Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Resource
2.2. Diet Quality Scores
2.3. Heavy Metal Measurements
2.4. Obesity
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 February 2022).
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Pi-Sunyer, F. Medical hazards of obesity. Ann. Intern. Med. 1993, 199, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef]
- Leasure, J.L.; Giddabasappa, A.; Chaney, S.; Johnson, J.E., Jr.; Pothakos, K.; Lau, Y.S.; Fox, D.A. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008, 116, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Hanao, N.; Nishiyama, K.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 2012, 258, 32–42. [Google Scholar] [CrossRef]
- Hou, Y.; Xue, P.; Woods, C.G.; Wang, X.; Fu, J.; Yarborough, K.; Qu, W.; Zhang, Q.; Andersen, M.E.; Pi, J. Association between arsenic suppression of adipogenesis and induction of CHOP10 via the endoplasmic reticulum stress response. Environ. Health Perspect. 2013, 121, 237–243. [Google Scholar] [CrossRef]
- Mailloux, R.; Lemire, J.; Appanna, V. Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: A link to obesity. Cell. Physiol. Biochem. 2007, 20, 627–638. [Google Scholar] [CrossRef]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Choe, N.H.; Schallert, D.L.; Forbis, A.K. The chemical engineering research laboratory as context for graduate students’ training: The role of lab structure and cultural climate in collaborative work. Learn. Cult. Soc. Interact. 2017, 13, 113–122. [Google Scholar] [CrossRef]
- Niehoff, N.M.; Keil, A.P.; O’Brien, K.M.; Jackson, B.P.; Karagas, M.R.; Weinberg, C.R.; White, A.J. Metals and trace elements in relation to body mass index in a prospective study of US women. Environ. Res. 2020, 184, 109396. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Wells, E.M.; Kopylev, L.; Nachman, R.; Radke, E.G.; Segal, D. Seafood, wine, rice, vegetables, and other food items associated with mercury biomarkers among seafood and non-seafood consumers: NHANES 2011–2012. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 504–514. [Google Scholar] [CrossRef]
- Krupa, Z.; Siedlecka, A.; Skórzynska-Polit, E.; Maksymiec, W. Heavy metal interactions with plant nutrients. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Prasad, M.N.V., Strzałka, K., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (accessed on 25 February 2022).
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, i–viii. [Google Scholar]
- Hunt, K.J.; St Peter, J.V.; Malek, A.M.; Vrana-Diaz, C.; Marriott, B.P.; Greenberg, D. Daily eating frequency in US adults: Associations with low-calorie sweeteners, body mass index, and nutrient intake (NHANES 2007–2016). Nutrients 2020, 12, 2566. [Google Scholar] [CrossRef]
- SSY, A.L.; Natto, Z.S.; Midle, J.B.; Gyurko, R.; O’Neill, R.; Steffensen, B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J. Periodontol. 2019, 90, 16–25. [Google Scholar]
- Gay, I.C.; Tran, D.T.; Paquette, D.W. Alcohol intake and periodontitis in adults aged ≥30 years: NHANES 2009–2012. J. Periodontol. 2018, 89, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended physical activity and all cause and cause specific mortality in US adults: Prospective cohort study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity Guidelines for Americans, 2nd ed. Available online: https://healthysd.gov/physical-activity-guidelines-for-americans-2nd-edition/ (accessed on 30 June 2022).
- Abete, I.; Astrup, A.; Martinez, J.A.; Thorsdottir, I.; Zulet, M.A. Obesity and the metabolic syndrome: Role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr. Rev. 2010, 68, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Kris-Etherton, P.M. Macronutrient Content of the Diet: What Do We Know About Energy Balance and Weight Maintenance? Curr. Obes. Rep. 2016, 5, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Willey, J.; Wakefield, M.; Silver, H.J. Exploring the Diets of Adults with obesity and type II diabetes from nine diverse countries: Dietary intakes, patterns, and quality. Nutrients 2020, 12, 2027. [Google Scholar] [CrossRef]
- Tande, D.L.; Magel, R.; Strand, B.N. Healthy Eating Index and abdominal obesity. Public Health Nutr. 2010, 13, 208–214. [Google Scholar] [CrossRef]
- Gao, S.K.; Beresford, S.A.; Frank, L.L.; Schreiner, P.J.; Burke, G.L.; Fitzpatrick, A.L. Modifications to the Healthy Eating Index and its ability to predict obesity: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2008, 88, 64–69. [Google Scholar] [CrossRef]
- Tardivo, A.P.; Nahas-Neto, J.; Nahas, E.A.; Maesta, N.; Rodrigues, M.A.; Orsatti, F.L. Associations between healthy eating patterns and indicators of metabolic risk in postmenopausal women. Nutr. J. 2010, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Mirmiran, P.; Rashidkhani, B.; Asghari-Jafarabadi, M.; Mehran, M.; Azizi, F. The association between diet quality indices and obesity: Tehran lipid and glucose study. Arch. Iran. Med. 2012, 15, 599–605. [Google Scholar]
- Sundararajan, K.; Campbell, M.K.; Choi, Y.H.; Sarma, S. The relationship between diet quality and adult obesity: Evidence from Canada. J. Am. Coll. Nutr. 2014, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, C.K.; Khadka, S. Heterogeneities in consumer diet quality and health outcomes of consumers by store choice and income. Nutrients 2021, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Olstad, D.L.; Nejatinamini, S.; Victorino, C.; Kirkpatrick, S.I.; Minaker, L.M.; McLaren, L. Trends in socioeconomic inequities in diet quality between 2004 and 2015 among a nationally representative sample of children in Canada. J. Nutr. 2021, 151, 3781–3794. [Google Scholar] [CrossRef]
- Asghari, G.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. A systematic review of diet quality indices in relation to obesity. Br. J. Nutr. 2017, 117, 1055–1065. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Korrick, S.A.; Fayad, R. The influence of obesity on blood mercury levels for U.S. non-pregnant adults and children: NHANES 2007–2010. Environ. Res. 2015, 138, 173–180. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Popova, E.V.; Sinitskii, A.I.; Nemereshina, O.N.; Gatiatulina, E.R.; Nikonorov, A.A.; Skalny, A.V. Mercury and metabolic syndrome: A review of experimental and clinical observations. Biometals 2015, 28, 231–254. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; Daniel, A.; Salazar, A.M.; Panico, P.; Ostrosky-Wegman, P.; Diaz-Villasenor, A. Effects of arsenic on adipocyte metabolism: Is arsenic an obesogen? Mol. Cell. Endocrinol. 2017, 452, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gade, M.; Comfort, N.; Re, D.B. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ. Res. 2021, 201, 111558. [Google Scholar] [CrossRef] [PubMed]
- Rechtman, E.; Curtin, P.; Papazaharias, D.M.; Renzetti, S.; Cagna, G.; Peli, M.; Levin-Schwartz, Y.; Placidi, D.; Smith, D.R.; Lucchini, R.G.; et al. Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl. Psychiatry 2020, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Joneidi, Z.; Mortazavi, Y.; Memari, F.; Roointan, A.; Chahardouli, B.; Rostami, S. The impact of genetic variation on metabolism of heavy metals: Genetic predisposition? Biomed. Pharmacother. 2019, 113, 108642. [Google Scholar] [CrossRef]
- Chang, L.; Shen, S.; Zhang, Z.; Song, X.; Jiang, Q. Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann. Transl. Med. 2018, 6, 320. [Google Scholar] [CrossRef]
- Gunderson, E.L. FDA Total Diet Study, July 1986-April 1991, dietary intakes of pesticides, selected elements, and other chemicals. J. AOAC Int. 1995, 78, 1353–1363. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhao, Q.; Du, H.; Gao, Y.; Bai, M.; Lv, J.; Guo, Y.; Li, L.; Sun, L.; et al. Urinary element profiles and associations with cardiometabolic diseases: A cross-sectional study across ten areas in China. Environ. Res. 2022, 205, 112535. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Wani, A.L.; Hammad Ahmad Shadab, G.G.; Afzal, M. Lead and zinc interactions—An influence of zinc over lead related toxic manifestations. J. Trace Elem. Med. Biol. 2021, 64, 126702. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Oh, H.; Hoang, N.H.M.; Jo, W.H.; Kim, M.S. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: A national cross-sectional study (2009–2017). Environ. Sci. Pollut. Res. Int. 2022, 29, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. Ann. Nutr. Metab. 2015, 66, 7–12. [Google Scholar] [CrossRef] [PubMed]

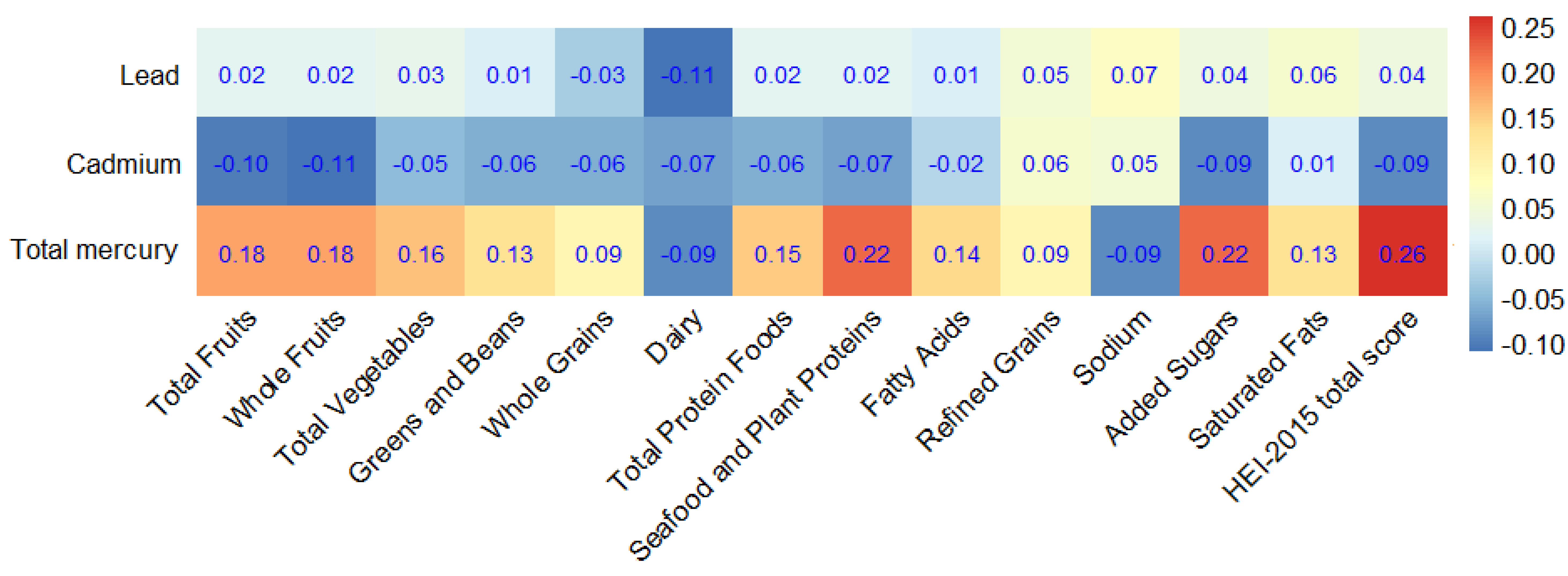
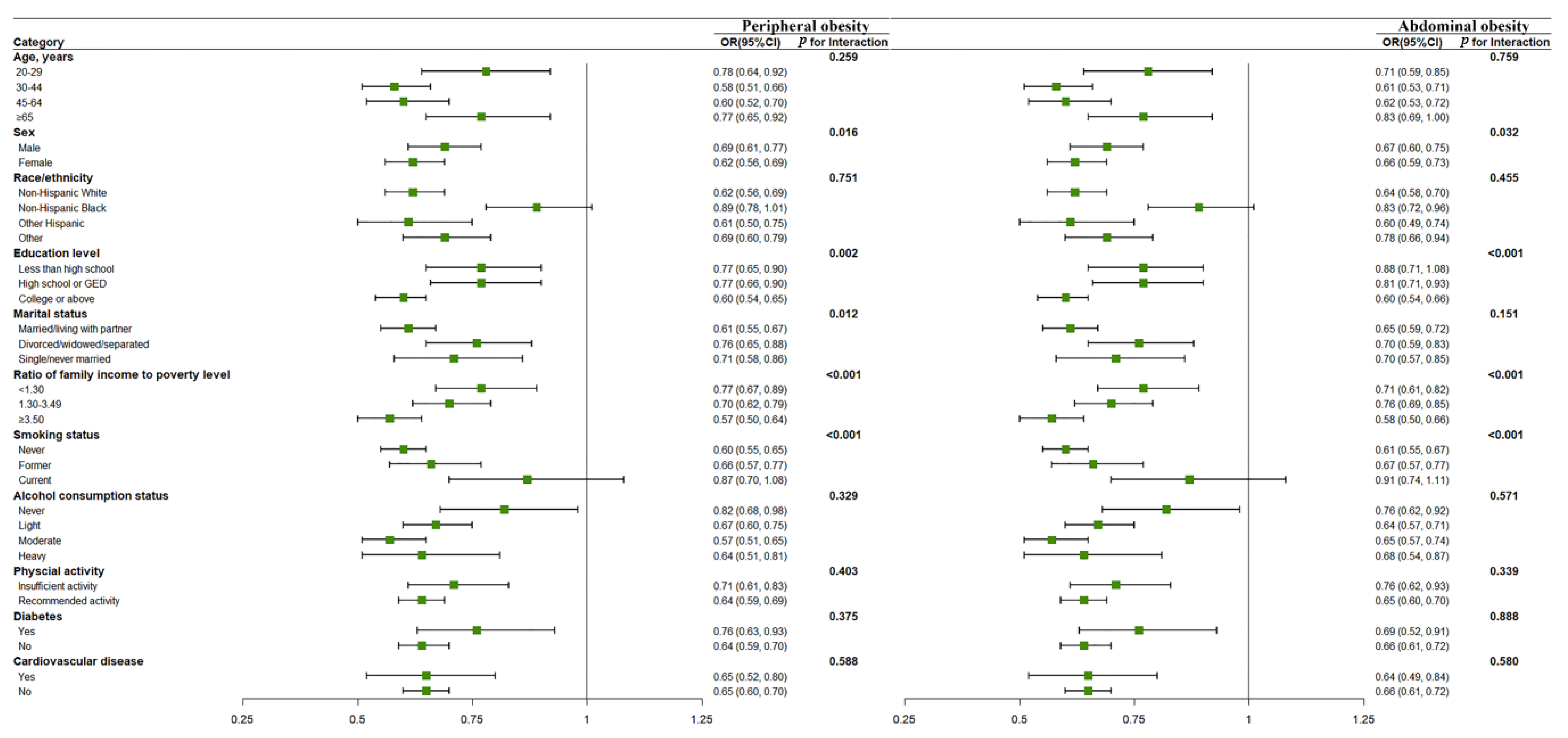
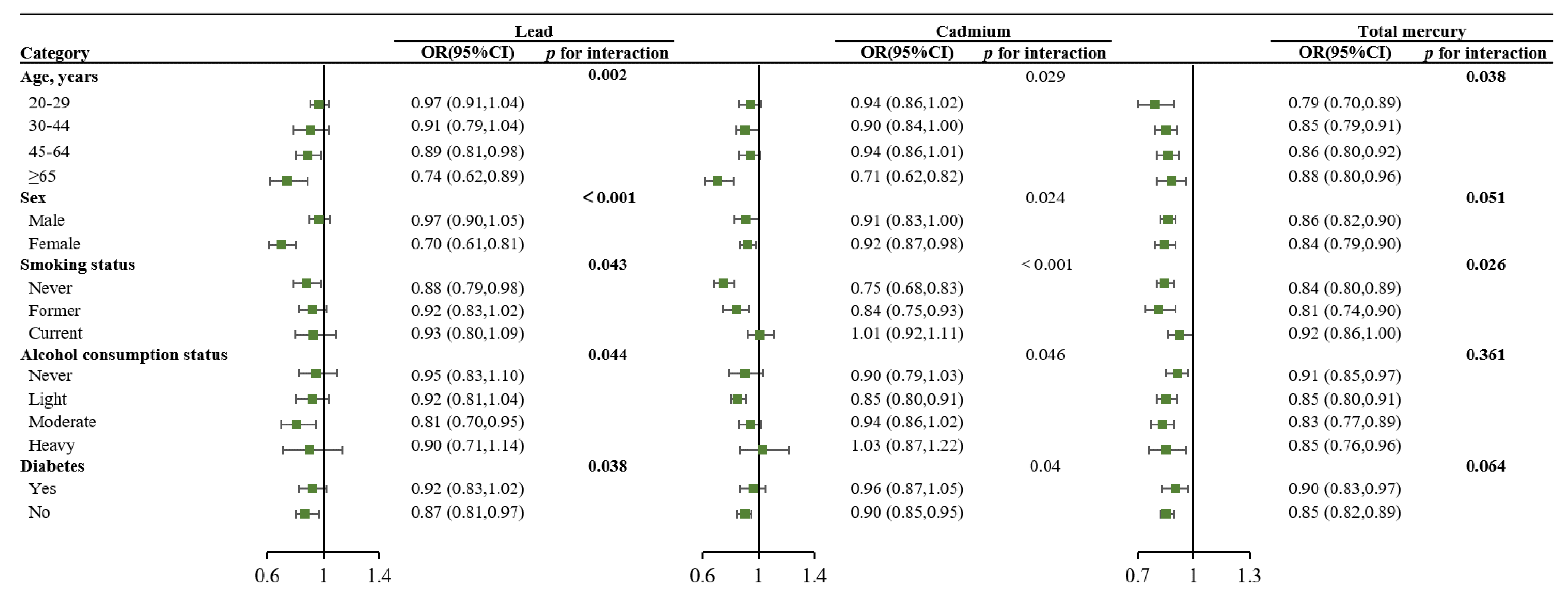
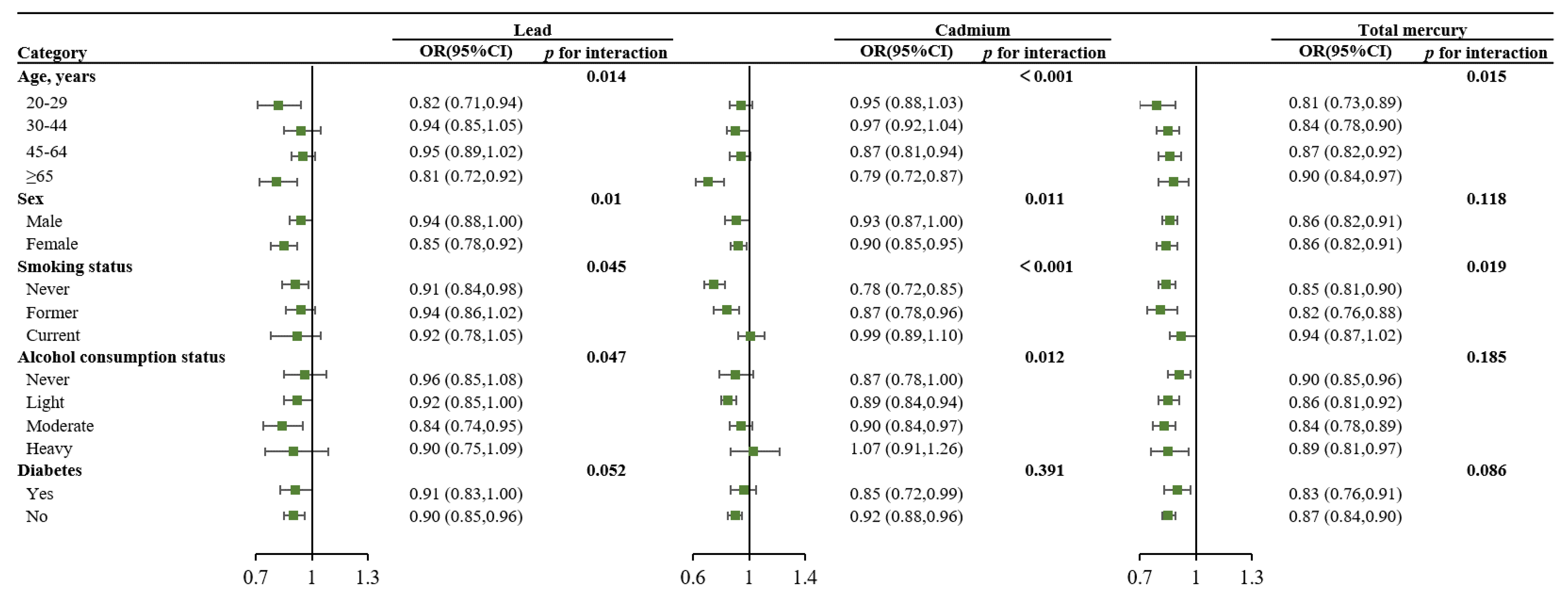
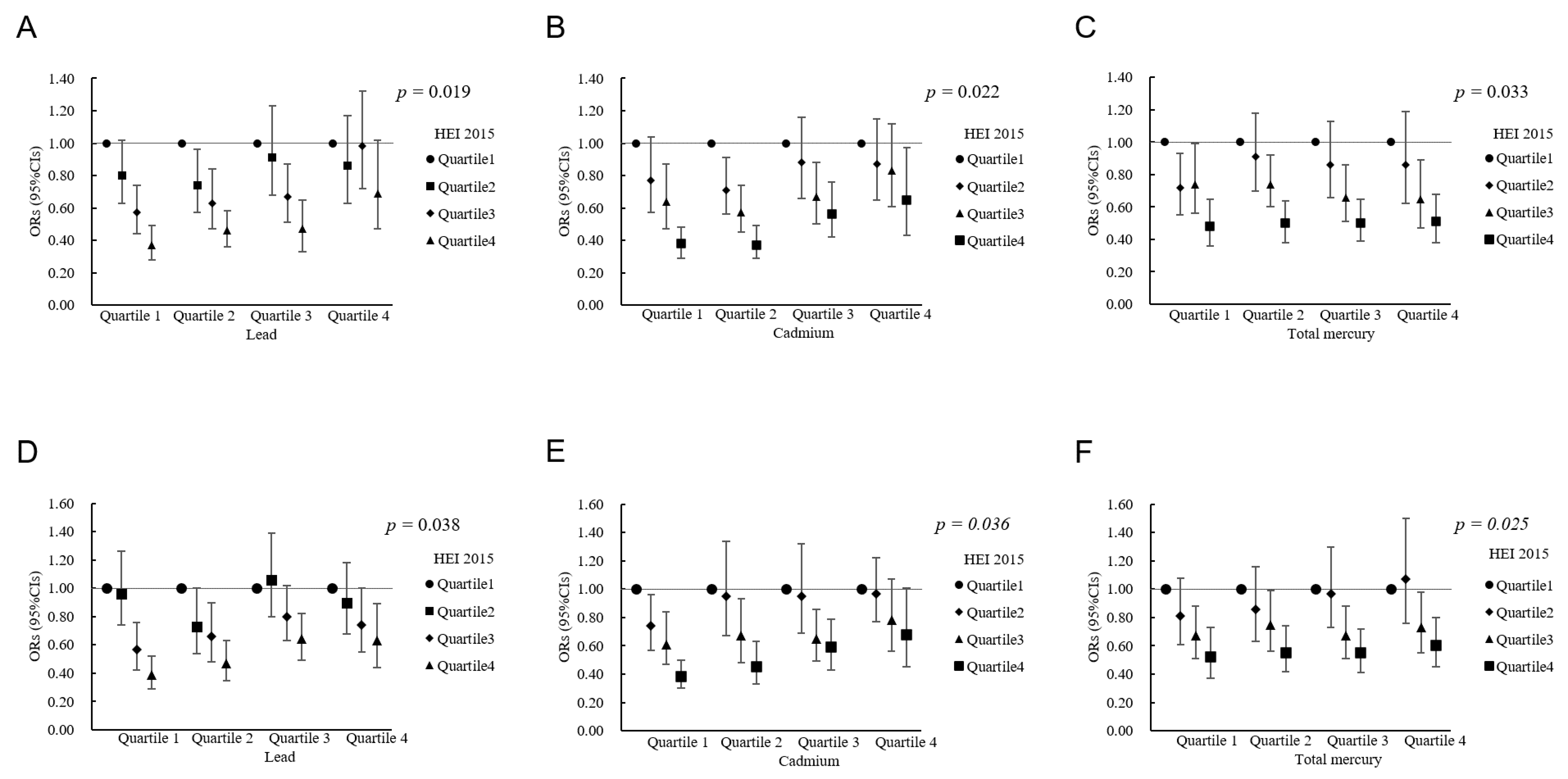
| Characteristics | Total (n = 15,959) | No Obesity (n = 10,160) | Obesity (n = 5799) | p Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 45.71 (16.31) | 45.27(16.74) | 46.53 (15.46) | 0.004 |
| Sex, n (%) | 0.509 | |||
| Male | 8415 (52.73) | 5582 (54.94) | 2833 (48.85) | |
| Female | 7544 (47.27) | 4578 (45.06) | 2966 (51.15) | |
| Race/ethnicity, n (%) | <0.001 | |||
| Non-Hispanic White | 7240 (45.37) | 4822 (47.46) | 2418 (41.70) | |
| Non-Hispanic Black | 3164 (19.83) | 1657 (16.31) | 1507 (25.99) | |
| Other Hispanic | 1509 (9.46) | 967 (9.52) | 542 (9.35) | |
| Others | 4046 (25.35) | 2714 (26.71) | 1332 (22.97) | |
| Education level, n (%) | <0.001 | |||
| Less than high school | 3231 (20.25) | 2030 (19.98) | 1201 (20.71) | |
| High school graduate or GED | 3584 (22.46) | 2151 (21.17) | 1433 (24.71) | |
| College or above | 9144 (57.30) | 5979 (58.85) | 3165 (54.58) | |
| Marital status, n (%) | <0.001 | |||
| Married/living with partner | 9648 (60.45) | 6154 (60.57) | 3494 (60.25) | |
| Divorced/widowed/separated | 3129 (19.61) | 1876 (18.46) | 1253 (21.61) | |
| Single/never married | 3182 (19.94) | 2130 (20.96) | 1052 (18.14) | |
| Ratio of family income to poverty level, n (%) | 0.005 | |||
| <1.30 | 4738 (29.69) | 2957 (29.10) | 1781 (30.71) | |
| 1.30–3.49 | 5936 (37.20) | 3670 (36.12) | 2266 (39.08) | |
| ≥3.50 | 5285 (33.12) | 3533 (34.77) | 1752 (30.21) | |
| Smoking status, n (%) | <0.001 | |||
| Never | 8844 (55.42) | 5599 (55.11) | 3245 (55.96) | |
| Former | 3818 (23.92) | 2286 (22.50) | 1532 (26.42) | |
| Current | 3297 (20.66) | 2275 (22.39) | 1022 (17.62) | |
| Alcohol consumption status, n (%) | <0.001 | |||
| Never | 2429 (15.22) | 1422 (14.00) | 1007 (17.37) | |
| Light | 6566 (41.14) | 3984 (39.21) | 2582 (44.52) | |
| Moderate | 5102 (31.97) | 3465 (34.10) | 1637 (28.23) | |
| Heavy | 1862 (11.67) | 1289 (12.69) | 573 (9.88) | |
| Physical activity, n (%) | <0.001 | |||
| Insufficient activity | 2853 (17.88) | 1698 (16.71) | 1155 (19.92) | |
| Recommended activity | 13,106 (82.12) | 8462 (83.29) | 4644 (80.08) | |
| Diabetes, n (%) | <0.001 | |||
| Yes | 2013 (12.61) | 832 (8.19) | 1181 (20.37) | |
| No | 13,946 (87.39) | 9328 (91.81) | 4618 (79.63) | |
| Cardiovascular disease, n (%) | <0.001 | |||
| Yes | 1318 (8.26) | 730 (7.19) | 588 (10.14) | |
| No | 14,641 (91.74) | 9430 (92.81) | 5211 (89.86) | |
| BMI (kg/m2), mean (SD) | 28.63 (6.51) | 24.88 (3.10) | 35.60 (5.34) | <0.001 |
| Waist circumference (cm), mean (SD) | 98.21 (16.08) | 89.64 (10.17) | 114.13 (12.52) | <0.001 |
| HEI-2015 total score, mean (SD) | 53.97 (13.62) | 55.17 (13.89) | 51.74 (12.82) | <0.001 |
| Cadmium (μg/L), GM (GSD) | 0.32 (1.40) | 0.33 (1.41) | 0.30 (1.38) | <0.001 * |
| Lead (μg/dL), GM (GSD) | 1.06 (1.26) | 1.11 (1.25) | 0.96 (1.26) | <0.001 * |
| Total mercury (μg/L), GM (GSD) | 0.92 (1.64) | 0.99 (1.67) | 0.80 (1.52) | <0.001 * |
| Exposure | Peripheral Obesity a | Abdominal Obesity b | ||||
|---|---|---|---|---|---|---|
| Model 1 c OR (95% CI) | Model 2 d OR (95% CI) | Model 3 e OR (95% CI) | Model 1 c OR (95% CI) | Model 2 d OR (95% CI) | Model 3 e OR (95% CI) | |
| HEI-2015 total score | ||||||
| Quartile 1 f | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 0.84 (0.73, 0.95) | 0.82 (0.71, 0.93) | 0.81 (0.70, 0.93) | 0.91 (0.78, 1.05) | 0.89 (0.76, 1.03) | 0.88 (0.75, 1.03) |
| Quartile 3 | 0.69 (0.60, 0.79) | 0.67 (0.58, 0.77) | 0.67 (0.58, 0.77) | 0.67(0.59, 0.79) | 0.66(0.57, 0.76) | 0.66 (0.57, 0.77) |
| Quartile 4 | 0.49 (0.44, 0.56) | 0.48 (0.42, 0.54) | 0.47 (0.41, 0.54) | 0.53 (0.46, 0.60) | 0.51(0.45, 0.58) | 0.51 (0.45, 0.57) |
| P for trend g | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Continuous (per IQR) | 0.67 (0.62, 0.71) | 0.65 (0.61, 0.70) | 0.65 (0.60, 0.70) | 0.67 (0.63, 0.73) | 0.66 (0.62, 0.71) | 0.66 (0.62, 0.71) |
| Pb | ||||||
| Quartile 1 f | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 0.79 (0.68, 0.91) | 0.81 (0.70, 0.93) | 0.83 (0.72, 0.96) | 0.83 (0.72, 0.95) | 0.83 (0.72, 0.96) | 0.85 (0.74, 0.98) |
| Quartile 3 | 0.54 (0.46, 0.62) | 0.57 (0.49, 0.66) | 0.62 (0.54, 0.72) | 0.68 (0.59, 0.79) | 0.69 (0.59, 0.81) | 0.74 (0.64, 0.87) |
| Quartile 4 | 0.39 (0.33, 0.47) | 0.42 (0.35, 0.50) | 0.48 (0.40, 0.57) | 0.49 (0.41, 0.57) | 0.49 (0.41, 0.59) | 0.55 (0.46, 0.65) |
| P for trend g | 0.001 | 0.004 | 0.013 | <0.001 | <0.001 | <0.001 |
| Continuous (per IQR) | 0.83 (0.75, 0.93) | 0.86 (0.78, 0.95) | 0.89 (0.82, 0.98) | 0.87 (0.82, 0.93) | 0.88 (0.83, 0.94) | 0.90 (0.86, 0.96) |
| Cd | ||||||
| Quartile 1 f | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 0.84 (0.74, 0.96) | 0.83 (0.73, 0.94) | 0.85 (0.75, 0.97) | 0.86 (0.76, 0.99) | 0.83 (0.73, 0.95) | 0.85 (0.74, 0.97) |
| Quartile 3 | 0.69 (0.60, 0.81) | 0.66 (0.57, 0.78) | 0.70 (0.60, 0.82) | 0.75 (0.64, 0.87) | 0.69 (0.59, 0.81) | 0.72 (0.61, 0.84) |
| Quartile 4 | 0.51 (0.45, 0.59) | 0.45 (0.38, 0.54) | 0.47 (0.39, 0.57) | 0.59 (0.52, 0.68) | 0.48 (0.40, 0.57) | 0.50 (0.42, 0.60) |
| P for trend g | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Continuous (per IQR) | 0.90 (0.86, 0.94) | 0.91 (0.87, 0.95) | 0.91 (0.87, 0.96) | 0.93 (0.89, 0.96) | 0.91 (0.88, 0.95) | 0.92 (0.88, 0.96) |
| Hg | ||||||
| Quartile 1 f | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 0.92 (0.81, 1.04) | 0.92 (0.81, 1.04) | 0.93 (0.82, 1.06) | 0.99 (0.85, 1.14) | 0.99 (0.85, 1.14) | 1.00 (0.86, 1.16) |
| Quartile 3 | 0.83 (0.72, 0.96) | 0.85 (0.73, 0.98) | 0.88 (0.75, 1.03) | 0.79 (0.67, 0.93) | 0.79 (0.67, 0.94) | 0.81 (0.69, 0.96) |
| Quartile 4 | 0.53 (0.45, 0.62) | 0.55 (0.47, 0.65) | 0.57 (0.49, 0.67) | 0.54 (0.46, 0.62) | 0.55 (0.47, 0.63) | 0.56 (0.49, 0.65) |
| p for trend g | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Continuous (per IQR) | 0.84 (0.80, 0.87) | 0.85 (0.81, 0.88) | 0.85 (0.82, 0.89) | 0.85 (0.82, 0.89) | 0.86 (0.83, 0.89) | 0.86 (0.83, 0.89) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yu, L.; Yang, Z.; Shen, P.; Lin, H.; Shui, L.; Tang, M.; Jin, M.; Chen, K.; Wang, J. Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients 2022, 14, 4038. https://doi.org/10.3390/nu14194038
Li T, Yu L, Yang Z, Shen P, Lin H, Shui L, Tang M, Jin M, Chen K, Wang J. Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients. 2022; 14(19):4038. https://doi.org/10.3390/nu14194038
Chicago/Turabian StyleLi, Tiezheng, Luhua Yu, Zongming Yang, Peng Shen, Hongbo Lin, Liming Shui, Mengling Tang, Mingjuan Jin, Kun Chen, and Jianbing Wang. 2022. "Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES)" Nutrients 14, no. 19: 4038. https://doi.org/10.3390/nu14194038
APA StyleLi, T., Yu, L., Yang, Z., Shen, P., Lin, H., Shui, L., Tang, M., Jin, M., Chen, K., & Wang, J. (2022). Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients, 14(19), 4038. https://doi.org/10.3390/nu14194038