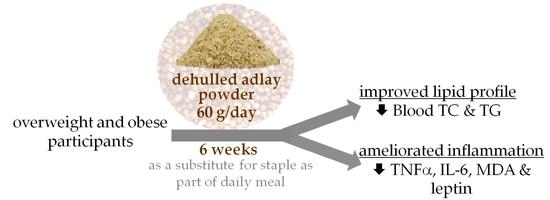
Consumption of Dehulled Adlay Improved Lipid Metabolism and Inflammation in Overweight and Obese Individuals after a 6-Week Single-Arm Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Dietary Record and Nutrients Analysis
2.4. Anthropometric Measurements
2.5. Blood Sampling and Biochemical Analysis
2.6. Blood Inflammatory Biomarkers Analysis
2.7. Statistical Analysis
3. Results
3.1. Diet Composition and Anthropometric Measurements
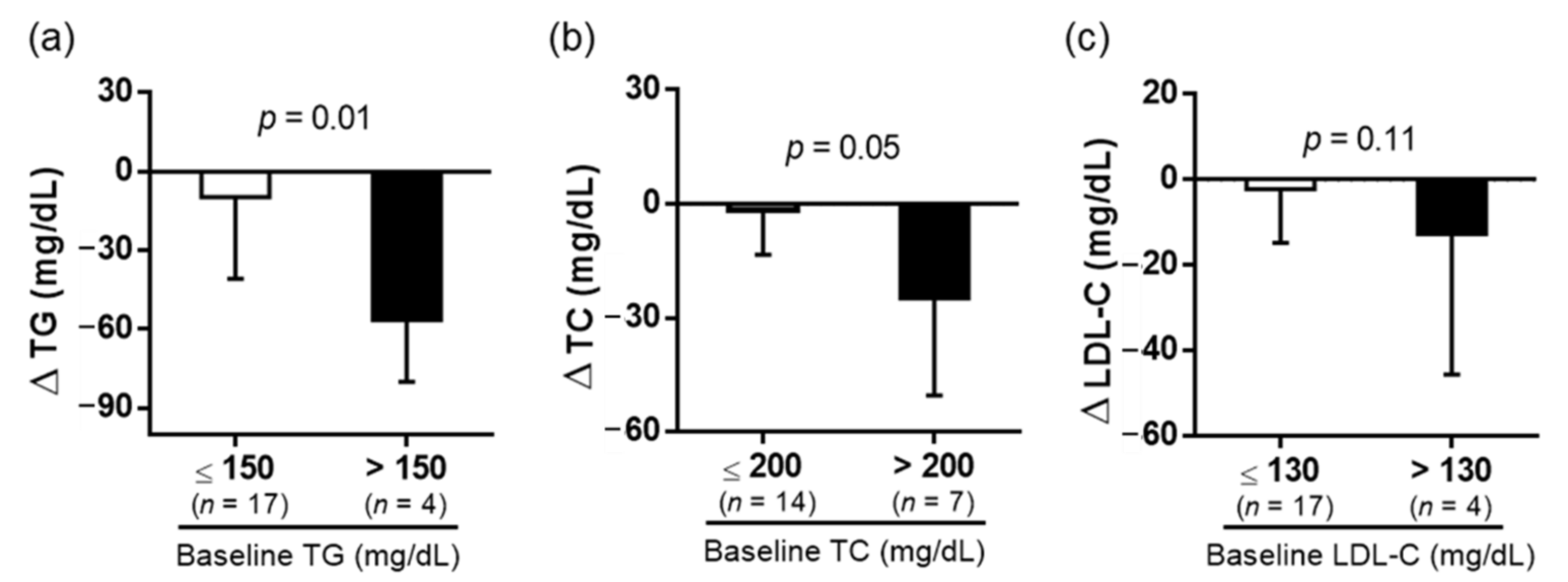
3.2. Biochemical Analysis
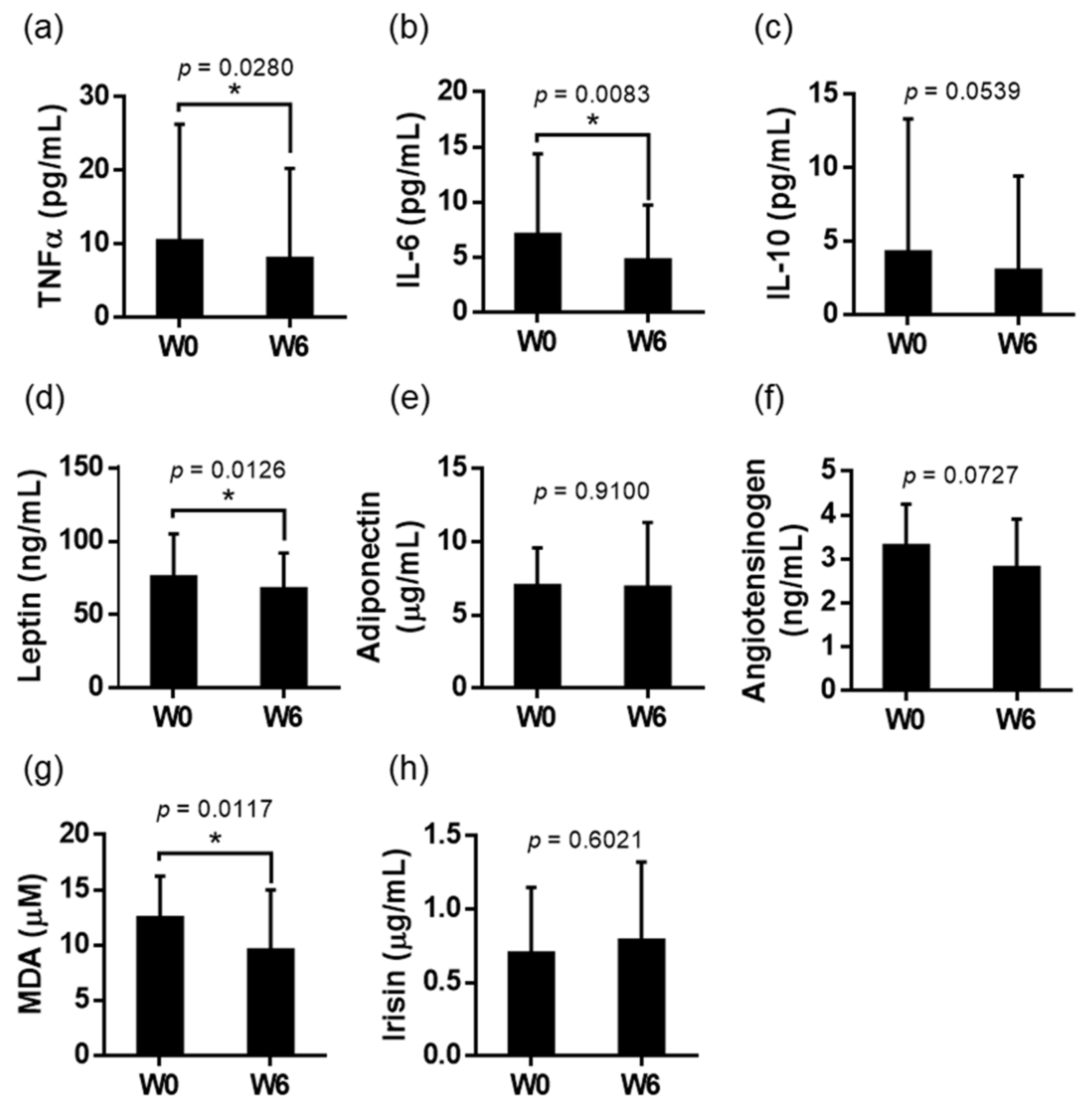
3.3. Blood Inflammatory Biomarkers Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Oliveira, L.F.; de Azevedo, L.G.; da Mota Santana, J.; de Sales, L.P.C.; Pereira-Santos, M. Obesity and overweight decreases the effect of vitamin D supplementation in adults: Systematic review and meta-analysis of randomized controlled trials. Rev. Endocr. Metab. Disord. 2020, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.B.; Kim, C.; Hwang, J.K. Whole grain cereal attenuates obesity-induced muscle atrophy by activating the PI3K/Akt pathway in obese C57BL/6N mice. Food Sci. Biotechnol. 2017, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.N.; Choi, J.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2013, 93, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Vielma, S.A.; Klein, R.L.; Levingston, C.A.; Young, M.R.I. Adipocytes as immune regulatory cells. Int. Immunopharmacol. 2013, 16, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Maiorino, M.I.; Bellastella, G.; Giugliano, D.; Esposito, K. From inflammation to sexual dysfunctions: A journey through diabetes, obesity, and metabolic syndrome. J. Endocrinol. Investig. 2018, 41, 1249–1258. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yang, C.C.; Li, P.C.; Chen, W.C.; Chien, C.T. Therapeutic potential of traditional chinese medicine on inflammatory diseases. J. Tradit. Complement. Med. 2013, 3, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.O.; Yun, S.J.; Jung, B.; Lee, H.; Hahm, D.H.; Shim, I.; Lee, H.J. Hypolipidemic effects of crude extract of adlay seed (Coix lachrymajobi var. mayuen) in obesity rat fed high fat diet: Relations of TNF-alpha and leptin mRNA expressions and serum lipid levels. Life Sci. 2004, 75, 1391–1404. [Google Scholar]
- Wang, C.Y.; Lin, H.T.; Wu, S.C. Influence of dietary supplementation with Bacillus-fermented adlay on lipid metabolism, antioxidant status and intestinal microflora in hamsters. J. Sci. Food Agric. 2011, 91, 2271–2276. [Google Scholar] [CrossRef]
- Chen, H.J.; Chung, C.P.; Chiang, W.; Lin, Y.L. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Q.; Liu, T.; Liu, Z. Coixol suppresses NF-kappaB, MAPK pathways and NLRP3 inflammasome activation in lipopolysaccharide-induced RAW 264.7 cells. Molecules 2020, 25, 894. [Google Scholar]
- Li, Y.; Tian, X.; Li, S.; Chang, L.; Sun, P.; Lu, Y.; Yu, X.; Chen, S.; Wu, Z.; Xu, Z.; et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-alpha induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [PubMed]
- Yeh, W.J.; Ko, J.; Cheng, W.Y.; Yang, H.Y. Diet containing dehulled adlay ameliorates hepatic steatosis, inflammation and insulin resistance in rats with non-alcoholic fatty liver disease. Br. J. Nutr. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.M.; Chang, W.C.; Liu, C.S.; Tsai, C.M. Effect of young barley leaf extract and adlay on plasma lipids and LDL oxidation in hyperlipidemic smokers. Biol. Pharm. Bull. 2004, 27, 802–805. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.; Chao, T.Y.; Chen, H.J.; Xie, G.R.; Chiang, W.; Hsieh, S.C. Investigating the impact of extruded dehulled adlay with specific in vitro digestion properties on blood lipids in subjects with mild to moderate dyslipidemia. Foods 2022, 11, 493. [Google Scholar] [CrossRef]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Zhou, J.; Zhao, D.; Wang, N.; Zeng, Z.; Wang, C.; Hao, L.; Peng, X. Effects of lutein supplementation on inflammatory biomarkers and metabolic risk factors in adults with central obesity: Study protocol for a randomised controlled study. Trials 2020, 21, 32. [Google Scholar] [CrossRef] [Green Version]
- Steffen, L.M.; Jacobs, D.R., Jr.; Murtaugh, M.A.; Moran, A.; Steinberger, J.; Hong, C.P.; Sinaiko, A.R. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am. J. Epidemiol. 2003, 158, 243–250. [Google Scholar]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur. J. Clin. Nutr. 2005, 59, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Wu, J.; Tang, J.; Wang, J.J.; Lu, C.H.; Wang, P.X. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among Chinese Rural-to-Urban migrant workers. Int. J. Environ. Res. Public Health 2015, 12, 4726–4738. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; He, J.; Wildman, R.P.; Reynolds, K.; Streiffer, R.H.; Whelton, P.K. A randomized controlled trial of dietary fiber intake on serum lipids. Eur. J. Clin. Nutr. 2006, 60, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorand, B.; Baumert, J.; Döring, A.; Herder, C.; Kolb, H.; Rathmann, W.; Giani, G.; Koenig, W.; KORA Group. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis 2006, 184, 216–224. [Google Scholar]
- Rossi, I.A.; Bochud, M.; Bovet, P.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Taffé, P. Sex difference and the role of leptin in the association between high-sensitivity C-reactive protein and adiposity in two different populations. Eur. J. Epidemiol. 2012, 27, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Canela, M.; Zazpe, I.; Shivappa, N.; Hébert, J.R.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Lamuela-Raventós, R.M.; Rekondo, J.; et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br. J. Nutr. 2015, 113, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Delaney, K.Z.; Santosa, S. Sex differences in regional adipose tissue depots pose different threats for the development of Type 2 diabetes in males and females. Obes. Rev. 2022, 23, e13393. [Google Scholar] [CrossRef]
- Chen, H.H.; Chiang, W.; Chang, J.Y.; Chien, Y.L.; Lee, C.K.; Liu, K.J.; Cheng, Y.T.; Chen, T.F.; Kuo, Y.H.; Kuo, C.C. Antimutagenic constituents of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) with potential cancer chemopreventive activity. J. Agric. Food Chem. 2011, 59, 6444–6452. [Google Scholar]
- Jeong, H.; Vacanti, N.M. Systemic vitamin intake impacting tissue proteomes. Nutr. Metab. 2020, 17, 73. [Google Scholar] [CrossRef]
- Chen, H.J.; Lo, Y.C.; Chiang, W. Inhibitory effects of adlay bran (Coix lachryma-jobi L. var. ma-yuen Stapf) on chemical mediator release and cytokine production in rat basophilic leukemia cells. J. Ethnopharmacol. 2012, 141, 119–127. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [Green Version]
- Minocci, A.; Savia, G.; Lucantoni, R.; Berselli, M.E.; Tagliaferri, M.; Calò, G.; Petroni, M.L.; de Medici, C.; Viberti, G.C.; Liuzzi, A. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1139–1144. [Google Scholar] [CrossRef] [Green Version]
- Reseland, J.E.; Anderssen, S.A.; Solvoll, K.; Hjermann, I.; Ural, P.; Holme, I.; Drevon, C.A. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am. J. Clin. Nutr. 2001, 73, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Oliveira, V.; Camara, N.O.; Moraes-Vieira, P.M. Adipokines as drug targets in diabetes and underlying disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Ouchi, N.; Shibata, R.; Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007, 74, 11–18. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Massiéra, F.; Bloch-Faure, M.; Ceiler, D.; Murakami, K.; Fukamizu, A.; Gasc, J.M.; Quignard-Boulange, A.; Negrel, R.; Ailhaud, G.; Seydoux, J.; et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001, 15, 2727–2729. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Moustaid-Moussa, N. The renin-angiotensin system: A link between obesity, inflammation and insulin resistance. Obes. Rev. 2012, 13, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Fan, Z.Y.; Wang, H.Y.; Wen, D.C.; Zhang, S.Y. Effect of polysaccharides from adlay seed on anti-diabetic and gut microbiota. Food Funct. 2019, 10, 4372–4380. [Google Scholar] [CrossRef] [PubMed]

| Parameters | W0 | W6 |
|---|---|---|
| Height (cm) | 162.3 ± 7.4 | |
| Weight (kg) | 78.5 ± 9.1 | 78.6 ± 8.9 |
| Fat mass (%) | 40.9 ± 6.9 | 37.7 ± 6.0 * |
| BMI | 29.8 ± 3.2 | 29.9 ± 2.9 |
| Waist (cm) | 92.0 ± 7.0 | 90.4 ± 8.4 |
| Hip (cm) | 108.9 ± 5.7 | 108.2 ± 4.7 |
| WHR | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Tricep (mm) | 27.7 ± 6.4 | 25.5 ± 4.2 |
| Fasting glucose (mg/dL) | 93.8 ±16.5 | 91.6 ± 8.2 |
| C-peptide (ng/dL) | 2.0 ± 0.5 | 2.3 ± 0.4 * |
| HbA1c (%) | 5.4 ± 0.9 | 5.3 ± 0.6 |
| TC (mg/dL) | 180.1 ± 34.5 | 170.8 ± 32.3 * |
| TG (mg/dL) | 114.0 ± 52.3 | 95.4 ± 43.1 * |
| HDL-C (mg/dL) | 45.8 ± 8.0 | 46.3 ± 9.0 |
| LDL-C (mg/dL) | 105.7 ± 29.6 | 101.5 ± 30.2 |
| AST (U/L) | 20.9 ± 9.0 | 20.6 ± 12.1 |
| ALT (U/L) | 28.7 ± 49.3 | 32.7 ± 56.8 * |
| hsCRP (mg/L) | 1.88 ± 1.56 | 2.42 ± 3.38 |
| Parameters | Change of Parameters | |
|---|---|---|
| Women (n = 15) | Men (n = 6) | |
| Body weight (kg) | −0.7 ± 2.0 | 2.2 ± 3.0 * |
| Fat mass (%) | −3.0 ± 3.8 | −0.3 ± 4.4 |
| Fasting glucose (mg/dL) | −3.6 ± 11.8 | 1.5 ± 2.4 |
| C-peptide (ng/dL) | 0.2 ± 0.5 | 0.5 ± 0.8 |
| HbA1c (%) | −0.2 ± 0.4 | 0.0 ± 0.2 |
| TC (mg/dL) | −13.5 ± 21.7 | 1.3 ± 12.8 |
| TG (mg/dL) | −11.5 ± 37.2 | −36.5 ± 21.3 |
| HDL-C (mg/dL) | 0.5 ± 5.7 | 0.5 ± 8.0 |
| LDL-C (mg/dL) | −8.1 ± 18.2 | 5.7 ± 11.9 |
| AST (U/L) | −1.0 ± 3.6 | 1.7 ± 6.2 |
| ALT (U/L) | 1.6 ± 3.4 | 10.2 ± 14.2 |
| hsCRP (mg/L) | −0.09 ± 1.46 | 2.13 ± 5.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-Y.; Yeh, W.-J.; Ko, J.; Huang, Y.-L.; Yang, H.-Y. Consumption of Dehulled Adlay Improved Lipid Metabolism and Inflammation in Overweight and Obese Individuals after a 6-Week Single-Arm Pilot Study. Nutrients 2022, 14, 2250. https://doi.org/10.3390/nu14112250
Cheng W-Y, Yeh W-J, Ko J, Huang Y-L, Yang H-Y. Consumption of Dehulled Adlay Improved Lipid Metabolism and Inflammation in Overweight and Obese Individuals after a 6-Week Single-Arm Pilot Study. Nutrients. 2022; 14(11):2250. https://doi.org/10.3390/nu14112250
Chicago/Turabian StyleCheng, Wei-Yi, Wan-Ju Yeh, Jung Ko, Ya-Ling Huang, and Hsin-Yi Yang. 2022. "Consumption of Dehulled Adlay Improved Lipid Metabolism and Inflammation in Overweight and Obese Individuals after a 6-Week Single-Arm Pilot Study" Nutrients 14, no. 11: 2250. https://doi.org/10.3390/nu14112250
APA StyleCheng, W.-Y., Yeh, W.-J., Ko, J., Huang, Y.-L., & Yang, H.-Y. (2022). Consumption of Dehulled Adlay Improved Lipid Metabolism and Inflammation in Overweight and Obese Individuals after a 6-Week Single-Arm Pilot Study. Nutrients, 14(11), 2250. https://doi.org/10.3390/nu14112250