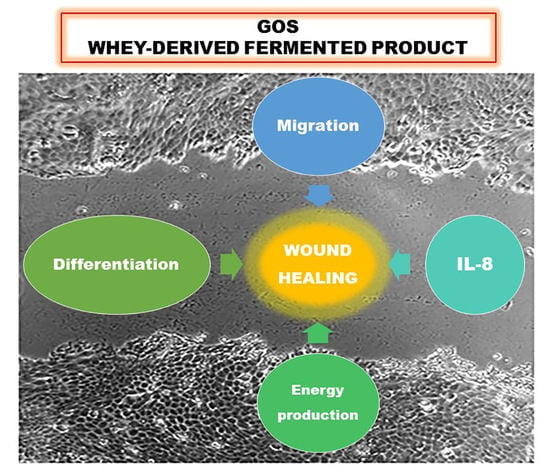
Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Preparation of Whey Derivatives
2.3. Cell Treatments
2.4. Cell Proliferation Assay
2.5. Cell Viability Assays
2.6. Wound Healing Assay
2.7. Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
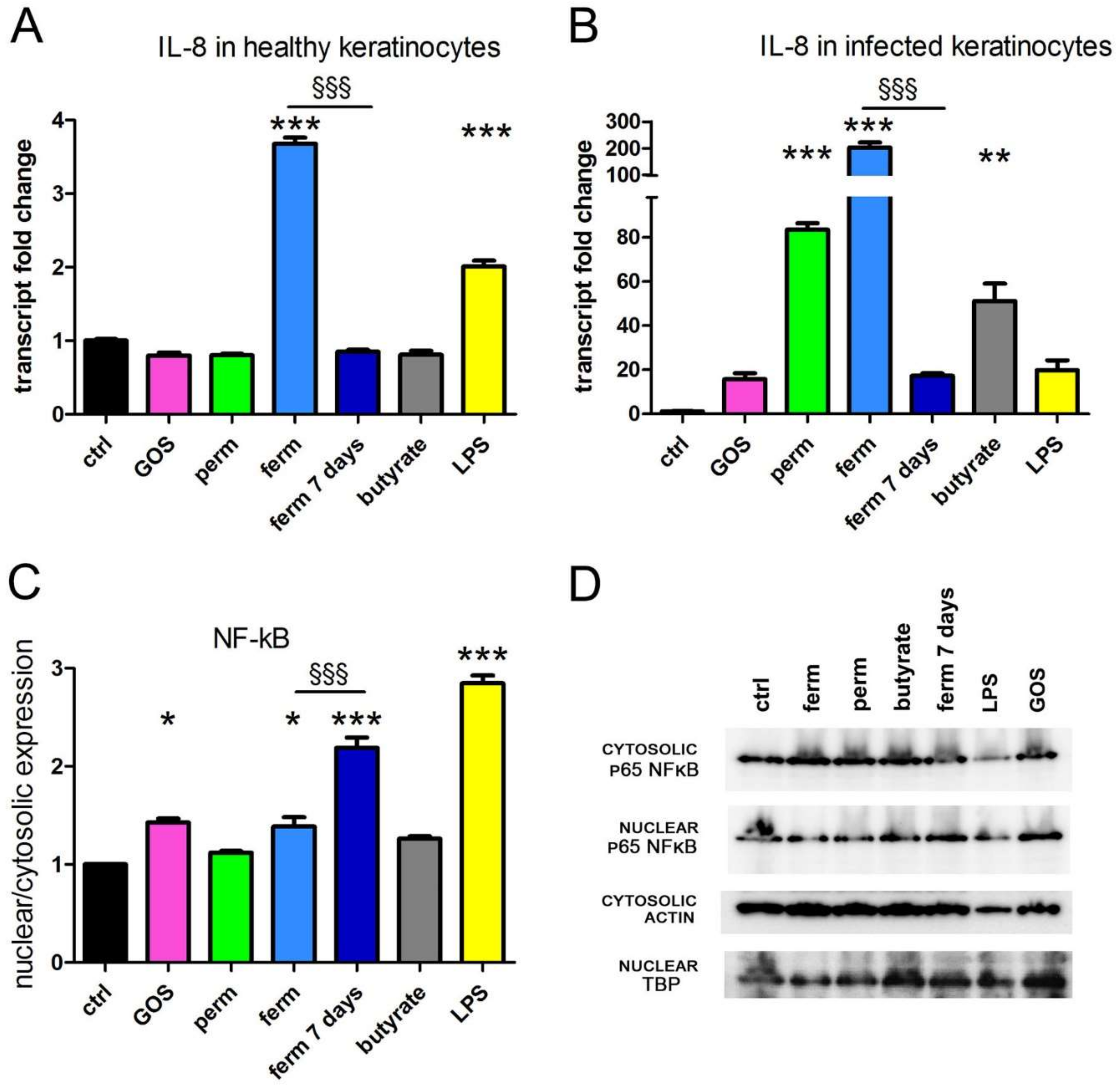
3.1. GOS Induce the Production of IL-8 through NF-kB Signaling
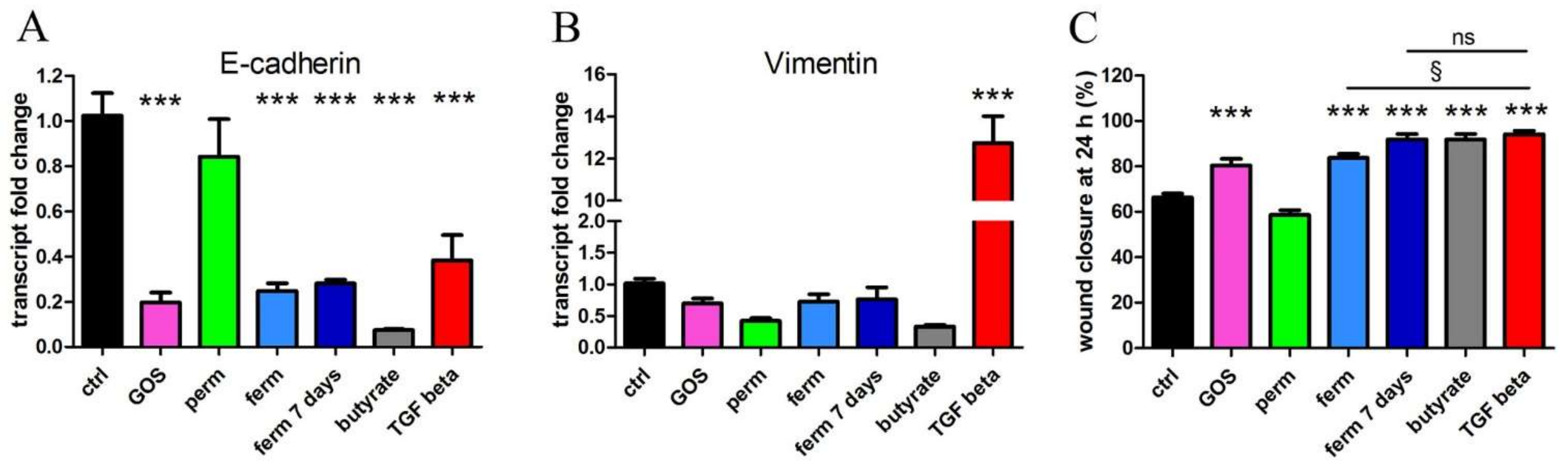
3.2. Whey Derivatives and GOS Increase the Motility of HaCaT Cells
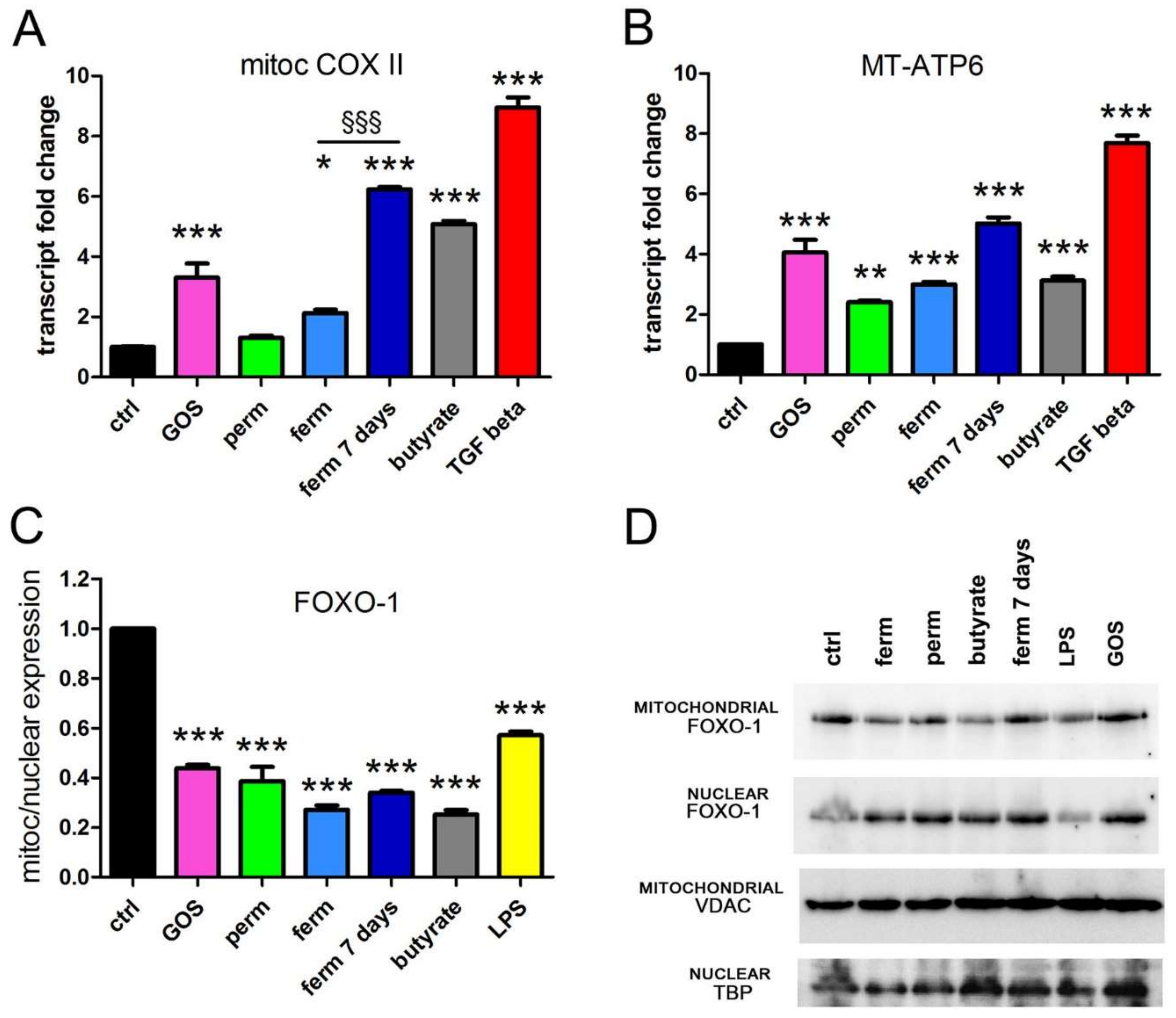
3.3. GOS Enhance Mitochondrial Activity in Association with Mitochondrial Export of FOXO-1
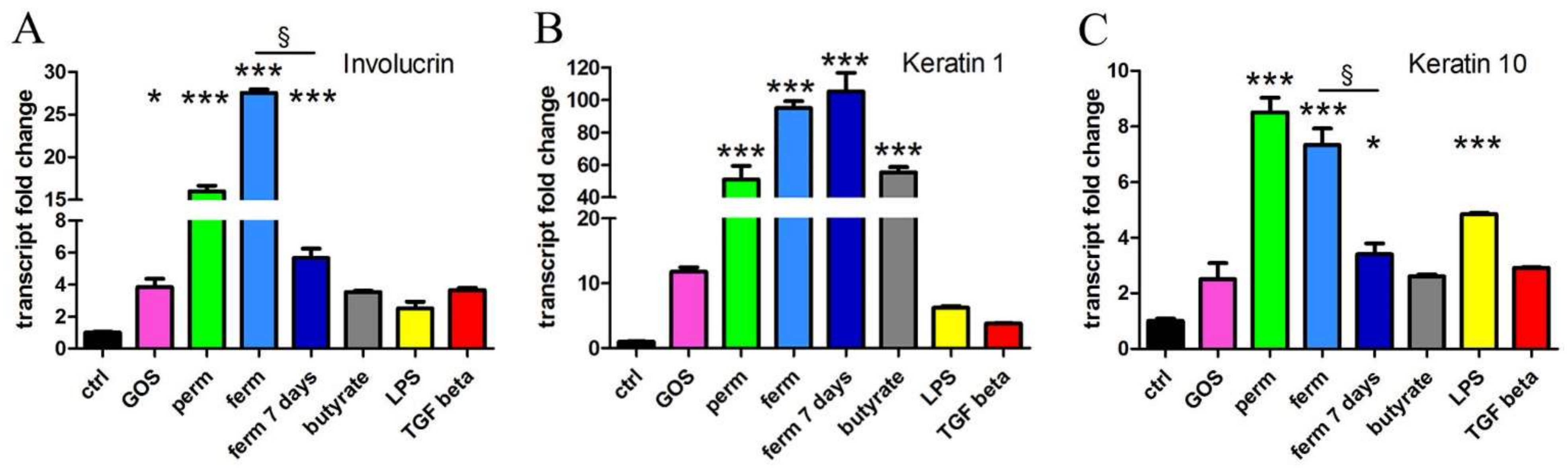
3.4. Whey Derivatives and GOS Increase the Differentiation of HaCaT Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, D.P.M.; do Pilar, F.; Gonçalves, M.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ko, J.S.; Leone, S.; Nanthakumar, N.N. Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6′-Galactosyllactose and Attenuate Inflammation in Human T84, NCM-460, and H4 Cells and Intestinal Tissue Ex Vivo. J. Nutr. 2016, 146, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; Difilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing Microbiota-Independent Effects of Oligosaccharides on Intestinal Epithelial Cells: Insight into the Role of Structure and Size: Structure-Activity Relationships of Non-Digestible Oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef]
- Bhatia, S.; Prabhu, P.N.; Benefiel, A.C.; Miller, M.J.; Chow, J.; Davis, S.R.; Gaskins, H.R. Galacto-Oligosaccharides May Directly Enhance Intestinal Barrier Function through the Modulation of Goblet Cells. Mol. Nutr. Food Res. 2015, 59, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Ocón, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Nondigestible Oligosaccharides Exert Nonprebiotic Effects on Intestinal Epithelial Cells Enhancing the Immune Response via Activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Ling, T.; Zhao, C.; Li, Y.; Geng, M.; Gai, S.; Qi, W.; Luo, X.; Chen, L.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-ΚB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics- a Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- SanMiguel, A.; Grice, E.A. Interactions between Host Factors and the Skin Microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. The Skin-Resident and Migratory Immune System in Steady State and Memory: Innate Lymphocytes, Dendritic Cells and T Cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef]
- Portou, M.J.; Baker, D.; Abraham, D.; Tsui, J. The Innate Immune System, Toll-like Receptors and Dermal Wound Healing: A Review. Vasc. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-H.; Staudt, L.M. Toll-like Receptor Signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-N.; Li, M. The Immune Function of Keratinocytes in Anti-Pathogen Infection in the Skin. Int. J. Dermatol. Venereol. 2020, 03, 231–238. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, F.; Zhang, Q.; Huang, X.; Wang, Z. Autophagy and Skin Wound Healing. Burn. Trauma 2022, 10, tkac003. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory Cytokines Regulate Epidermal Stem Cells in Wound Epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Gloushankova, N.A.; Rubtsova, S.N.; Zhitnyak, I.Y. Cadherin-Mediated Cell-Cell Interactions in Normal and Cancer Cells. Tissue Barriers 2017, 5, e1356900. [Google Scholar] [CrossRef] [PubMed]
- Sou, P.W.; Delic, N.C.; Halliday, G.M.; Lyons, J.G. Snail Transcription Factors in Keratinocytes: Enough to Make Your Skin Crawl. Int. J. Biochem. Cell Biol. 2010, 42, 1940–1944. [Google Scholar] [CrossRef]
- Domingos, P.L.B.; Souza, M.G.; Guimarães, T.A.; Santos, E.S.; Farias, L.C.; de Carvalho Fraga, C.A.; Jones, K.M.; Santos, S.H.S.; de Paula, A.M.B.; Guimarães, A.L.S. Hypoxia Reduces the E-Cadherin Expression and Increases OSCC Cell Migration Regardless of the E-Cadherin Methylation Profile. Pathol. Res. Pract. 2017, 213, 496–501. [Google Scholar] [CrossRef]
- Das, V.; Bhattacharya, S.; Chikkaputtaiah, C.; Hazra, S.; Pal, M. The Basics of Epithelial–Mesenchymal Transition (EMT): A Study from a Structure, Dynamics, and Functional Perspective. J. Cell. Physiol. 2019, 234, 14535–14555. [Google Scholar] [CrossRef]
- Golowczyc, M.; Vera, C.; Santos, M.; Guerrero, C.; Carasi, P.; Illanes, A.; Gómez-Zavaglia, A.; Tymczyszyn, E. Use of Whey Permeate Containing in Situ Synthesised Galacto-Oligosaccharides for the Growth and Preservation of Lactobacillus Plantarum. J. Dairy Res. 2013, 80, 374–381. [Google Scholar] [CrossRef]
- Vera, C.; Guerrero, C.; Conejeros, R.; Illanes, A. Synthesis of Galacto-Oligosaccharides by β-Galactosidase from Aspergillus Oryzae Using Partially Dissolved and Supersaturated Solution of Lactose. Enzym. Microb. Technol. 2012, 50, 188–194. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Rai, P.; Duke, M. Cheese Whey to Biohydrogen and Useful Organic Acids: A Non-Pathogenic Microbial Treatment by L. Acidophilus. Sci. Rep. 2019, 9, 8320. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.-H.; Kim, I.S.; Yu, D.Y.; Kim, S.C.; Lee, S.H.; Lee, S.S.; Yun, C.-H.; Choi, I.S.; Cho, K.K. Anti-Inflammatory Effects of a Mixture of Lactic Acid Bacteria and Sodium Butyrate in Atopic Dermatitis Murine Model. J. Med. Food 2018, 21, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Hamza, A.A.; Daoud, S.; Hamza, W. Spirulina Protects against Cadmium-Induced Hepatotoxicity in Rats. Am. J. Pharmacol. Toxicol. 2006, 1, 21–25. [Google Scholar] [CrossRef]
- Abdalla, A.; Murali, C.; Amin, A. Safranal Inhibits Angiogenesis via Targeting HIF-1α/VEGF Machinery: In Vitro and Ex Vivo Insights. Front. Oncol. 2021, 11, 789172. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Mahmoud-Ghoneim, D.; Adeghate, E.; Al-Akhras, M.A.; Al-Saadi, M.; Al-Rahmoun, S.; Hameed, R. Pancreas-Protective Effects of Chlorella in STZ-Induced Diabetic Animal Model: Insights into the Mechanism. J. Diabetes Mellit. 2011, 1, 36–45. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Murali, C.; Madhoon, A.A.; Amin, A. Salvadora Persica (Miswak): Antioxidant and Promising Antiangiogenic Insights. Am. J. Plant Sci. 2018, 9, 1228–1244. [Google Scholar] [CrossRef][Green Version]
- Al-Shamsi, M.; Amin, A.; Adeghate, E. Effect of Vitamin C on Liver and Kidney Functions in Normal and Diabetic Rats. Ann. N. Y. Acad. Sci. 2006, 1084, 371–390. [Google Scholar] [CrossRef]
- Juaid, N.; Amin, A.; Abdalla, A.; Reese, K.; Alamri, Z.; Moulay, M.; Abdu, S.; Miled, N. Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights. Int. J. Mol. Sci. 2021, 22, 10774. [Google Scholar] [CrossRef]
- Fauquant, J.; Vieco, E.; Brule, G.; Maubois, J.-L. Clarification des lactosérums doux par agrégation thermocalcique de la matière grasse résiduelle. Lait 1985, 65, 1–20. [Google Scholar] [CrossRef]
- Pereira, C.D.; Diaz, O.; Cobos, A. Valorization of By-Products from Ovine Cheese Manufacture: Clarification by Thermocalcic Precipitation/Microfiltration before Ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile Organic Compounds Profile during Milk Fermentation by Lactobacillus Pentosus and Correlations between Volatiles Flavor and Carbohydrate Metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Pati, M.L.; Hornick, J.R.; Niso, M.; Berardi, F.; Spitzer, D.; Abate, C.; Hawkins, W. Sigma-2 Receptor Agonist Derivatives of 1-Cyclohexyl-4-[3-(5-Methoxy-1,2,3,4-Tetrahydronaphthalen-1-Yl)Propyl]Piperazine (PB28) Induce Cell Death via Mitochondrial Superoxide Production and Caspase Activation in Pancreatic Cancer. BMC Cancer 2017, 17, 51. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Viano, M.; Bergandi, L.; Aldieri, E.; Silvagno, F. Vitamin D Inhibits the Epithelial-Mesenchymal Transition by a Negative Feedback Regulation of TGF-β Activity. J. Steroid Biochem. Mol. Biol. 2019, 187, 97–105. [Google Scholar] [CrossRef]
- Bergandi, L.; Mungo, E.; Morone, R.; Bosco, O.; Rolando, B.; Doublier, S. Hyperglycemia Promotes Chemoresistance Through the Reduction of the Mitochondrial DNA Damage, the Bax/Bcl-2 and Bax/Bcl-XL Ratio, and the Cells in Sub-G1 Phase Due to Antitumoral Drugs Induced-Cytotoxicity in Human Colon Adenocarcinoma Cells. Front. Pharmacol. 2018, 9, 866. [Google Scholar] [CrossRef]
- Silvagno, F.; Consiglio, M.; Foglizzo, V.; Destefanis, M.; Pescarmona, G. Mitochondrial Translocation of Vitamin D Receptor Is Mediated by the Permeability Transition Pore in Human Keratinocyte Cell Line. PLoS ONE 2013, 8, e54716. [Google Scholar] [CrossRef]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 Gene Expression and Production in Human Keratinocytes and Their Modulation by UVB. J. Investig. Dermatol. 1993, 101, 690–694. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 Chemokine Family and Its Receptors in Inflammatory Diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Lanao, A.E.; Chakraborty, R.K.; Pearson-Shaver, A.L. Mycoplasma Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Arabatzis, M.; Velegraki, A. Evidence for the Presence of a Human Saprophytic Oral Bacterium, Mycoplasma Faucium, in the Skin Lesions of a Psoriatic Patient. J. Cutan. Pathol. 2022, 49, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.W.; Gally, F.; Thaikoottathil, J.; Janssen-Heininger, Y.M.; Wu, Q.; Zhang, G.; Reisdorph, N.; Case, S.; Minor, M.; Smith, S.; et al. SPLUNC1 Regulation in Airway Epithelial Cells: Role of Toll-like Receptor 2 Signaling. Respir. Res. 2010, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.A.; Chang, T.-H.; Winter, V.T.; Coalson, J.J.; Cagle, M.P.; Pandranki, L.; Bose, S.; Baseman, J.B.; Kannan, T.R. NLRP3 Is a Critical Regulator of Inflammation and Innate Immune Cell Response during Mycoplasma Pneumoniae Infection. Infect. Immun. 2018, 86, e00548-17. [Google Scholar] [CrossRef] [PubMed]
- Fiz, C.; Apprato, G.; Ricca, C.; Aillon, A.; Bergandi, L.; Silvagno, F. TGF Beta Induces Vitamin D Receptor and Modulates Mitochondrial Activity of Human Pancreatic Cancer Cells. Cancers 2021, 13, 2932. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Ioannilli, L.; Aquilano, K.; Ciccarone, F.; Rosina, M.; Ciriolo, M.R. FoxO1 Localizes to Mitochondria of Adipose Tissue and Is Affected by Nutrient Stress. Metabolism 2019, 95, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, G.; Moschidis, A.; Ortiz, J.; Benakanakere, M.R.; Kinane, D.F.; Graves, D.T.P. Gingivalis Modulates Keratinocytes through FOXO Transcription Factors. PLoS ONE 2013, 8, e78541. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masuoka, N.; Kano, M.; Iizuka, R. Bifidobacterium Fermented Milk and Galacto-Oligosaccharides Lead to Improved Skin Health by Decreasing Phenols Production by Gut Microbiota. Benef. Microbes 2014, 5, 121–128. [Google Scholar] [CrossRef]
- Kano, M.; Masuoka, N.; Kaga, C.; Sugimoto, S.; Iizuka, R.; Manabe, K.; Sone, T.; Oeda, K.; Nonaka, C.; Miyazaki, K.; et al. Consecutive Intake of Fermented Milk Containing Bifidobacterium Breve Strain Yakult and Galacto-Oligosaccharides Benefits Skin Condition in Healthy Adult Women. Biosci. Microbiot. Food Health 2013, 32, 33–39. [Google Scholar] [CrossRef]
- Murali, C.; Mudgil, P.; Gan, C.-Y.; Tarazi, H.; El-Awady, R.; Abdalla, Y.; Amin, A.; Maqsood, S. Camel Whey Protein Hydrolysates Induced G2/M Cellcycle Arrest in Human Colorectal Carcinoma. Sci. Rep. 2021, 11, 7062. [Google Scholar] [CrossRef]
- Kennedy-Crispin, M.; Billick, E.; Mitsui, H.; Gulati, N.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.M.; Suárez-Fariñas, M.; Krueger, J.G. Human Keratinocytes’ Response to Injury Upregulates CCL20 and Other Genes Linking Innate and Adaptive Immunity. J. Investig. Dermatol. 2012, 132, 105–113. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of Interleukin-8 (IL-8) and IL-8 Receptors on the Migration of Human Keratinocytes, the Role of PLC-γ and Potential Clinical Implications. Exp. Ther. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chatterjee, M.P. A Review of the Influence of Growth Factors and Cytokines in in Vitro Human Keratinocyte Migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, M.; Jiang, P.; Sun, N.; Lin, S. Peptides Derived from Sea Cucumber Accelerate Cells Proliferation and Migration for Wound Healing by Promoting Energy Metabolism and Upregulating the ERK/AKT Pathway. Eur. J. Pharmacol. 2022, 921, 174885. [Google Scholar] [CrossRef]
- Green, A.; Hossain, T.; Eckmann, D.M. Hyperbaric Oxygen Alters Intracellular Bioenergetics Distribution in Human Dermal Fibroblasts. Life Sci. 2021, 278, 119616. [Google Scholar] [CrossRef]
- Ioannilli, L.; Ciccarone, F.; Ciriolo, M.R. Adipose Tissue and FoxO1: Bridging Physiology and Mechanisms. Cells 2020, 9, 849. [Google Scholar] [CrossRef]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 Promotes Wound Healing through the Up-Regulation of TGF-Β1 and Prevention of Oxidative Stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Li, Y.; Zhang, X. The Functions of FoxO Transcription Factors in Epithelial Wound Healing. Australas. J. Dermatol. 2019, 60, 105–109. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, Z.; Yu, Y.; Qu, H.; Koch, M.; Aumailley, M. Integrin A3 Subunit Regulates Events Linked to Epithelial Repair, Including Keratinocyte Migration and Protein Expression. Wound Repair Regen. 2010, 18, 325–334. [Google Scholar] [CrossRef]
- Weng, Q.; Liu, Z.; Li, B.; Liu, K.; Wu, W.; Liu, H. Oxidative Stress Induces Mouse Follicular Granulosa Cells Apoptosis via JNK/FoxO1 Pathway. PLoS ONE 2016, 11, e0167869. [Google Scholar] [CrossRef]
- Shen, B.; Chao, L.; Chao, J. Pivotal Role of JNK-Dependent FOXO1 Activation in Downregulation of Kallistatin Expression by Oxidative Stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1048–H1054. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.-F.; Chan, E.C.; Liu, G.-S. Differentiation of Human Adipose-Derived Stem Cells into Fat Involves Reactive Oxygen Species and Forkhead Box O1 Mediated Upregulation of Antioxidant Enzymes. Stem Cells Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Li, X.; Yan, Q.; Ma, J.; Jiang, Z. Curdlan (Alcaligenes Faecalis) (1→3)-β-d-Glucan Oligosaccharides Drive M1 Phenotype Polarization in Murine Bone Marrow-Derived Macrophages via Activation of MAPKs and NF-ΚB Pathways. Molecules 2019, 24, 4251. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergandi, L.; Flutto, T.; Valentini, S.; Thedy, L.; Pramotton, R.; Zenato, S.; Silvagno, F. Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways. Nutrients 2022, 14, 2888. https://doi.org/10.3390/nu14142888
Bergandi L, Flutto T, Valentini S, Thedy L, Pramotton R, Zenato S, Silvagno F. Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways. Nutrients. 2022; 14(14):2888. https://doi.org/10.3390/nu14142888
Chicago/Turabian StyleBergandi, Loredana, Tania Flutto, Sabina Valentini, Laura Thedy, Rita Pramotton, Simona Zenato, and Francesca Silvagno. 2022. "Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways" Nutrients 14, no. 14: 2888. https://doi.org/10.3390/nu14142888
APA StyleBergandi, L., Flutto, T., Valentini, S., Thedy, L., Pramotton, R., Zenato, S., & Silvagno, F. (2022). Whey Derivatives and Galactooligosaccharides Stimulate the Wound Healing and the Function of Human Keratinocytes through the NF-kB and FOXO-1 Signaling Pathways. Nutrients, 14(14), 2888. https://doi.org/10.3390/nu14142888