Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Biomarkers Modulated by Lipid-Soluble Vitamins in Glioblastoma Cells
2.4. Survival Analysis of Biomarkers
3. Results
3.1. Literature Search Results
3.2. Molecular Mechanisms Modulated by LIPID-Soluble Vitamins in GBM
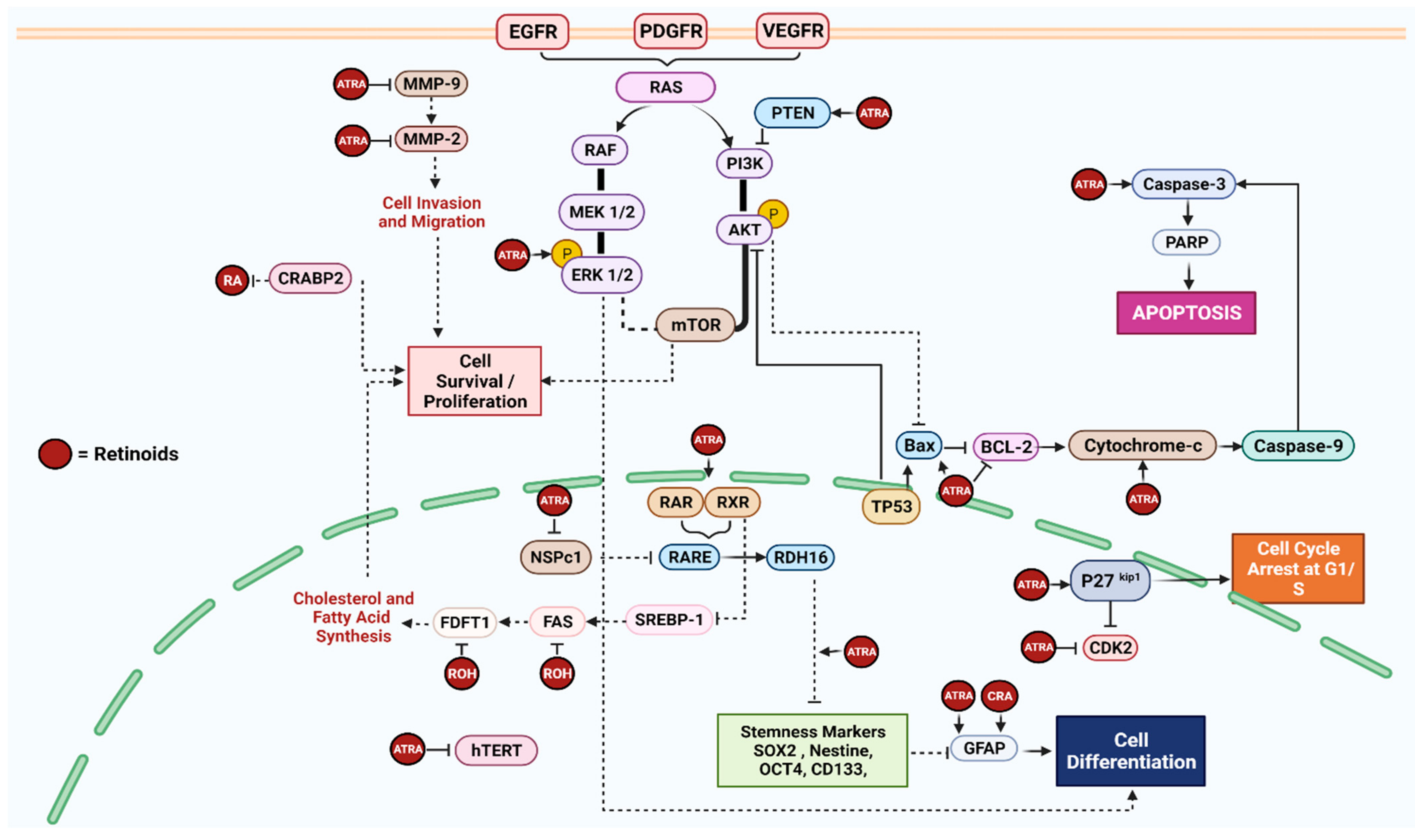
3.2.1. Retinoids (Vitamin A)
| Ref. | Aim of the Study | Glioblastoma Cell Line(s) | Vitamin | Intervention | Main Outcome | Other Outcomes |
|---|---|---|---|---|---|---|
| [18] | To test the effect of phytol and retinol on the viability of human GBM cell lines To determine the effect of phytol and retinol on the expression of signaling pathway and key proteins belong to cholesterol and/or fatty acid biosynthesis | U87MG, A172, and T98G | Retinol | U87MG-41.8 µM, A172–10.4 µM, and T98G-244.2 µM for 72 h | Transcriptome analysis revealed the downregulation of genes involved in the cholesterol and/or fatty acid biosynthetic pathway by retinol IC50 Retinol significantly reduced the level of (FAS and FDFT1) key proteins involved in tumor lipogenesis and cholesterol synthesis | Retinol exhibited a dose-dependent cytotoxic effect on GBM cell lines |
| [19] | To correlate the increased expression of NSPc1 and increase in tumor growth in stem-like cells (SLC) of GBM cell lines To test the effect of ATRA on SLCs and expression of NSPc1 and stemness markers | U87MG-SLC and U251-SLC | ATRA | 10 μM (6–12 days) | ATRA treatment partially reversed NSPc1 induced-stemness markers (CD133 and Sox2), resulting in the ATRA-induced differentiation of GBM stem cells through the activation of the RDH16 protein | ATRA partially reversed glioma sphere growth in stem-like cancer cells and promoted differentiation in U87MG-SLC cells. NSPc1 knockdown resulted in impaired neurospheres’ formation, self-renewal abilities, and the downregulation of stemness markers CD133 and Sox2 NSPc1 epigenetically repressed the expression of RDH16 by directly binding to the RDH16 promoter |
| [20] | To investigate the role of mTOR in CSC maintenance and to establish the mechanism of targeting GBM CSCs using differentiating agents along with inhibitors of the mTOR pathway | U87MG and LN18 | ATRA | 10 μM for 2, 6, and 24 h | ATRA induced the differentiation of CSCs resulting in (1) suppression in the stem cell marker Nestin and (2) the enhanced expression of activated extracellular signal-regulated kinase 1/2 (pERK1/2) “independent of mTOR pathway inhibitors” | The combination of AT-RA, PI3K inhibitor, and mTOR inhibitor synergistically resulted in reduced CSC proliferation and migration |
| [21] | To demonstrate the presence of CRABP2 predominantly in the cytoplasm of GBM To correlate the relationship between CRABP2 level and RA treatment | U251 and M049 | RA | 0.5–5 µM for 6 h | Following RA treatment, CRABP2 accumulate in the cytoplasm of GBM cells, blocking the action of RA and activating anti-apoptotic pathway proteins (Cyclin E/CDK2, CRYAB, GFAP, and FABP7) | Knockdown of CRABP2 reduced proliferation rate, restored RA function, and downregulated the expression of anti-apoptotic proteins (Cyclin E, CRYAB, GFAP, and FABP7) |
| [22] | To investigate the effect of ATRA treatment on the migration, invasion, apoptosis, and proliferation of glioma cells | U87MG and SHG44 | ATRA | 5, 10, 20, or 40 µmol/L for 24 h | ATRA significantly downregulated the expression of invasion-mediated factors (MMP-2 and MMP-9) in a dose-dependent manner in all lineages. However, MMP-2 expression in U87MG cell line was only lowered following a high dose treatment of ATRA (20 and 40 µmol/L) | ATRA significantly inhibits the migration, invasion, proliferation, and promotes the apoptosis of GBM cells following treatment with various concentrations for 24 h in a dose-dependent manner |
| [23] | To investigate and compare the effect of ATRA and/or Interferon-γ on different GBM cell lines, LN18 (PTEN-proficient) and U87MG (PTEN-deficient) | LN18 and U87MG | ATRA | 1 μM for 7 days | In LN18 cells, ATRA induced cell differentiation followed by the elevation of GFAP and apoptosis by increasing the Bax: Bcl-2 ratio, the mitochondrial release of cytochrome c into the cytosol, and calpain and caspase-3 activity, which is triggered by PTEN expression. While U87MG failed to show apoptotic biomarkers, it still exhibited high levels of GFAP, reflecting differentiation. ATRA also elevated p27kip1 and decreased CDK2 levels in both cell lines, indicating cell cycle arrest at the G1/S phase | The combination of ATRA and Interferon-γ control the growth of both GBM cell lines (PTEN-proficient and PTEN-deficient) by inducing cell differentiation, apoptosis, and cell cycle arrest |
| [24] | To test whether cell differentiation induced by the retinoids (ATRA or 13-CRA) affects GBM cells’ sensitivity to the microtubule-binding drug Taxol (TXL) and triggers apoptosis | T98G and U87MG | ATRA and 13 CRA | 1 μM ATRA or 13-CRA for 7 days | GBM cells treated with ATRA or 13-CRA induced astrocytic differentiation, followed by the overexpression of GFAP and the downregulation of hTERT expression and activity, but no effects on apoptotic pathway proteins were found | The combination of retinoids with TXL effectively enhanced cell differentiation and apoptosis |
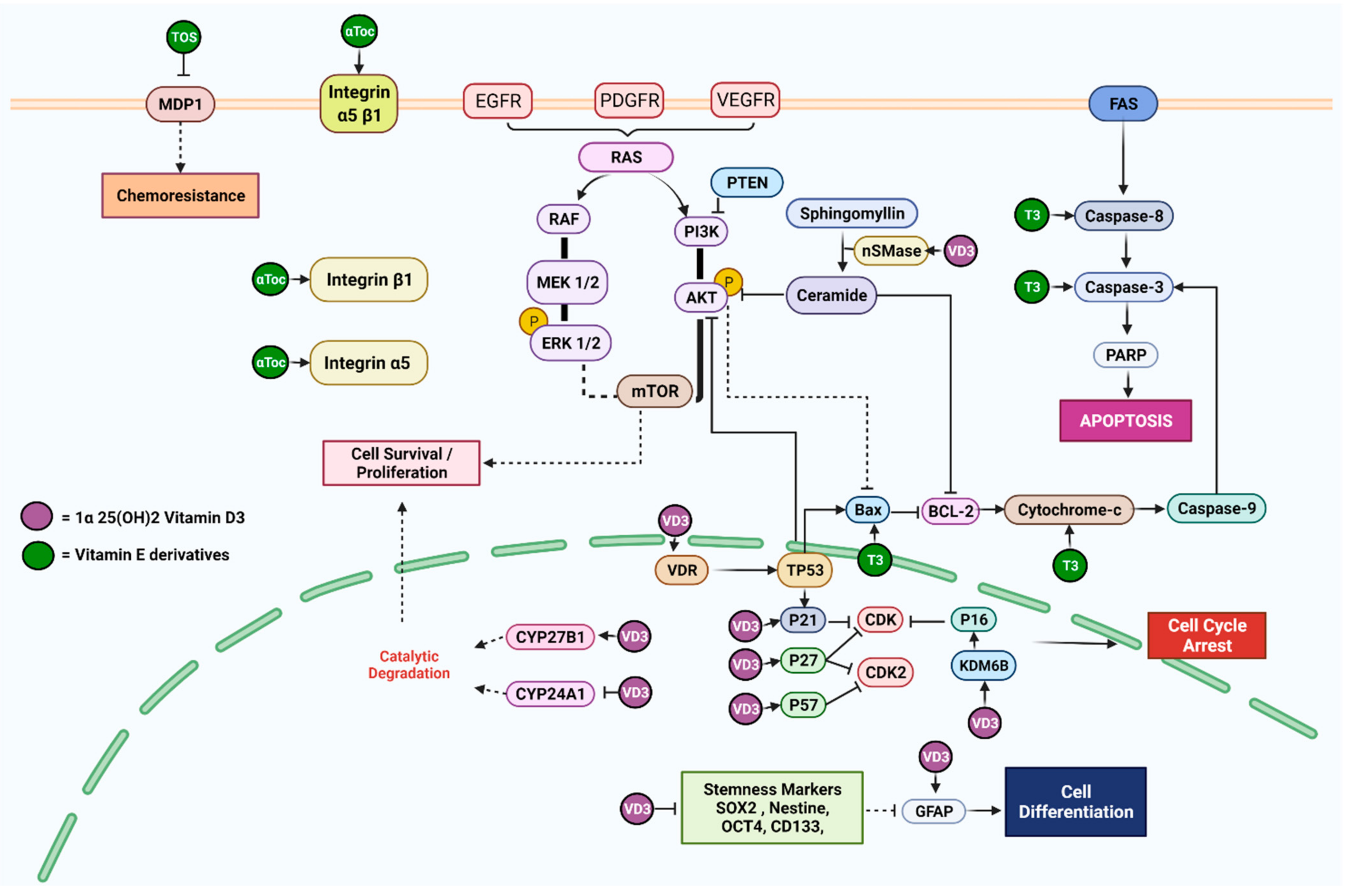
| [25] | To interrogate the possible functions of 1α,25(OH)2 vitamin D3 on mutant P53 and wild-type GBM cell lines | GL15 (wild-type P53), U251, and LN18 (mutant P53) | VD3 | 100 nM and 400 nM for 24 h | VD3 act via vitamin D receptor (VDR) in GL15 cells at a concentration of 100 nM as well as neutral sphingomyelinase1 by increasing the expression nSMase, aSMase, and GFAP, while increasing ceramide levels in U251 and LN18 cells at a concentration of 400 nM | The differentiation of mutant p53 (U251 and LN18) cells induced by neutral Sphingomyelinase1 enzyme was observed following a 24 h treatment with 400 nM (high dose) VD3 using the immunofluorescence method |
| [26] | To investigate the effects of 1α,25(OH)2 vitamin D3 on the expression of stemness markers in stem cell-like glioma cells in an acidic microenvironment | U87MG, T98G, and U251 | VD3 | 10 nM and 100 nM in 4, 8, 12, 24, and 48 h intervals | VD3 10 nM or 100 nM treatment for 4–24 h suppressed the expression of stemness markers (Nestin, Oct4, and Sox2) on stem-like glioma cells | Acidosis induced the self-renewal ability of neurospheres, which were markedly reduced when treated with 10 or 100 nM VD3 under pH 7.4 and pH 6.8 conditions. VD3 inhibited the ATP production of mitochondria and rescued the acidosis triggered ATP enrichment |
| [27] | To investigate the effects of 1α,25(OH)2 vitamin D3 on the expression of senescence markers in glioma cells | U87MG and U251 | VD3 | 10, 100, or 500 nM for 48 h | VD3 significantly increased the senescent markers INK4A (p16) and CDKN1A (p21) and promoted the expression of histone demethylase KDM6B glioma cells, while it does not affect vitamin D receptor expression | KDM6B knockdown attenuated VD3 and induced the senescence of glioma and reduced INK4A and CDKN1A upregulation |
| [28] | To investigate the expression of vitamin D receptor (VDR) in human glioma tissues To evaluate the effect of 1α,25(OH)2 vitamin D3 on cell survival and the modulation of the cell cycle in GBM cell lines | U251, U87MG, and T98G | VD3 | 1 μM for 96 h | VD3 reduced cell survival and induced cell cycle arrest witnessed by the increase in the expression of p57, p27, and p21 and a decrease in Cyclin D1 expression | An increase in the expression of VDR was denoted in human GBM cells as compared with the non-malignant control |
| [29] | To analyze five 1,25(OH)2 VD3-resistant GBM cell lines for key components of notch-signaling pathways using conventional RT-PCR | TX3868, U373, U118, TX3095, and U87 | VD3 | 10−6 mol/L (4 h) 10−8 mol/L (24 h) | Treatment with various concentrations of VD3 failed to modulate the expression of any key component of the notch-signaling pathway | Combination treatment of VD3 with TSA or 5-aza did not enhance antiproliferative effect. but in fact reduced it, indicating a protective antagonizing effect of VD3 against the effect of other treatments |
| [30] | To detect the metabolism of vitamin D3 by tracking the expression of CYP27B1 splice variants To investigate the effect of vitamin D3 metabolites on GBM cell proliferation | TX3868 and TX3095 | VD3 | 10–8 mol/L (24 h.) | VD3 increased the expression of CYP27B1, 1α25-dihydroxy vitamin D3-24 hydroxylase (CYP24), showing that GBM cell lines were able to metabolize VD3, while it showed no effect on the expression of VDR | VD3 metabolites increased the proliferation of GBM cell lines in a dose-dependent manner |
| [31] | To investigate the anticancer effects of vitamin C/E and Methotrexate on GBM | DBTRG | α-Toc | 5 µM (24, 48, and 92 h) | α-Toc did not reveal any changes in the expression of proteins related to the caspase-3 death pathway (Cleaved PARP, Caspase-3, and Cleaved Caspase-3) on its own in the GBM cell line | The combination of vitamin E with low dose (0.01 µM) methotrexate displayed a significant anti-cancer effect on the GBM cell line through the activation of the Caspase-3 pathway |
| [32] | To apply combinatorial approach with the joint application of γ-Tocotrienol and jerantinine A to minimize toxicity towards non-cancerous cells and improve potency on brain cancer cells | U87MG | γ-T3 | 3.17 μg/mL (24 h) | Individual treatment with γ-T3 induced anti-proliferative effect on U87MG cells through multiple mechanisms: upregulation of pro-apoptotic protein Bax, TRAIL and Caspase-3 and Caspase-8 enzymatic activity, while inducing cell cycle arrest at G0/G1 phase and double-stranded breaks | The combined use of γ-T3 and jerantinine A induced the disruption of the microtubules network and Fas and p53 activation, triggering apoptosis. This demonstrated an improved potency of γ-T3-induced apoptosis through death receptor and mitochondrial pathways |
| [33] | To compare the cytotoxicity potency of alpha-, gamma-, and delta-tocotrienol and to explore the resultant apoptotic mechanism in glioblastoma U87MG cells | U87MG | α-, γ-, and δ-T3 | 24 h IC50 concentrations: α-T3 (2.0 µM), γ-T3 (3.0 µM), and δ-T3 (1.0 µM) | α-, γ-, and δ-T3 efficiently inhibited cell growth in a time- and concentration-dependent manner by triggering both intrinsic (Bax, Bid, and Cytochrome c), which was confirmed by a decrease in mitochondrial membrane permeability (MMP), and extrinsic (Caspase-8) pathways of apoptosis downstream signaling components | δ-T3 was found to be the most potent isomer among the tested isomers of tocotrienol |
| [34] | To evaluate the effect of γ- and α-tocopherol on the proliferation, integrin expression, adhesion, and migration of human GBM cells | U87MG | γ-Toc and α-Toc | 50 μM for 6 h | Both γ- and α-toc increased the expression of integrin α5 and β1 protein and cell surface heterodimer integrin α5 β1, resulting in decreased proliferation and adhesion to fibronectin | γ-Toc exerted more anti-proliferative effect than α-Toc, while having a controversial impact on cell migration |
| [35] | To evaluate the effect of α-tocopheryl succinate on the expression of MRP1 and intracellular glutathione level in GMB when co-treated with VP-16 | U87MG, T98G and U251MG, SNU 489 | α-TOS | 50, 100, and 150 μM | α-TOS decreased the expression of MDP1 and resulted in chemosensitization of GBM cells to VP-16 | α-TOS decreased the intracellular concentration of glutathione |
| [36] | To characterize the efficacy and possible mechanisms of the combination of sorafenib and vitamin K1 on glioma cell lines | BT325 and U251 | VK1 | 50 μM for 24 h | VK1 as an individual treatment failed to induce apoptosis or changes to the Raf/MEK/ERK signaling pathway | VK1 enhanced the cytotoxicity of sorafenib by repressing the Raf/MEK/ERK signaling pathway and inducing apoptosis in GBM cell lines |
3.2.2. Vitamin D3
3.2.3. Vitamin E
3.2.4. Vitamin K1
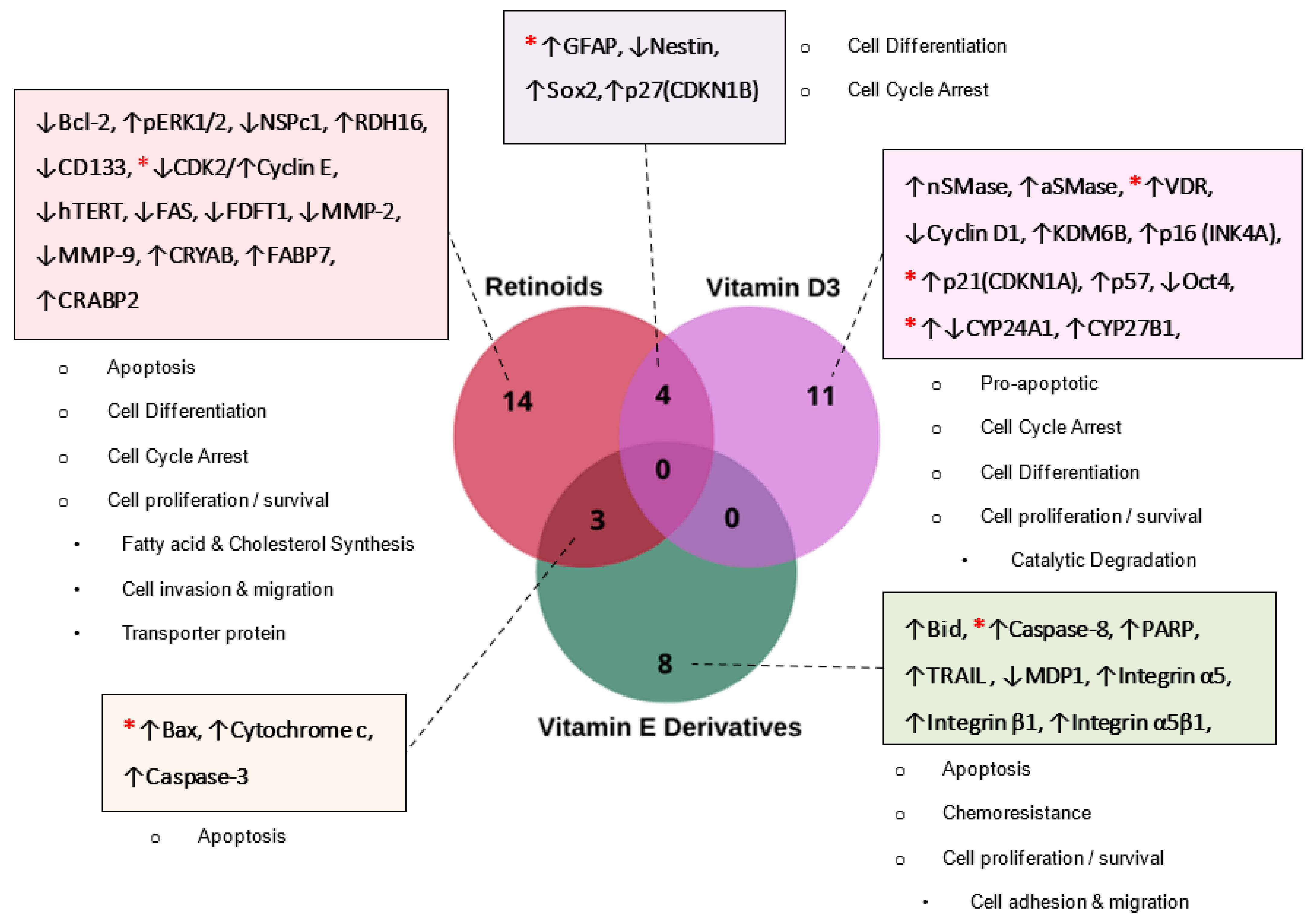
3.3. Differentially Expressed and Replicable Biomarkers Modulated by Lipid-Soluble Vitamins in GBM
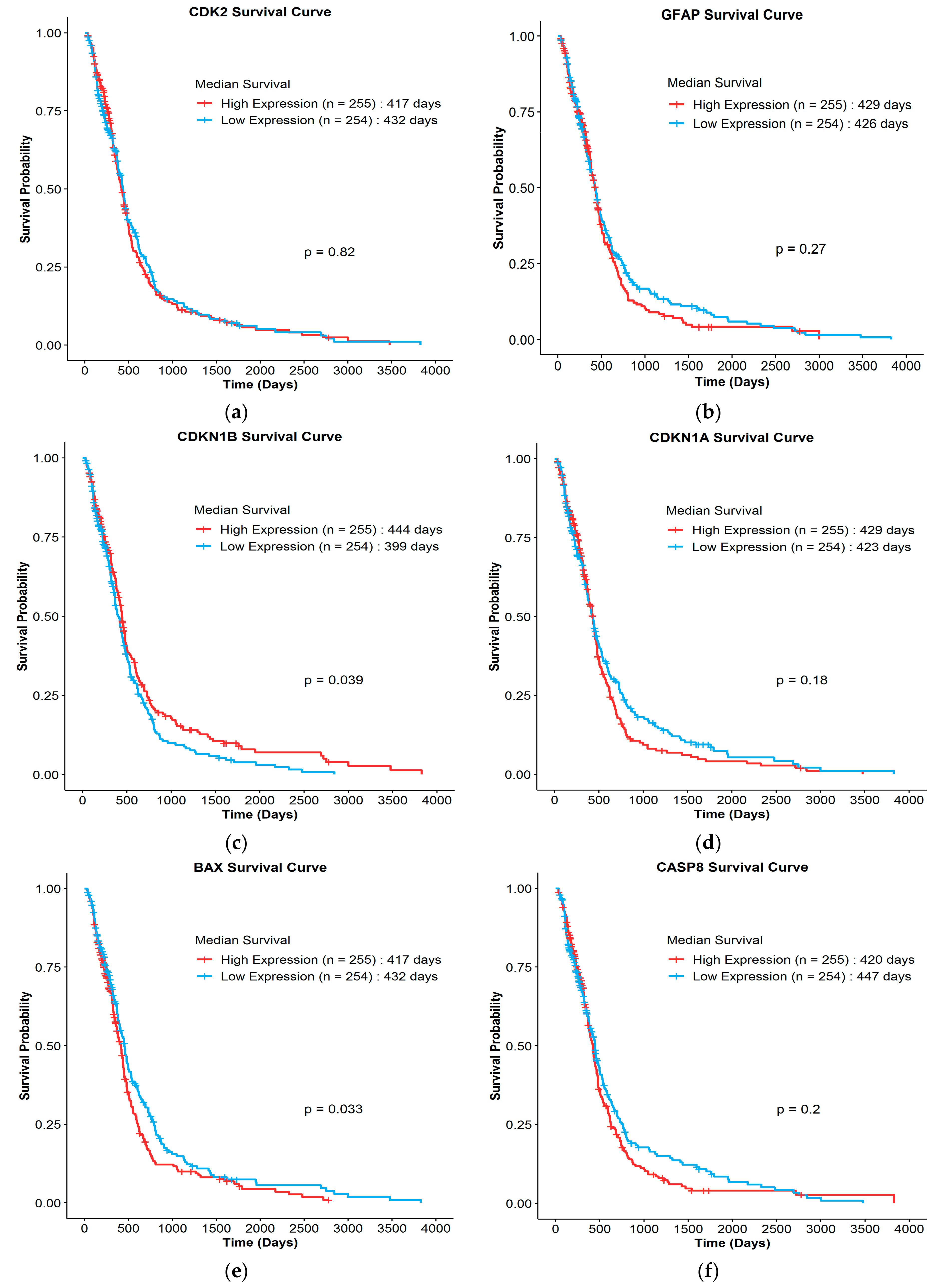
3.4. Clinical Survival Correlation of Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 13 CRA | 13-cis retinoic acid |
| ATRA | all-trans retinoic acid |
| aSMase | acidic sphingomyelinases |
| BBB | blood–brain barrier |
| Bax | BCL2-associated X protein |
| Bcl-2 | B-cell lymphoma |
| Bid | BH3 interacting domain death agonist |
| CDK2 | cyclin-dependent kinase 2 |
| CDKN1 | cyclin-dependent kinase inhibitors |
| CDKN1A | cyclin-dependent kinase inhibitor 1A |
| CDKN1B | cyclin-dependent kinase inhibitor 1B |
| CRYAB | alpha-crystallin B chain |
| CSCs | cancer stem cells |
| CYP24 | cytochrome P450 family 24 |
| CYP24A1 | cytochrome P450 family 24 subfamily A member 1 |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| FABP7 | fatty acid binding protein 7 |
| FAS | fatty acid synthase |
| Fas | FS-7-associated surface antigen |
| FDFT1 | farnesyl-diphosphate farnesyltransferase 1 |
| GBM | glioblastoma |
| GFAP | glial fibrillary acidic protein |
| hTERT | human telomerase protein reverse transcriptase |
| IC50 | half maximum inhibitory concentration |
| IDH | isocitrate dehydrogenase |
| KDM6B | lysine demethylase 6B |
| MDP1 | multidrug resistance protein 1 |
| MEK | mitogen-activated protein kinase |
| MGMT | O6-methylguanine methyl transferase |
| MMP-2 | metalloproteinase 2 |
| MMP-9 | metalloproteinase 9 |
| Nestin | neuroepithelial stem cell protein |
| nSMase 1 | neutral sphingomyelinase 1 |
| NSPc1 | nervous system polycomb |
| Oct4 | octamer-binding transcription factor 4 |
| p16INK4a | cyclin-dependent kinase inhibitor 2A |
| p53 | tumor suppressor protein |
| PARP | Poly (ADP-ribose) polymerase |
| PERK1/2 | activated extracellular signal-regulated kinase ½ |
| PKC | protein kinase C |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| PTEN | phosphatase and tensin homolog |
| RA | retinoic acid |
| Raf | rapidly accelerated fibrosarcoma |
| RAR | retinoic acid receptor |
| RDH16 | retinol dehydrogenase 16 |
| RXR | retinoid x receptor |
| SCL | stem cell-like |
| Sox2 | sex-determining region Y-box 2 |
| TCGA | the cancer genome atlas |
| TMZ | temozolomide |
| T3 | tocotrienol |
| TOC | tocopherol |
| TOS | alpha tocopheryl succinate |
| TRAIL | TNF-related apoptosis-inducing ligand |
| VDR | vitamin D receptor |
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhanwar-Uniyal, M.; Labagnara, M.; Friedman, M.; Kwasnicki, A.; Murali, R. Glioblastoma: Molecular pathways, stem cells and therapeutic targets. Cancers 2015, 7, 538–555. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, A.L.V.; Gomes, I.N.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, V.; Islam, M.M.; Yang, M.; Slaby, R.; Ramirez, H.M.; Mirza, S.P. Glioblastoma: Pathogenesis and current status of chemotherapy and other novel treatments. Cancers 2020, 12, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Miranda, A.; Blanco-Prieto, M.; Sousa, J.; Pais, A.; Vitorino, C. Breaching barriers in glioblastoma. Part I: Molecular pathways and novel treatment approaches. Int. J. Pharm. 2017, 531, 372–388. [Google Scholar] [CrossRef]
- Mamede, A.C.; Tavares, S.D.; Abrantes, A.M.; Trindade, J.; Maia, J.M.; Botelho, M.F. The role of vitamins in cancer: A review. Nutr. Cancer 2011, 63, 479–494. [Google Scholar] [CrossRef]
- Jain, A.; Tiwari, A.; Verma, A.; Jain, S. Vitamins for cancer prevention and treatment: An insight. Curr. Mol. Med. 2017, 17, 321–340. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Innovation, V.H. Covidence Systematic Review Software: Melbourne, Australia. 2014. Available online: www.covidence.org (accessed on 22 June 2021).
- Hupe, M. EndNote X9. J. Electron. Resour. Med. Libr. 2019, 16, 117–119. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data; AACR: Philadelphia, PA, USA, 2012. [Google Scholar]
- Zhang, D.; Holmes, W.F.; Wu, S.; Soprano, D.R.; Soprano, K.J. Retinoids and ovarian cancer. J. Cell. Physiol. 2000, 185, 1–20. [Google Scholar] [CrossRef]
- Trump, D. Retinoids in bladder, testis and prostate cancer: Epidemiologic, pre-clinical and clinical observations. Leukemia 1994, 8, S50–S54. [Google Scholar]
- Facchini, G.; Ignarro, R.S.; Rodrigues-Silva, E.; Vieira, A.S.; Lopes-Cendes, I.; Castilho, R.F.; Rogerio, F. Toxic effects of phytol and retinol on human glioblastoma cells are associated with modulation of cholesterol and fatty acid biosynthetic pathways. J. Neurooncol. 2018, 136, 435–443. [Google Scholar] [CrossRef]
- Hu, P.S.; Xia, Q.S.; Wu, F.; Li, D.K.; Qi, Y.J.; Hu, Y.; Wei, Z.Z.; Li, S.S.; Tian, N.Y.; Wei, Q.F.; et al. NSPc1 promotes cancer stem cell self-renewal by repressing the synthesis of all-trans retinoic acid via targeting RDH16 in malignant glioma. Oncogene 2017, 36, 4706–4718. [Google Scholar] [CrossRef]
- Friedman, M.D.; Jeevan, D.S.; Tobias, M.; Murali, R.; Jhanwar-Uniyal, M. Targeting cancer stem cells in glioblastoma multiforme using mTOR inhibitors and the differentiating agent all-trans retinoic acid. Oncol. Rep. 2013, 30, 1645–1650. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.Z.; Li, S.; Garcia, E.; Glubrecht, D.D.; Poon, H.Y.; Easaw, J.C.; Godbout, R. Association between cytoplasmic CRABP2, altered retinoic acid signaling, and poor prognosis in glioblastoma. Glia 2016, 64, 963–976. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Yang, L.; Guo, S.W. All-trans retinoic acid inhibits migration, invasion and proliferation, and promotes apoptosis in glioma cells in vitro. Oncol. Lett. 2015, 9, 2833–2838. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Banik, N.L.; Ray, S.K. Combination of all-trans retinoic acid and interferon-gamma upregulated p27kip1 and down regulated CDK2 to cause cell cycle arrest leading to differentiation and apoptosis in human glioblastoma LN18 (PTEN-proficient) and U87MG (PTEN-deficient) cells. Cancer Chemother. Pharmacol. 2008, 62, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. Retinoids induced astrocytic differentiation with down regulation of telomerase activity and enhanced sensitivity to taxol for apoptosis in human glioblastoma T98G and U87MG cells. J. Neuro-Oncol. 2008, 87, 9–22. [Google Scholar] [CrossRef]
- Cataldi, S.; Arcuri, C.; Lazzarini, A.; Nakashidze, I.; Ragonese, F.; Fioretti, B.; Ferri, I.; Conte, C.; Codini, M.; Beccari, T.; et al. Effect of 1α,25(OH)2 vitamin D3 in mutant P53 glioblastoma cells: Involvement of neutral sphingomyelinase1. Cancers 2020, 12, 3163. [Google Scholar] [CrossRef]
- Hu, P.; Li, S.; Tian, N.; Wu, F.; Hu, Y.; Li, D.; Qi, Y.; Wei, Z.; Wei, Q.; Li, Y.; et al. Acidosis enhances the self-renewal and mitochondrial respiration of stem cell-like glioma cells through CYP24A1-mediated reduction of vitamin D. Cell Death Dis. 2019, 10, 25. [Google Scholar] [CrossRef]
- Sui, A.; Xu, Y.; Pan, B.; Guo, T.; Wu, J.; Shen, Y.; Yang, J.; Guo, X. Histone demethylase KDM6B regulates 1,25-dihydroxyvitamin D3-induced senescence in glioma cells. J. Cell. Physiol. 2019, 234, 17990–17998. [Google Scholar] [CrossRef]
- Salomón, D.G.; Fermento, M.E.; Gandini, N.A.; Ferronato, M.J.; Arévalo, J.; Blasco, J.; Andrés, N.C.; Zenklusen, J.C.; Curino, A.C.; Facchinetti, M.M. Vitamin D receptor expression is associated with improved overall survival in human glioblastoma multiforme. J. Neurooncol 2014, 118, 49–60. [Google Scholar] [CrossRef]
- Reichrath, S.; Müller, C.S.; Gleissner, B.; Pfreundschuh, M.; Vogt, T.; Reichrath, J. Notch- and vitamin D signaling in 1,25(OH)2D3-resistant glioblastoma multiforme (GBM) cell lines. J. Steroid Biochem. Mol. Biol. 2010, 121, 420–424. [Google Scholar] [CrossRef]
- Diesel, B.; Radermacher, J.; Bureik, M.; Bernhardt, R.; Seifert, M.; Reichrath, J.; Fischer, U.; Meese, E. Vitamin D(3) metabolism in human glioblastoma multiforme: Functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin. Cancer Res. 2005, 11, 5370–5380. [Google Scholar] [CrossRef] [Green Version]
- Yiang, G.T.; Chen, T.Y.; Chen, C.; Hung, Y.T.; Hsueh, K.C.; Wu, T.K.; Pan, Y.R.; Chien, Y.C.; Chen, C.H.; Yu, Y.L. Antioxidant vitamins promote anticancer effects on low-concentration methotrexate-treated glioblastoma cells via enhancing the caspase-3 death pathway. Food Sci. Nutr. 2021, 9, 3308–3316. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Lim, K.H.; Kam, T.S.; Loh, H.S. Enhancement of apoptotic activities on brain cancer cells via the combination of gamma-tocotrienol and jerantinine A. Phytomedicine 2017, 30, 74–84. [Google Scholar] [CrossRef]
- Lim, S.W.; Loh, H.S.; Ting, K.N.; Bradshaw, T.D.; Zeenathul, N.A. Cytotoxicity and apoptotic activities of alpha-, gamma- and delta-tocotrienol isomers on human cancer cells. BMC Complement. Altern. Med. 2014, 14, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samandari, E.; Visarius, T.; Zingg, J.M.; Azzi, A. The effect of γ-tocopherol on proliferation, integrin expression, adhesion, and migration of human glioma cells. Biochem. Biophys. Res. Commun. 2006, 342, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Lee, E.; Youk, H.J.; Kim, S.H.; Lee, H.J.; Park, Y.G.; Lim, S.J. Potentiation by alpha-tocopheryl succinate of the etoposide response in multidrug resistance protein 1-expressing glioblastoma cells. Cancer Lett. 2005, 217, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, J.R.; Wang, D.L.; Gong, K.; Zhang, Q.J. Vitamin K1 enhances sorafenib-induced growth inhibition and apoptosis of human malignant glioma cells by blocking the Raf/MEK/ERK pathway. World J. Surg. Oncol. 2012, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Raval-Pandya, M.; Wernyj, R.P.; Yang, W. Genomic mechanisms involved in the pleiotropic actions of 1, 25-dihydroxyvitamin D3. Biochem. J. 1996, 316, 361–371. [Google Scholar] [CrossRef]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the central nervous system. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef]
- Cardenas, E.; Ghosh, R. Vitamin E: A dark horse at the crossroad of cancer management. Biochem. Pharmacol. 2013, 86, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Constantinou, C.; Charalambous, C.; Kanakis, D. Vitamin E and cancer: An update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 2020, 59, 845–857. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Pédeboscq, S.; Rey, C.; Petit, M.; Harpey, C.; De Giorgi, F.; Ichas, F.; Lartigue, L. Non-antioxidant properties of α-tocopherol reduce the anticancer activity of several protein kinase inhibitors in vitro. PLoS ONE 2012, 7, e36811. [Google Scholar] [CrossRef]
- Suttie, J. The importance of menaquinones in human nutrition. Annu. Rev. Nutr. 1995, 15, 399–417. [Google Scholar] [CrossRef]
- Zou, J.; Lei, T.; Guo, P.; Yu, J.; Xu, Q.; Luo, Y.; Ke, R.; Huang, D. Mechanisms shaping the role of ERK1/2 in cellular senescence. Mol. Med. Rep. 2019, 19, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef] [Green Version]
- Venneti, S.; Mischel, P. Metabolic reprogramming in cancer–a coordinated effort. Brain Pathol. 2015, 25, 753–754. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.Y.; Sekar, K.; Seshachalam, P.; Shen, J.; Chow, W.Y.; Lau, C.C.; Yang, H.; Park, J.; Kang, S.G.; Li, X.; et al. Relevance of a TCGA-derived glioblastoma subtype gene-classifier among patient populations. Sci. Rep. 2019, 9, 7442. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, D.E.-R.; Phon, B.W.S.; Kamarudin, M.N.A.; Ponnampalam, S.N.; Radhakrishnan, A.K.; Bhuvanendran, S. Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma. Nutrients 2022, 14, 2873. https://doi.org/10.3390/nu14142873
Osman DE-R, Phon BWS, Kamarudin MNA, Ponnampalam SN, Radhakrishnan AK, Bhuvanendran S. Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma. Nutrients. 2022; 14(14):2873. https://doi.org/10.3390/nu14142873
Chicago/Turabian StyleOsman, Dina El-Rabie, Brandon Wee Siang Phon, Muhamad Noor Alfarizal Kamarudin, Stephen Navendran Ponnampalam, Ammu Kutty Radhakrishnan, and Saatheeyavaane Bhuvanendran. 2022. "Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma" Nutrients 14, no. 14: 2873. https://doi.org/10.3390/nu14142873
APA StyleOsman, D. E.-R., Phon, B. W. S., Kamarudin, M. N. A., Ponnampalam, S. N., Radhakrishnan, A. K., & Bhuvanendran, S. (2022). Biomarkers Regulated by Lipid-Soluble Vitamins in Glioblastoma. Nutrients, 14(14), 2873. https://doi.org/10.3390/nu14142873






