Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Quantitative and Qualitative Characteristics of the Mother–Child Dyad
3.2. Maternal Anthropometric Characteristics and Bacterial Phyla in the Human Milk
3.3. Comparison of the Medians of the Percentages of Bacterial Phyla in Human Milk According to the Maternal Anthropometric Indices and Indicators
3.4. Correlations and Explained Values between Bacterial Phyla of Human Milk and Maternal Anthropometric Variables
3.5. Multiple Linear Regressions (Stepwise Method) Were Performed with the Anthropometric Variables That Showed Important Correlations with the Percentages of Bacterial Phyla
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human milk microbiota profiles in relation to Birthing Method, Gestation and Infant Gender. Microbiome 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and Next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.V.; Leonard, M.M.; Fiechtner, L.; Fasano, A. Transitioning from Descriptive to Mechanistic Understanding of the Microbiome: The Need for a Prospective Longitudinal Approach to Predicting Disease. J. Pediatr. 2016, 179, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Martos, I.; Jiménez, E.; de Andrés, J.; Rodríguez-Alcalá, L.M.; Tavárez, S.; Manzano, S.; Fernández, L.; Alonso, E.; Fontecha, J.; Rodríguez, J. Milk and blood biomarkers associated to the clinical efficacy of a probiotic for the treatment of infectious mastitis. Benef. Microbes 2016, 7, 305–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togo, A.; Dufour, J.; Lagier, J.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of human breast and milk microbiota: A systematic review. Future Microbiol. 2019, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical Mother-Neonate Transfer of Maternal Gut Bacteria via Breastfeeding. Environ. Microbiol. 2013, 16, 2891–2904. [Google Scholar] [CrossRef]
- Rodriguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Osorio, L.M.; Umbarila, A.S. Microbiota de la glándula mamaria. Pediatría 2015, 48, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Olivares, M.; Boza, J.; Jiménez, J.; Fernández, L.; Xaus, J.; et al. The Commensal Microflora of Human Milk: New Perspectives for Food Bacteriotherapy and Probiotics. Trends Food Sci. Technol. 2004, 15, 121–127. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between Milk Microbiota, Bacterial Load, Macronutrients, and Human Cells during Lactation. Front. Microbiol. 2016, 7, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Rubio, R.; Mira-Pascual, L.; Mira, A.; Collado, M.C. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health Dis. 2015, 7, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avershina, E.; Angell, I.L.; Simpson, M.; Storrø, O.; Øien, T.; Johnsen, R.; Rudi, K. Low Maternal Microbiota Sharing across Gut, Breast Milk and Vagina, as Revealed by 16S rRNA Gene and Reduced Metagenomic Sequencing. Genes 2018, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Monks, J.; Orlicky, D.; Stefanski, A.L.; Libby, A.E.; Bales, E.S.; Rudolph, M.C.; Johnson, G.C.; Sherk, V.D.; Jackman, M.R.; Williamson, K.; et al. Maternal obesity during lactation may protect offspring from high fat diet-induced metabolic dysfunction. Nutr. Diabetes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Gordon-Larsen, P.; Adair, L.S.; Suchindran, C.M. Maternal obesity is associated with younger age at obesity onset in U.S. adolescent offspring followed into adulthood. Obesity 2007, 15, 2790–2796. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal Weight and Excessive Weight Gain during Pregnancy Modify the Immunomodulatory Potential of Breast Milk. Pediatr. Res. 2012, 72, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mantrana, I.; Collado, M.C. Obesity and overweight: Impact on maternal and milk microbiome and their role for infant health and nutrition. Mol. Nutr. Food Res. 2016, 60, 1865–1875. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The Composition of Human Milk and Infant Faecal Microbiota over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [Green Version]
- Diario Oficial de la Federación. NORMA Oficial Mexicana NOM-043-SSA2-2012, Servicios Básicos de Salud. Promoción y Educación Para la Salud en Materia Alimentaria. Criterios Para Brindar Orientación. 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5285372&fecha=22/01/2013#gsc.tab=0 (accessed on 8 February 2022).
- Marfell-Jones, M.J.; Stewart, A.D.; de Ridder, J.H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011; pp. 26–68. ISBN 0620362073. [Google Scholar]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; The National Academies Collection—Reports funded by National Institutes of Health. In Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M.; Yaktine, E.L. (Eds.) National Academies Press: Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32801/ (accessed on 25 May 2022).
- Gallagher, D.; Heymsfield, S.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Siri, W. Body Composition from Fluid Spaces and Density: Analysis of Methods. 1961. Nutrition 1993, 9, 480–492. [Google Scholar] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 1, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct Patterns in Human Milk Microbiota and Fatty Acid Profiles across Specific Geographic Locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlesfsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct Composition of Gut Microbiota during Pregnancy in Overweight and Normal-Weight Women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- den-Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Avellaneda-Guimarães, R.C.; Hiane, P.A.; Bogo, D.; Zorgetto-Pinheiro, V.A.; Silva-de Oliveira, L.C.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- Rial, S.A.; Karelis, A.D.; Bergeron, K.; Mounier, C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients 2016, 8, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indian, C.; Rizzardi, K.; Castelo, P.; Ferraz, L.; Darrieux, M.; Parisotto, T. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; et al. What’s Normal? Microbiomes in Human Milk and Infant Feces Are Related to Each Other but Vary Geographically: The INSPIRE Study. Front. Nutr. 2019, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Marín-Gómez, W.; Grande, M.J.; Pérez-Pulido, R.; Galvez, A.; Lucas, R. Changes in the Bacterial Diversity of Human Milk during Late Lactation Period (Weeks 21 to 48). Foods 2020, 279, 1184. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Alba, C.; García-Carral, C.; Jiménez, E.A.; Lackey, K.A.; McGuire, M.K.; Meehan, C.L.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; et al. Comparison of Two Approaches for the Metataxonomic Analysis of the Human Milk Microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 622550. [Google Scholar] [CrossRef] [PubMed]
- Diario Oficial de la Federación. NORMA Oficial Mexicana NOM-087-ECOL-SSA1-2002, Protección Ambiental-Salud Ambiental-Residuos Peligrosos Biológicoinfecciosos—Clasificación y Especificaciones de Manejo. 2002. Available online: https://www.cndh.org.mx/DocTR/2016/JUR/A70/01/JUR-20170331-NOR14.pdf (accessed on 8 February 2022).
| Variable | Median | IQR 25–75 |
|---|---|---|
| Percentage of Firmicutes (%) | 9.0 | 5.12–18.8 |
| Percentage of Bacteroidetes (%) | 4.4 | 1.9–13.8 |
| Percentage of Actinobacteria (%) | 2.4 | 1–5.4 |
| Percentage of others (%) | 73.5 | 56.3–86.7 |
| F/B ratio 1 | 2.07 | 0.33–5.4 |
| Variable | Median | IQR 25–75 |
|---|---|---|
| Prepregnancy weight (kg) | 62 | 55–70.6 |
| Prepregnancy BMI (kg/m2) | 24.7 | 21.6–27.3 |
| Weight gain during pregnancy (kg) | 12 | 9–14.6 |
| Weight (kg) | 64.7 | 57.3–73.2 |
| Height (m) | 1.60 | 1.5–1.6 |
| Waist circumference (cm) | 81.3 | 76.8–87.7 |
| MUAC (cm) | 28.3 | 26.5–31.8 |
| TSF (mm) | 24.5 | 16–29.2 |
| SSF (mm) | 21 | 15.7–26.5 |
| BSF (mm) | 15 | 11.6–22 |
| SISF (mm) | 24 | 14.7–30.2 |
| BMI (Kg/m2) | 24.9 | 22.8–28.6 |
| Total body fat per BIA 1 (kg) | 22.2 | 18.1–30.2 |
| Fat percentage by BIA 1 (%) | 34.9 | 30.4–38.7 |
| Lean mass per BIA 1 (kg) | 40.8 | 38–44.3 |
| Lean mass percentage by BIA 1 (%) | 65 | 61.1–69.6 |
| Fat percentage calculated with SIRI 2 (%) | 36.4 | 30.9–39.1 |
| Phyla (%) | n | r | R2 | p | |||
|---|---|---|---|---|---|---|---|
| Anthropometric Variables | Median | IQR (25–75) | |||||
| Firmicutes | 24 | Weight gain in pregnancy > Recommended | 14.3 | 12–17 | −0.399 1 | 0.159 | 0.054 |
| 60 | BSF ≤ 1 DE | 14 | 10–18 | 0.190 1 | 0.036 | 0.150 | |
| Bacteroidetes | 37 | Pregestational BMI ≤ 24.9 kg/m2 | 22 | 19.6–23.6 | 0.238 2 | 0.056 | 0.168 |
| 33 | Pregestational BMI ≥ 25 kg/m2 | 28 | 25.8–31.3 | −0.402 1 | 0.162 | 0.023 | |
| 36 | Current BMI ≤ 24.9 kg/m2 | 22.8 | 21–23.9 | 0.375 2 | 0.140 | 0.029 | |
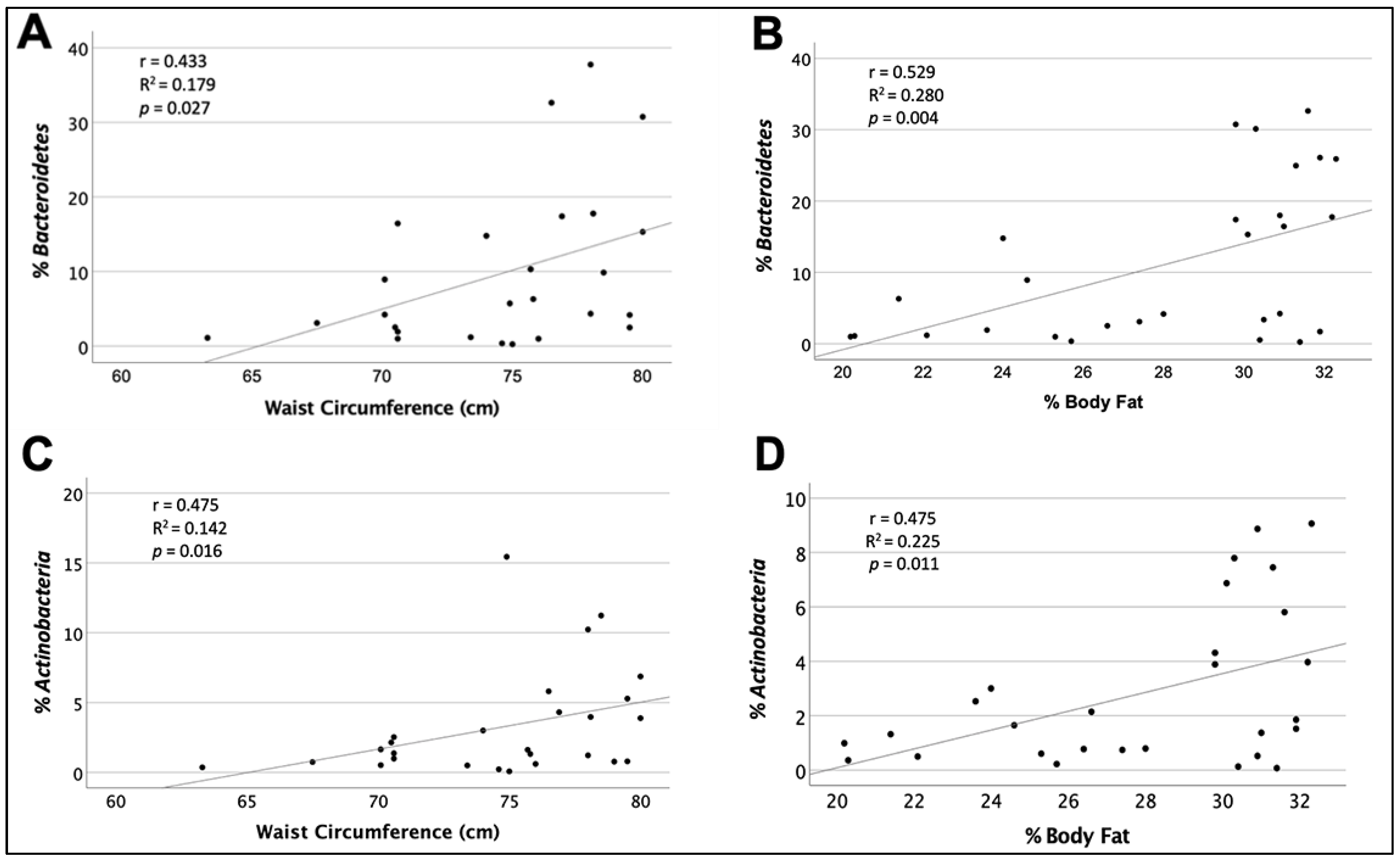
| 27 | Waist circumference ≤80 cm | 75.8 | 70.6–78 | 0.433 1 | 0.187 | 0.027 | |
| 43 | Waist circumference >80 cm | 86 | 81.7–91.6 | −0.210 2 | 0.044 | 0.187 | |
| 29 | Percentage of fat ≤33% | 29.8 | 25–31.2 | 0.529 2 | 0.280 | 0.004 | |
| 41 | Percentage of fat >33% | 38.4 | 36.6–38.4 | −0.054 2 | 0.003 | 0.742 | |
| 7 | TSF >1 DE | 37.1 | 34–42 | 0.575 2 | 0.330 | 0.177 | |
| 11 | BSF >1 DE | 26.5 | 25–34.5 | 0.693 1 | 0.480 | 0.026 | |
| 11 | SSF >1 DE | 31 | 31–39 | 0.893 2 | 0.797 | <0.001 | |
| Actinobacteria | 37 | Pregestational BMI ≤ 24.9 kg/m2 | 22 | 19.6–23.6 | −0.228 2 | 0.052 | 0.188 |
| 36 | Current BMI ≤ 24.9 kg/m2 | 22.8 | 21–23.9 | 0.344 2 | 0.118 | 0.047 | |
| 27 | Waist circumference ≤80 cm | 75.8 | 70.6–78 | 0.457 2 | 0.208 | 0.016 | |
| 29 | Percentage of fat ≤33 % | 29.8 | 25–31.2 | 0.475 2 | 0.226 | 0.011 | |
| 63 | TSF ≤1 DE | 23 | 16–28 | 0.252 1 | 0.063 | 0.052 | |
| 59 | BSF ≤1 DE | 14 | 10–18 | 0.255 2 | 0.065 | 0.054 | |
| 10 | BSF >1 DE | 26.5 | 25–34.5 | 0.820 2 | 0.672 | 0.004 | |
| 56 | SISF≤1 DE | 19 | 12.3–25.8 | 0.303 2 | 0.092 | 0.026 | |
| F/B Ratio | 27 | Waist circumference ≤80 cm | 75.8 | 70.6–78 | 0.299 2 | 0.089 | 0.139 |
| 11 | BSF >1 DE | 26.5 | 25–34.5 | 0.517 1 | 0.267 | 0.126 | |
| 11 | SSF >1 DE | 31 | 31–39 | 0.478 1 | 0.228 | 0.137 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavoya-Guardado, M.A.; Vasquez-Garibay, E.M.; Ruiz-Quezada, S.L.; Ramírez-Cordero, M.I.; Larrosa-Haro, A.; Castro-Albarran, J. Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity. Nutrients 2022, 14, 2887. https://doi.org/10.3390/nu14142887
Chavoya-Guardado MA, Vasquez-Garibay EM, Ruiz-Quezada SL, Ramírez-Cordero MI, Larrosa-Haro A, Castro-Albarran J. Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity. Nutrients. 2022; 14(14):2887. https://doi.org/10.3390/nu14142887
Chicago/Turabian StyleChavoya-Guardado, Martha Alejandra, Edgar Manuel Vasquez-Garibay, Sandra Luz Ruiz-Quezada, María Inés Ramírez-Cordero, Alfredo Larrosa-Haro, and Jorge Castro-Albarran. 2022. "Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity" Nutrients 14, no. 14: 2887. https://doi.org/10.3390/nu14142887
APA StyleChavoya-Guardado, M. A., Vasquez-Garibay, E. M., Ruiz-Quezada, S. L., Ramírez-Cordero, M. I., Larrosa-Haro, A., & Castro-Albarran, J. (2022). Firmicutes, Bacteroidetes and Actinobacteria in Human Milk and Maternal Adiposity. Nutrients, 14(14), 2887. https://doi.org/10.3390/nu14142887






