Hepatic Steatosis in Patients with Celiac Disease: The Role of Packaged Gluten-Free Foods
Abstract
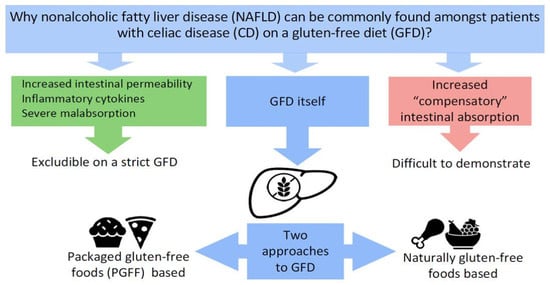
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subclassification of PGFF
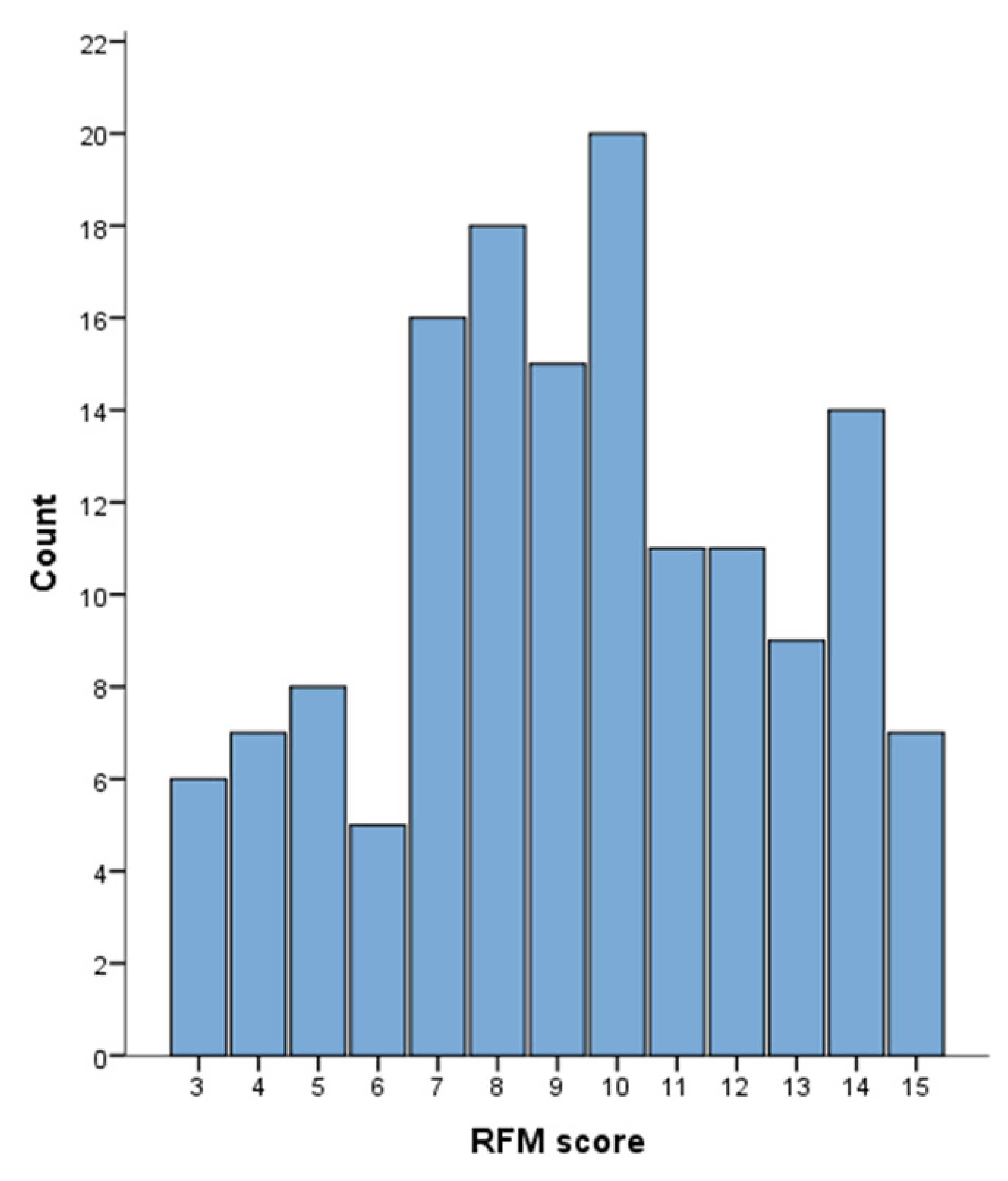
2.3. RFM Analysis
2.4. Ultrasound Evaluation and Diagnosis of NAFLD
2.5. Sample size Calculation
2.6. Ethics
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Consumption of Packaged Gluten-Free Foods
3.3. Prevalence and Relative Risk of NAFLD
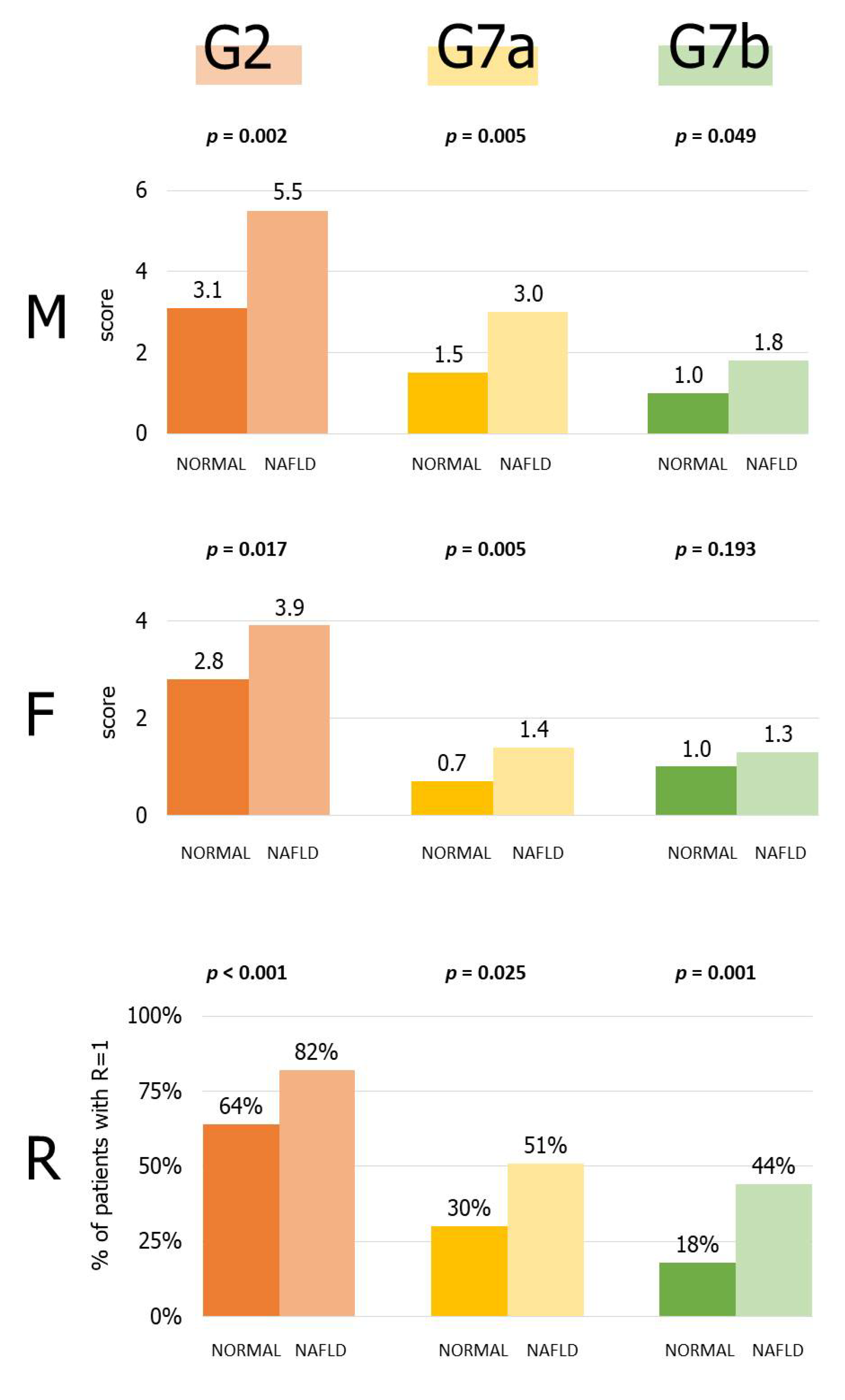
3.4. Role of Single PGFF Categories
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo Definitions for Coeliac Disease and Related Terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Reilly, N.R.; Aguilar, K.; Hassid, B.G.; Cheng, J.; Defelice, A.R.; Kazlow, P.; Bhagat, G.; Green, P.H. Celiac Disease in Normal-Weight and Overweight Children: Clinical Features and Growth Outcomes Following a Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac Disease and Obesity: Need for Nutritional Follow-up after Diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norsa, L.; Shamir, R.; Zevit, N.; Verduci, E.; Hartman, C.; Ghisleni, D.; Riva, E.; Giovannini, M. Cardiovascular Disease Risk Factor Profiles in Children with Celiac Disease on Gluten-Free Diets. World J. Gastroenterol. 2013, 19, 5658–5664. [Google Scholar] [CrossRef]
- Reilly, N.R.; Lebwohl, B.; Hultcrantz, R.; Green, P.H.R.; Ludvigsson, J.F. Increased Risk of Non-Alcoholic Fatty Liver Disease after Diagnosis of Celiac Disease. J. Hepatol. 2015, 62, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Tovoli, F.; Negrini, G.; Farì, R.; Guidetti, E.; Faggiano, C.; Napoli, L.; Bolondi, L.; Granito, A. Increased Risk of Nonalcoholic Fatty Liver Disease in Patients with Coeliac Disease on a Gluten-Free Diet: Beyond Traditional Metabolic Factors. Aliment. Pharmacol. Ther. 2018, 48, 538–546. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hartjes, K.; Shah, U.; Khalili, H.; Arnell, H.; Ludvigsson, J.F. Non-Alcoholic Fatty Liver Disease in Children and Young Adults Is Associated with Increased Long-Term Mortality. J. Hepatol. 2021, 75, 1034–1041. [Google Scholar] [CrossRef]
- Tortora, R.; Capone, P.; De Stefano, G.; Imperatore, N.; Gerbino, N.; Donetto, S.; Monaco, V.; Caporaso, N.; Rispo, A. Metabolic Syndrome in Patients with Coeliac Disease on a Gluten-Free Diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-Related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisarò, F.; Astegiano, M.; et al. A Score That Verifies Adherence to a Gluten-Free Diet: A Cross-Sectional, Multicentre Validation in Real Clinical Life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef]
- Silvester, J.A.; Graff, L.A.; Rigaux, L.; Walker, J.R.; Duerksen, D.R. Symptomatic Suspected Gluten Exposure Is Common among Patients with Coeliac Disease on a Gluten-Free Diet. Aliment. Pharmacol. Ther. 2016, 44, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Biagi, F.; Andrealli, A.; Bianchi, P.I.; Marchese, A.; Klersy, C.; Corazza, G.R. A Gluten-Free Diet Score to Evaluate Dietary Compliance in Patients with Coeliac Disease. Br. J. Nutr. 2009, 102, 882–887. [Google Scholar] [CrossRef] [Green Version]
- Missbach, B.; Schwingshackl, L.; Billmann, A.; Mystek, A.; Hickelsberger, M.; Bauer, G.; König, J. Gluten-Free Food Database: The Nutritional Quality and Cost of Packaged Gluten-Free Foods. PeerJ 2015, 3, e1337. [Google Scholar] [CrossRef]
- Vergeer, L.; Franco-Arellano, B.; Tjong, G.B.; Bernstein, J.T.; L’Abbé, M.R. The Level of Processing, Nutritional Composition and Prices of Canadian Packaged Foods and Beverages with and without Gluten-Free Claims. Nutrients 2021, 13, 1183. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Slydahl, M.; Hellmann, M.; Garnweidner-Holme, L.; Lundin, K.E.A.; Henriksen, C.; Telle-Hansen, V.H. Nutritional Quality and Costs of Gluten-Free Products: A Case-Control Study of Food Products on the Norwegian Marked. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Lavriša, Ž.; Hribar, M.; Kušar, A.; Žmitek, K.; Pravst, I. Nutritional Composition of Gluten-Free Labelled Foods in the Slovenian Food Supply. Int. J. Environ. Res. Public Health 2020, 17, 8239. [Google Scholar] [CrossRef]
- Guennouni, M.; Khoudri, N.E.; Bourrhouat, A.; Hilali, A. Nutritional Quality of Gluten-Free Products in Moroccan Supermarkets and e-Commerce Platforms. Cereal Chem. 2020, 97, 912–920. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J.; American Gastroenterological Association; American Association for the Study of Liver Diseases; et al. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [CrossRef] [Green Version]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body Composition and Dietary Intakes in Adult Celiac Disease Patients Consuming a Strict Gluten-Free Diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A Comparison of the Nutritional Status between Adult Celiac Patients on a Long-Term, Strictly Gluten-Free Diet and Healthy Subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of Nutrients in Italian Children with Celiac Disease and the Role of Commercially Available Gluten-Free Products. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2013, 26, 436–444. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Weir, M.; Gougeon, L. Canadian Packaged Gluten-Free Foods Are Less Nutritious than Their Regular Gluten-Containing Counterparts. PeerJ 2018, 6, e5875. [Google Scholar] [CrossRef] [Green Version]
- Romão, B.; Botelho, R.B.A.; Alencar, E.R.; da Silva, V.S.N.; Pacheco, M.T.B.; Zandonadi, R.P. Chemical Composition and Glycemic Index of Gluten-Free Bread Commercialized in Brazil. Nutrients 2020, 12, 2234. [Google Scholar] [CrossRef]
- Brandi, G.; De Lorenzo, S.; Candela, M.; Pantaleo, M.A.; Bellentani, S.; Tovoli, F.; Saccoccio, G.; Biasco, G. Microbiota, NASH, HCC and the Potential Role of Probiotics. Carcinogenesis 2017, 38, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Triarico, S.; Dionisi, T.; Gasbarrini, G.B.; Gasbarrini, A.; Mele, M.C. The Healthy Gluten-Free Diet: Practical Tips to Prevent Metabolic Disorders and Nutritional Deficiencies in Celiac Patients. Gastroenterol. Insights 2021, 12, 166–182. [Google Scholar] [CrossRef]

| Variable | |
|---|---|
| Age (years) | 42 (28–56) |
| Female sex | 107 (81.7) |
| Body mass index (BMI) | 22.0 (19.8–24.0) |
| BMI categorization | |
| -Underweight (<18.5) | 9 (6.9) |
| -Normal weight (18.5–25) | 93 (71.0) |
| -Overweight (25–30) | 24 (18.3) |
| -Obesity (>30) | 5 (3.8) |
| Total cholesterol (mg/dL) | 196 (171–220) |
| Total cholesterol >200 mg/dL | 62 (47.3) |
| HDL cholesterol (mg/dL) | 57 (53–64) |
| HDL cholesterol low 1 | 19 (14.5) |
| Triglycerides (mg/dL) | 79 (64–98) |
| Triglycerides >150 mg/dL | 8 (6.1) |
| Diabetes | 11 (8.4) |
| AST (UI/L) | 19 (16–24) |
| AST high 2 | 3 (2.0) |
| ALT (UI/L) | 17 (13–22) |
| ALT high 2 | 10 (6.8) |
| Raw scores | Q1 | Q2 | Q3 | Q4 | Q5 |
|---|---|---|---|---|---|
| M | 2–13 | 14–18 | 19–21 | 22–31 | 32–71 |
| F | 1–15 | 16–19 | 20–23 | 24–27 | 28–52 |
| R | 1–2 | 3 | 4 | 5 | 6–9 |
| Univariate Analysis | Variable | Multivariable Analysis | ||
|---|---|---|---|---|
| Exp(B) (95% CI) | p | Exp(B) (95% CI) | p | |
| 1.050 (1.023–1.078) | <0.001 | Age (years) | 1.039 (1.007–1.072) | 0.017 |
| 0.399 (0.160–0.995) | 0.049 | Sex (F = 1) | 0.744 (0.204–2.714) | 0.654 |
| 1.294 (1.137–1.473) | <0.001 | Body mass index | 1.228 (1.053–1.432) | 0.009 |
| 1.013 (1.002–1.024) | 0.023 | Total cholesterol (mg/dL) | 1.012 (0.998–1.027) | 0.099 |
| 0.963 (0.932–0.996) | 0.028 | HDL cholesterol (mg/dL) | 0.996 (0.986–1.005) | 0.375 |
| 1.034 (1.016–1.051) | <0.001 | Triglycerides (mg(dL) | 1.030 (1.010–1.050) | 0.003 |
| 2.197 (0.628–7.688) | 0.218 | Diabetes (No = 0, Yes = 1) | - | - |
| 1.080 (1.019–1.144) | 0.009 | AST (UI/L) * | - | - |
| 1.046 (1.010–1.085) | 0.013 | ALT (UI/L) | 1.041 (0.970–1.017) | 0.266 |
| 1.166 (1.030–1.321) | 0.016 | RFM score (units) | 1.242 (1.057–1.459) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiteri, A.; Granito, A.; Faggiano, C.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Hepatic Steatosis in Patients with Celiac Disease: The Role of Packaged Gluten-Free Foods. Nutrients 2022, 14, 2942. https://doi.org/10.3390/nu14142942
Raiteri A, Granito A, Faggiano C, Giamperoli A, Catenaro T, Negrini G, Tovoli F. Hepatic Steatosis in Patients with Celiac Disease: The Role of Packaged Gluten-Free Foods. Nutrients. 2022; 14(14):2942. https://doi.org/10.3390/nu14142942
Chicago/Turabian StyleRaiteri, Alberto, Alessandro Granito, Chiara Faggiano, Alice Giamperoli, Teresa Catenaro, Giulia Negrini, and Francesco Tovoli. 2022. "Hepatic Steatosis in Patients with Celiac Disease: The Role of Packaged Gluten-Free Foods" Nutrients 14, no. 14: 2942. https://doi.org/10.3390/nu14142942