modulating Drugs in Obese Patients with Plaque-Type Psoriasis: A Randomized Control Clinical Trial. Clin. Nutr. 2014, 33, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Illescas, O.; Rodríguez-Sosa, M.; Gariboldi, M. Mediterranean Diet to Prevent the Development of Colon Diseases: A Meta-Analysis of Gut Microbiota Studies. Nutrients 2021, 13, 2234. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.; Touvier, M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Wolkenstein, P.; Chosidow, O.; Ezzedine, K.; Sbidian, E. Association Between Mediterranean Anti-Inflammatory Dietary Profile and Severity of Psoriasis. JAMA Dermatol. 2018, 154, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and Psoriasis: Is There Any Association between the Severity of the Disease and Adherence to the Mediterranean Diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Polo, T.C.F.; Corrente, J.E.; Miot, L.D.B.; Papini, S.J.; Miot, H.A. Dietary Patterns of Patients with Psoriasis at a Public Healthcare Institution in Brazil. An. Bras. de Dermatol. 2020, 95, 452–458. [Google Scholar] [CrossRef]
- Ungprasert, P.; Wijarnpreecha, K.; Kittanamongkolchai, W. Psoriasis and Risk of Celiac Disease: A Systematic Review and Meta-Analysis. Indian J. Dermatol. 2017, 62, 41–46. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M. Association between Psoriasis and Celiac Disease: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2019, 82, 1376–1385. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2018, 127, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Agarwala, A.; Makharia, G.; Bhatnagar, S.; Tandon, N. Effect Of Gluten-Free Diet On Metabolic Control And Anthropometric Parameters In Type 1 Diabetes With Subclinical Celiac Disease: A Randomized Controlled Trial. Endocr. Pr. 2020, 26, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Drucker, A.M.; Qureshi, A.A.; Thompson, J.M.; Li, T.; Cho, E. Gluten Intake and Risk of Psoriasis, Psoriatic Arthritis, and Atopic Dermatitis among United States Women. J. Am. Acad. Dermatol. 2019, 82, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Siegel, M.; Bagel, J.; Cordoro, K.; Garg, A.; Gottlieb, A.B.; Green, L.J.; Gudjonsson, J.E.; Koo, J.; Lebwohl, M.; et al. Dietary Recommendations for Adults With Psoriasis or Psoriatic Arthritis From the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2018, 154, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Afifi, L.; Danesh, M.J.; Lee, K.M.; Beroukhim, K.; Farahnik, B.; Ahn, R.S.; Yan, D.; Singh, R.K.; Nakamura, M.; Koo, J.; et al. Dietary Behaviors in Psoriasis: Patient-Reported Outcomes from a U.S. National Survey. Dermatol. Ther. 2017, 7, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Tuck, C.J.; Caminero, A.; Vargas, N.N.J.; Soltys, C.L.; Polanco, J.O.J.; Lopez, C.D.L.; Constante, M.; Lourenssen, S.R.; Verdu, E.F.; Muir, J.G.; et al. The Impact of Dietary Fermentable Carbohydrates on a Postinflammatory Model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2019, 31, e13675. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseri, K.; Dabiri, H.; Rostami-Nejad, M.; Yadegar, A.; Houri, H.; Olfatifar, M.; Sadeghi, A.; Saadati, S.; Ciacci, C.; Iovino, P.; et al. Influence of Low FODMAP-Gluten Free Diet on Gut Microbiota Alterations and Symptom Severity in Iranian Patients with Irritable Bowel Syndrome. BMC Gastroenterol. 2021, 21, 292. [Google Scholar] [CrossRef]
- Probiotics: What You Need To Know|NCCIH. Available online: https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know (accessed on 9 April 2022).
- Wieërs, G.; Verbelen, V.; van den Driessche, M.; Melnik, E.; Vanheule, G.; Marot, J.C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota with Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. public Heal. 2021, 8, 578089. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Synbiotics—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/immunology-and-microbiology/synbiotics (accessed on 9 April 2022).
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The Microbiome in Allergic Disease: Current Understanding and Future Opportunities-2017 PRACTALL Document of the American Academy of Allergy, Asthma and Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef] [Green Version]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus Rhamnosus and Lactobacillus Casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Deng, Y.; Fang, Z.; Zhai, Q.; Cui, S.; Zhao, J.; Chen, W.; Zhang, H. Potential Role of Probiotics in Ameliorating Psoriasis by Modulating Gut Microbiota in Imiquimod-Induced Psoriasis-Like Mice. Nutrients 2021, 13, 2010. [Google Scholar] [CrossRef] [PubMed]
- Vijayashankar, M.; Raghunath, N. Pustular Psoriasis Responding to Probiotics—A New Insight. Our Dermatol. Online 2012, 3, 326–329. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium Infantis 35624 Modulates Host Inflammatory Processes beyond the Gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Moludi, J.; Fathollahi, P.; Khedmatgozar, H.; Tabrizi, F.P.F.; Zare, A.G.; Razmi, H.; Amirpour, M. Probiotics Supplementation Improves Quality of Life, Clinical Symptoms, and Inflammatory Status in Patients With Psoriasis. J. Drugs Dermatol. 2022, 21, 637–644. [Google Scholar] [CrossRef]
- Lin, C.; Zeng, T.; Deng, Y.; Yang, W.; Xiong, J. Treatment of Psoriasis Vulgaris Using Bacteroides Fragilis BF839: A Single-Arm, Open Preliminary Clinical Study. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3828–3835. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscà, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.-S.; Zhang, B.; Gao, Z.L.; Zheng, R.-P.; Marcellin, D.F.H.M.; Saro, A.; Pan, J.; Chu, L.; Wang, T.-S.; Huang, J.-F. Altered Diversity and Composition of Gut Microbiota in Patients with Allergic Rhinitis. Microb. Pathog. 2021, 161, 105272. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.-W.; You, H.J.; Park, S.-J.; Lee, J.; Ju, J.H.; Park, M.S.; Jin, H.; Cho, M.-L.; Kwon, B.; et al. Gut Microbial Composition and Function Are Altered in Patients with Early Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 693. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Jin, W.; Mao, Z.; Dong, S.; Zhang, Q.; Yang, Y.; Chen, B.; Wu, H.; Zeng, M. Microbiome and Butyrate Production Are Altered in the Gut of Rats Fed a Glycated Fish Protein Diet. J. Funct. Foods 2018, 47, 423–433. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Zhong, Y.; Li, C.; Huang, X.; Geng, F.; Nie, S. Microbiota-Related Effects of Prebiotic Fibres in Lipopolysaccharide-Induced Endotoxemic Mice: Short Chain Fatty Acid Production and Gut Commensal Translocation. Food Funct. 2021, 12, 7343–7357. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Cephas, K.D.; Holscher, H.; Kerr, K.R.; Mangian, H.F.; Tappenden, K.; Swanson, K.S. Nondigestible Fructans Alter Gastrointestinal Barrier Function, Gene Expression, Histomorphology, and the Microbiota Profiles of Diet-Induced Obese C57BL/6J Mice. J. Nutr. 2016, 146, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takahashi, K.; Abe, S.; Yamada, K.; Suzuki, M.; Masahisa, M.; Endo, M.; Abe, K.; Inoue, R.; Hoshi, H. Improvement of Psoriasis by Alteration of the Gut Environment by Oral Administration of Fucoidan from Cladosiphon Okamuranus. Mar. Drugs 2020, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Taheri, M.; Ebrahimi, B.; Alirezaei, P.; Doosti-Irani, A.; Soleimani, M.; Nouri, F. Evaluation of Lactocare® Synbiotic Administration on the Serum Electrolytes and Trace Elements Levels in Psoriasis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial Study. Biol. Trace Elem. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Lampousi, A.-M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive Oil in the Prevention and Management of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies and Intervention Trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef] [Green Version]
- Istas, G.; Wood, E.; le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of Long-Term Consumption of Broccoli Sprouts on Inflammatory Markers in Overweight Subjects. Clin. Nutr. 2018, 38, 745–752. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary Supplementation of Black Rice Anthocyanin Extract Regulates Cholesterol Metabolism and Improves Gut Microbiota Dysbiosis in C57BL/6J Mice Fed a High-Fat and Cholesterol Diet. Mol. Nutr. Food Res. 2020, 64, e1900876. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the Treatment of Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; de Sanctis, P.; Giovannini, C.; d’Archivio, M.; Masella, R. Anti-Inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2017, 15, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.H.; Pérez, J.A.S.; Arjona, J.A.; Visioli, F. An Olive Polyphenol-Based Nutraceutical Improves Cutaneous Manifestations of Psoriasis in Humans. Pharma Nutr. 2016, 4, 151–153. [Google Scholar] [CrossRef]
- Vetrani, C.; Maukonen, J.; Bozzetto, L.; della Pepa, G.; Vitale, M.; Costabile, G.; Riccardi, G.; Rivellese, A.A.; Saarela, M.; Annuzzi, G. Diets Naturally Rich in Polyphenols and/or Long-Chain n-3 Polyunsaturated Fatty Acids Differently Affect Microbiota Composition in High-Cardiometabolic-Risk Individuals. Acta Diabetol. 2020, 57, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-Mediated Immune System Imprinting Early in Life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle Curcumin Ameliorates Experimental Colitis via Modulation of Gut Microbiota and Induction of Regulatory T Cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Cas, M.D.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Carrion-Gutierrez, M.; Ramirez-Bosca, A.; Navarro-Lopez, V.; Martinez-Andres, A.; Asín-Llorca, M.; Bernd, A.; de la Parte, J.F.H. Effects of Curcuma Extract and Visible Light on Adults with Plaque Psoriasis. Eur. J. Dermatol. 2015, 25, 240–246. [Google Scholar] [CrossRef]
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome Through Sex Steroid Hormones—Microbiota—Inflammation Axis in Rats. Front. Endocrinol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Tveit, K.; Brokstad, K.; Berge, R.; Sæbø, P.; Hallaråker, H.; Brekke, S.; Meland, N.; Bjørndal, B. A Randomized, Double-Blind, Placebo-Controlled Clinical Study to Investigate the Efficacy of Herring Roe Oil for Treatment of Psoriasis. Acta Derm. Venereol. 2020, 100, adv00154. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nakkarach, A.; Foo, H.L.; Song, A.A.-L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-Cancer and Anti-Inflammatory Effects Elicited by Short Chain Fatty Acids Produced by Escherichia Coli Isolated from Healthy Human Gut Microbiota. Microb. Cell Factories 2021, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms Underlying the Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A Combination of Quercetin and Resveratrol Reduces Obesity in High-Fat Diet-Fed Rats by Modulation of Gut Microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol Reduces Obesity in High-Fat Diet-Fed Mice via Modulating the Composition and Metabolic Function of the Gut Microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Cai, T.-T.; Ye, X.-L.; Li, R.-R.; Chen, H.; Wang, Y.-Y.; Yong, H.-J.; Pan, M.-L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and Resveratrol Regulate Intestinal Bacteria and Alleviate Intestinal Inflammation in Weaned Piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic Acid and Resveratrol Alleviate Oxidative Stress with Modulation of Gut Microbiota in High-Fat Diet-Fed Rats. Food Res. Int. 2018, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int. J. Obes. 2019, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic Analysis of Gut Microbiota in Non-Treated Plaque Psoriasis Patients Stratified by Disease Severity: Development of a New Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities. Crit. Rev. Food Sci. Nutr. 2019, 60, 3290–3303. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive Effect of Quercetin on Dendritic Cell Activation and Function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [Green Version]
- Chirumbolo, S. The Role of Quercetin, Flavonols and Flavones in Modulating Inflammatory Cell Function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin Disrupts Tyrosine-Phosphorylated Phosphatidylinositol 3-Kinase and Myeloid Differentiation Factor-88 Association and Inhibits MAPK/AP-1 and IKK/NF-ΚB-Induced Inflammatory Mediators Production in RAW 264.7 Cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Tan, Y.; Tam, C.; Rolston, M.; Alves, P.; Chen, L.; Meng, S.; Hong, H.; Chang, S.; Yokoyama, W. Quercetin Ameliorates Insulin Resistance and Restores Gut Microbiome in Mice on High-Fat Diets. Antioxidants 2021, 10, 1251. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.-Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin Ameliorates Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice via the NF-ΚB Pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, H.; Liu, Y.; Zhu, Z.; Wei, Q. Quercitrin Extracted from Tartary Buckwheat Alleviates Imiquimod-Induced Psoriasis-like Dermatitis in Mice by Inhibiting the Th17 Cell Response. J. Funct. Foods 2017, 38, 9–19. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.F.; Sun, Y.F.; Ding, X.; Zeng, J.Q.; Zhang, T.; Peng, L.H.; Yang, Y.S.; Zhao, H. Fecal Microbiota Transplantation as a Novel Therapy for Severe Psoriasis. Zhonghua Nei Ke Za Zhi 2019, 58, 782–785. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [Green Version]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [Green Version]
- Hvas, C.L.; Jørgensen, S.M.D.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium Difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef] [Green Version]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Möller, S.; et al. Safety and Efficacy of Faecal Microbiota Transplantation for Active Peripheral Psoriatic Arthritis: An Exploratory Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef]
| Therapy | Study Population | Intervention | Outcomes | Reference |
|---|---|---|---|---|
| Low-FODMAP diet | Crohn’s disease or ulcerative colitis patients Randomized n = 52 No previous probiotics, prebiotics, azathioprine, mercaptopurine, methotrexate, or biologics | Low-FODMAP diet for 4 weeks | ↓Bifidobacterium adolescentis, ↓Bifidobacterium longum, ↓Faecalibacterium prausnitzii | Selina R. Cox et al. [87] |
| Omega-3 fatty acids | 6-week-old female rats | 1 mg/kg/day of flaxseed oil by gavage for 8 weeks | ↑Allobaculum, ↑Lactobacillus, ↑Butyrivibrio, ↑Desulfovibrio, ↑Bifidobacterium, ↑Faecalibacterium, ↑Parabacteroides ↓Actinobacteria, ↓Bacteroides, ↓Proteobacteria, ↓Streptococcus, ↓Firmicutes/Bacteroidetes ratio | Ting Wang et al. [124] |
| Resveratrol | Diabetic nephropathy mice | Oral administration of 10 mg/kg/day resveratrol for 12 weeks | ↑Bacteroides, ↑Alistipes, ↑Rikenella, ↑Odoribacter, ↑Parabacteroides, ↑Alloprevotella | Ting-Ting Cai et al. [133] |
| High-fat diet-fed rats | 400 mg/kg/day resveratrol, 200 mg/kg/day sinapic acid or both for 8 weeks | ↑Blauta spp. ↑Dorea spp. ↓Bacteroides spp. ↓Desulfovibrionaceae spp. | ChenYang et al. [135] | |
| High-fat diet-fed mice | 300 mg/kg/day resveratrol for 16 weeks | ↑Lachnospiraceae family | Pan Wang et al. [136] | |
| Quercetin | Monosodium glutamate-induced abdominal obese mice | 5 mg/kg quercetin dissolved in 0.15% carboxymethylcellulose sodium, administrated by gavage for 6 weeks | ↓Firmicutes/Bacteroidetes ratio ↓Firmicutes ↓Bacteroides spp. ↓Lachnospiraceae spp., ↓Ruminicoccaceae spp. | Lijun Zhao et al. [144] |
| Therapy | Study Population | Design | Intervention | Outcomes | Reference | |
|---|---|---|---|---|---|---|
| Probiotics | 47-year-old woman with psoriasis, having pustules all over her body; non-responsive to the anti-psoriatic treatment | 6 month case report | Lactobacillus probiotic one sachet thrice daily with biotin 10 mg once daily for 6 months | In 15 days, the lesions started involuting; reduced blood sugar level After 6 months she was free of lesions | Metikurke Vijayashankar et al. [97] | |
| Psoriasis patients n = 26 PASI < 16 Healthy subjects n = 22 No previous immunosuppresant therapy | 8 week RCCT 1 | Bifidobacterium infantis 35,624 | ↓IL-6, ↓TNF-α, ↓CRP | Groeger David et al. [98] | ||
| Psoriasis patients n = 50 Randomized | 8 week RCCT | Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium langum 1.8 × 109 CFU/capsule | ↑DLQI 2, ↑TAC 3, ↓PASI score, ↓PSS 4, ↓CRP, ↓IL-6 | Jalal Moludi et al. [66] | ||
| Psoriasis patients n = 46 Randomized | 2 month RCCT | Probiotic capsules with multi-strain bacteria 1.6 × 109 CFU/g | ↑QOL 5, ↓serum LPS levels, ↓CRP, ↓IL-1β | Jalal Moludi et al. [99] | ||
| Psoriasis patients n = 27 Received anti-psoriatic treatment | 12 week single-arm, clinical trial | Bifidobacterium fragilis BF839 | 1 patient was excluded from the trial; ↓PASI score 1 case of side effect: constipation | Chuhui Lin et al. [100] | ||
| Psoriasis patients receiving topical anti-psoriatic treatment, age 18–70, PASI > 6 n = 90, Randomized | 12 week double-blind, RCCT | Bifidobacterium longum CECT 7347, B. lactis CECT 8145 and Lactobacillus rhamnosus CECT 8361 with a total of 1 × 109 CFU/capsule | 2 patients did not complete the study ↓PASI score; loss of the genera ↓Micromonospora, ↓Rhodococcus, ↑Collinsella, ↑Lactobacillus | Vicente Navarro-López et al. [101] | ||
| Synbiotic | Psoriasis patients n = 64 | 12 week double-blind RCCT | Lactobacillus casei, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, Streptococcus thermophiles and FOS | 8 patients from the intervention group and 18 patients from the control discontinued the study; ↑ serum levels of Fe, Ca, Mg, P, and Zn due to favorable effects on the gastrointestinal system | Ali Akbarzadeh et al. [108] | |
| Curcumin | Healthy human subjects n = 30 randomized No previous antibiotic, topical medication, or oral turmeric/curcuma supplement | 8 week double-blind RCCT | Supplementation with 6000 mg/daily Curcuma longa extract | ↑Clostridium spp., ↑Bacteroides spp., ↑Citrobacter spp. ↑Cronobacter spp. ↑Enterobacter spp., ↑Enterococcus spp., ↑Klebsiella spp., ↑Parabacteroides spp., ↑Pseudomonas spp., ↓ Blautia spp., ↓Ruminococcus spp. | Christine T. Peterson et al. [119] | |
| Psoriasis patients n = 63, PASI < 10. Randomized Receiving anti-psoriatic treatment | 12 week double-blind RCCT | 2 g/day of curcumin | ↓PASI score, ↓ IL-22 serum levels | Emiliano Antiga et al. [121] | ||
| Omega 3 fatty acids | Psoriasis patients n = 64. Randomized PASI < 10 53% of subjects used local anti-psoriatic maintenance treatment | 26 week double-blind RCCT | Herring roe oil (containing 292 mg of polyunsaturated fatty acids omega-3), Daily dose: 2,6 g EPA and DHA | 6 patients from the interventional group did not complete the trial ↓PASI score No difference in inflammatory markers | Kåre Steinar Tveit et al. [125] | |
| Healthy subjects n = 69 Randomized No previous treatment | 6 week randomized interventional trial | Daily dose of 500 mg of omega 3 (165 mg EPA, 110 mg DHA) vs. 20 g inulin | Inulin: ↑Bifidobacterium spp. ↑Lachnospiraceae spp. ↑iso-valerate ↑iso-butyrate ↑butyrate | Omega 3: ↑iso-valerate ↑iso-butyrate ↑ Coprococcus ↑ Bacteroides ↓Colinsella. | Amrita Vijay et al. [126] | |
| Fecal microbiota transplantation | Severe plaque psoriasis and IBS patient n = 1 | 5 week interventional clinical trial | FMT upper endoscopy and colonoscopy | ↓BSA 6, ↓PASI, ↑DLQI, ↓TNF-α Improved intestinal symptoms | G. Yin et al. [147] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhaș, M.C.; Gavrilaș, L.I.; Candrea, R.; Cătinean, A.; Mocan, A.; Miere, D.; Tătaru, A. Gut Microbiota in Psoriasis. Nutrients 2022, 14, 2970. https://doi.org/10.3390/nu14142970
Buhaș MC, Gavrilaș LI, Candrea R, Cătinean A, Mocan A, Miere D, Tătaru A. Gut Microbiota in Psoriasis. Nutrients. 2022; 14(14):2970. https://doi.org/10.3390/nu14142970
Chicago/Turabian StyleBuhaș, Mihaela Cristina, Laura Ioana Gavrilaș, Rareș Candrea, Adrian Cătinean, Andrei Mocan, Doina Miere, and Alexandru Tătaru. 2022. "Gut Microbiota in Psoriasis" Nutrients 14, no. 14: 2970. https://doi.org/10.3390/nu14142970
APA StyleBuhaș, M. C., Gavrilaș, L. I., Candrea, R., Cătinean, A., Mocan, A., Miere, D., & Tătaru, A. (2022). Gut Microbiota in Psoriasis. Nutrients, 14(14), 2970. https://doi.org/10.3390/nu14142970







