Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto’s Thyroiditis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methods
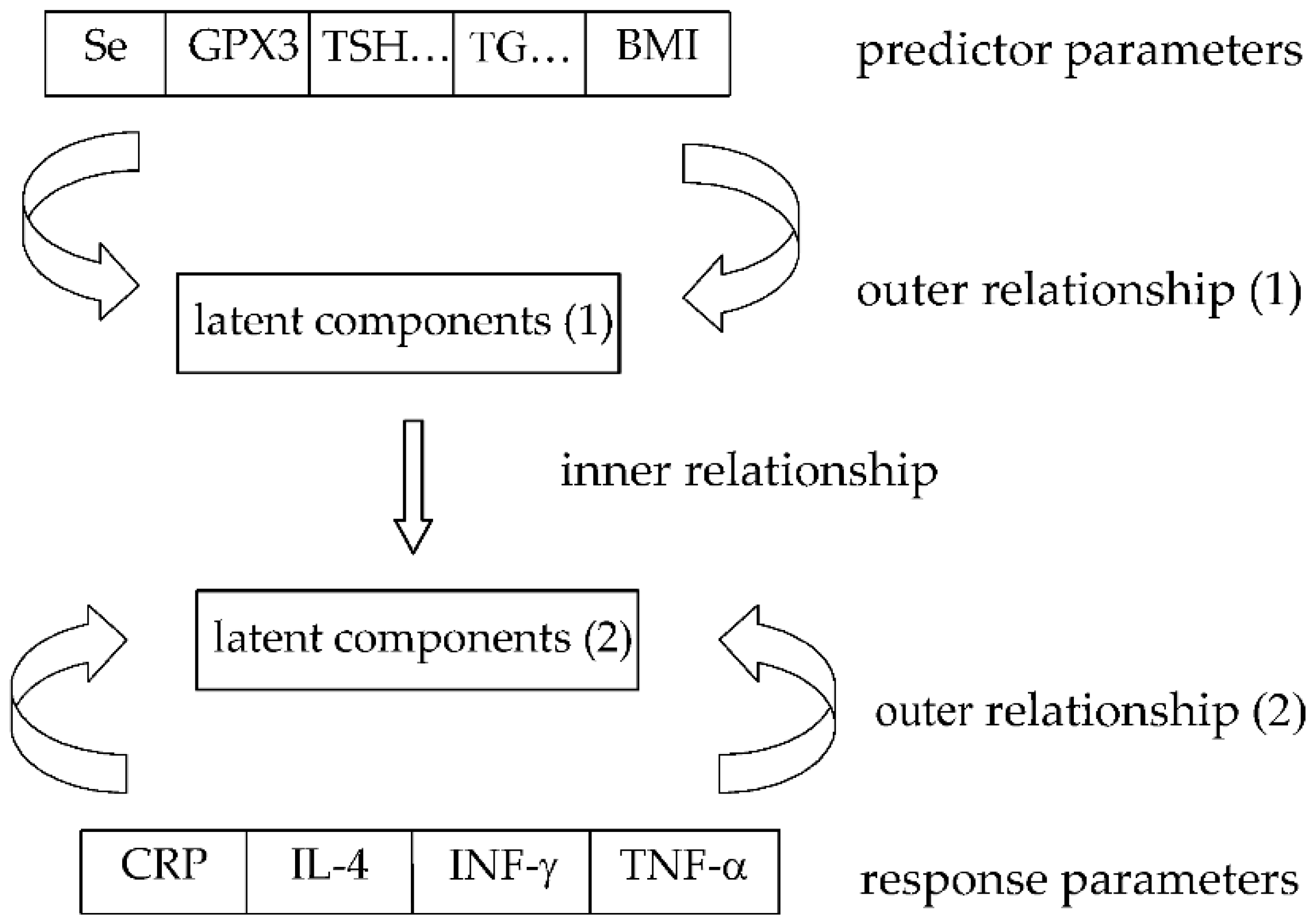
2.3. Statistical Approach
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayter, S.M.; Cook, M.C. Updated Assessment of the Prevalence, Spectrum and Case Definition of Autoimmune Disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.A.; Cooper, D.S. The Incidence and Prevalence of Thyroid Autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmune Thyroid Disease: Old and New Players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; di Domenicantonio, A.; Fallahi, P. Autoimmune Thyroid Disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Colin, I.M.; Isaac, J.; Dupret, P.; Ledant, T.; D’Hautcourt, J.L. Functional Lymphocyte Subset Assessment of the Th1/Th2 Profile in Patients with Autoimmune Thyroiditis by Flowcytometric Analysis of Peripheral Lymphocytes. J. Biol. Regul. Homeost. Agents. 2004, 18, 72–76. [Google Scholar]
- Nanba, T.; Watanabe, M.; Inoue, N.; Iwatani, Y. Increases of the Th1/Th2 Cell Ratio in Severe Hashimoto’s Disease and in the Proportion of Th17 Cells in Intractable Graves’ Disease. Thyroid 2009, 19, 495–501. [Google Scholar] [CrossRef]
- Horie, I.; Abiru, N.; Nagayama, Y.; Kuriya, G.; Saitoh, O.; Ichikawa, T.; Iwakura, Y.; Eguchi, K. T Helper Type 17 Immune Response Plays an Indispensable Role for Development of Iodine-Induced Autoimmune Thyroiditis in Nonobese Diabetic-H2 h4 Mice. Endocrinology 2009, 150, 5135–5142. [Google Scholar] [CrossRef]
- Hemdan, N.Y.; Birkenmeier, G.; Wichmann, G.; Abu El-Saad, A.M.; Krieger, T.; Conrad, K.; Sack, U. Interleukin-17-Producing T Helper Cells in Autoimmunity. Autoimmun. Rev. 2010, 9, 785–792. [Google Scholar] [CrossRef]
- Konca Degertekin, C.; Aktas Yilmaz, B.; Balos Toruner, F.; Kalkanci, A.; Turhan Iyidir, O.; Fidan, I.; Yesilyurt, E.; Cakır, N.; Kustimur, S.; Arslan, M. Circulating Th17 Cytokine Levels Are Altered in Hashimoto’s Thyroiditis. Cytokine 2016, 80, 13–17. [Google Scholar] [CrossRef]
- Bending, D.; de La Peña, H.; Veldhoen, M.; Phillips, J.M.; Uyttenhove, C.; Stockinger, B.; Cooke, A. Highly Purified Th17 Cells from BDC2.5NOD Mice Convert into Th1-like Cells in NOD/SCID Recipient Mice. J. Clin. Investig. 2009, 119, 565–572. [Google Scholar] [CrossRef]
- Bossowski, A.; Moniuszko, M.; Dąbrowska, M.; Sawicka, B.; Rusak, M.; Jeznach, M.; Wójtowicz, J.; Bodzenta-Lukaszyk, A.; Bossowska, A. Lower Proportions of CD4+CD25high and CD4+FoxP3, but Not CD4+CD25+CD127low FoxP3+T Cell Levels in Children with Autoimmune Thyroid Diseases. Autoimmunity 2013, 46, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, T.; Shi, R.; Zhou, X.; Qu, J.; Xu, J.; Shan, Z.; Teng, W. Effect of Iodine Excess on Th1, Th2, Th17, and Treg Cell Subpopulations in the Thyroid of NOD.H-2h4 Mice. Biol. Trace Elem. Res. 2014, 159, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, X.; Tian, J.; Zhu, C.; Peng, H.; Rui, K.; Wang, Y.; Mao, C.; Ma, J.; Lu, L.; et al. Th17/Treg Cells Imbalance and GITRL Profile in Patients with Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2014, 15, 21674–21686. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, W.; Li, Y.; Shan, Z.; Li, Y.; Teng, X.; Gao, Y.; Fan, C.; Teng, W. Selenium Upregulates CD4 (+) CD25 (+) Regulatory T Cells in Iodine-Induced Autoimmune Thyroiditis Model of NOD.H-2(H4) Mice. Endocr. J. 2010, 57, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Johansson, C.C.; Kiessling, R. Naturally Occurring Regulatory T Cells Show Reduced Sensitivity toward Oxidative Stress-Induced Cell Death. Blood 2009, 113, 3542–3545. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Johansson, C.C.; Jitschin, R.; Bottcher, M.; Kiessling, R. Increased Thioredoxin-1 Production in Human Naturally Occurring Regulatory T Cells Confers Enhanced Tolerance to Oxidative Stress. Blood 2011, 117, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Balázs, C.; Kaczur, V. Effect of Selenium on HLA-DR Expression of Thyrocytes. Autoimmune Dis. 2012, 2012, 374635. [Google Scholar] [CrossRef]
- Nettore, I.C.; de Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium Supplementation Modulates Apoptotic Processes in Thyroid Follicular Cells. BioFactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Prochownik, E.; Błażewska-Gruszczyk, A.; Słowiaczek, M.; Sun, Q.; Schomburg, L.; Ochab, E.; Bartyzel, M. Positive Effects of Selenium Supplementation in Women with Newly Diagnosed Hashimoto’s Thyroiditis in an Area with Low Selenium Status. Int. J. Clin. Pract. 2021, 75, e14484. [Google Scholar] [CrossRef]
- Boulesteix, A.L.; Strimmer, K. Partial Least Squares: A Versatile Tool for the Analysis of High-Dimensional Genomic Data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in Metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. IFN-Gamma, IL-5, IL-6 and IgE in Patients Infected with Giardia Intestinalis. Folia Histochem. Cytobiol. 2009, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rybacka-Chabros, B.; Pietrzak, A.; Milanowski, J.; Chabros, P. Influence of Prednisone on Serum Level of Tumor Necrosis Factor Alpha, Interferon Gamma and Interleukin-1 Beta, in Active Pulmonary Tuberculosis. Pol. J. Public Health 2014, 124, 42–45. [Google Scholar] [CrossRef]
- Undén, A.-L.; Andréasson, A.; Elofsson, S.; Brismar, K.; Mathsson, L.; Rönnelid, J.; Lekander, M. Inflammatory Cytokines, Behaviour and Age as Determinants of Self-Rated Health in Women. Clin. Sci. 2007, 112, 363–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hueso, L.; Ortega, R.; Selles, F.; Wu-Xiong, N.Y.; Ortega, J.; Civera, M.; Ascaso, J.F.; Sanz, M.-J.; Real, J.T.; Piqueras, L. Upregulation of Angiostatic Chemokines IP-10/CXCL10 and I-TAC/CXCL11 in Human Obesity and Their Implication for Adipose Tissue Angiogenesis. Int. J. Obes. 2018, 42, 1406–1417. [Google Scholar] [CrossRef]
- Iwanowski, P.; Losy, J.; Kramer, L.; Wójcicka, M.; Kaufman, E. CXCL10 and CXCL13 Chemokines in Patients with Relapsing Remitting and Primary Progressive Multiple Sclerosis. J. Neurol. Sci. 2017, 380, 22–26. [Google Scholar] [CrossRef]
- Zajkowska, A.; Garkowski, A.; Świerzbińska, R.; Kułakowska, A.; Król, M.E.; Ptaszyńska-Sarosiek, I.; Nowicka-Ciełuszecka, A.; Pancewicz, S.; Czupryna, P.; Moniuszko, A.; et al. Evaluation of Chosen Cytokine Levels among Patients with Herpes Zoster as Ability to Provide Immune Response. PLoS ONE 2016, 11, e0150301. [Google Scholar] [CrossRef]
- Mikos, H.; Mikos, M.; Rabska-Pietrzak, B.; Niedziela, M. The Clinical Role of Serum Concentrations of Selected Cytokines: IL-1 β, TNF-α and IL-6 in Diagnosis of Autoimmune Thyroid Disease (AITD) in Children. Autoimmunity 2014, 47, 466–472. [Google Scholar] [CrossRef]
- Phenekos, C.; Vryonidou, A.; Gritzapis, A.D.; Baxevanis, C.N.; Goula, M.; Papamichail, M. Th1 and Th2 Serum Cytokine Profiles Characterize Patients with Hashimoto’s Thyroiditis (Th1) and Graves’ Disease (Th2). Neuroimmunomodulation 2004, 11, 209–213. [Google Scholar] [CrossRef]
- Altay, M.; Ateş, İ.; Yılmaz, F.M.; Topçuoğlu, C.; Kaplan, M. The role of circulating sTWEAK in the pathogenesis of Hashimoto’s thyroiditis—A pilot study. Endokrynol. Pol. 2016, 67, 562–566. [Google Scholar] [CrossRef][Green Version]
- Xue, H.; Yu, X.; Ma, L.; Song, S.; Li, Y.; Zhang, L.; Yang, T.; Liu, H. The Possible Role of CD4+CD25highFoxp3+/CD4+IL-17A+ Cell Imbalance in the Autoimmunity of Patients with Hashimoto Thyroiditis. Endocrine 2015, 50, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Gutkowski, K.; Gutkowska, D.; Kiszka, J.; Partyka, M.; Kacperek-Hartleb, T.; Kajor, M.; Hartleb, M. Serum IL-17 Level Predicts Inflammatory Activity in Patients with Autoimmune Hepatitis. Pol. Arch. Intern. Med. 2018, 128, 150–156. [Google Scholar] [CrossRef]
- Pearce, E.N.; Bogazzi, F.; Martino, E.; Brogioni, S.; Pardini, E.; Pellegrini, G.; Parkes, A.B.; Lazarus, J.H.; Pinchera, A.; Braverman, L.E. The Prevalence of Elevated Serum C-Reactive Protein Levels in Inflammatory and Noninflammatory Thyroid Disease. Thyroid 2003, 13, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, C.; Bagnasco, M.; Pesce, G.; Montagna, P.; Brizzolara, R.; Altrinetti, V.; Richiusa, P.; Galluzzo, A.; Giordano, C. Regulation of Apoptosis in Endocrine Autoimmunity: Insights from Hashimoto’s Thyroiditis and Graves’ Disease. Ann. N. Y. Acad. Sci. 2002, 966, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fazle Akbar, S.M.; Zhen, Z.; Luo, Y.; Deng, L.; Huang, H.; Chen, L.; Li, W. Analysis of the Expression of Fas, FasL and Bcl-2 in the Pathogenesis of Autoimmune Thyroid Disorders. Cell. Mol. Immunol. 2004, 1, 224–228. [Google Scholar] [PubMed]
- Wang, S.H.; van Antwerp, M.; Kuick, R.; Gauger, P.G.; Doherty, G.M.; Fan, Y.Y.; Baker, J.R. Microarray Analysis of Cytokine Activation of Apoptosis Pathways in the Thyroid. Endocrinology 2007, 148, 4844–4852. [Google Scholar] [CrossRef] [PubMed]
- Marique, L.; van Regemorter, V.; Gérard, A.C.; Craps, J.; Senou, M.; Marbaix, E.; Rahier, J.; Daumerie, C.; Mourad, M.; Lengelé, B.; et al. The Expression of Dual Oxidase, Thyroid Peroxidase, and Caveolin-1 Differs According to the Type of Immune Response (TH1/TH2) Involved in Thyroid Autoimmune Disorders. J. Clin. Endocrinol. Metab. 2014, 99, 1722–1732. [Google Scholar] [CrossRef]
- Karanikas, G.; Schuetz, M.; Kontur, S.; Duan, H.; Kommata, S.; Schoen, R.; Antoni, A.; Kletter, K.; Dudczak, R.; Willheim, M. No Immunological Benefit of Selenium in Consecutive Patients with Autoimmune Thyroiditis. Thyroid 2008, 18, 7–12. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopien, B. The Effect of Levothyroxine and Selenomethionine on Lymphocyte and Monocyte Cytokine Release in Women with Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 2011, 96, 2206–2215. [Google Scholar] [CrossRef]
- Pilli, T.; Cantara, S.; Schomburg, L.; Cenci, V.; Cardinale, S.; Heid, E.C.D.; Kühn, E.C.; Cevenini, G.; Sestini, F.; Fioravanti, C.; et al. IFNγ-Inducible Chemokines Decrease upon Selenomethionine Supplementation in Women with Euthyroid Autoimmune Thyroiditis: Comparison between Two Doses of Selenomethionine (80 or 160 Μg) versus Placebo. Eur. Thyroid J. 2015, 4, 226–233. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Giuggioli, D.; Ferrannini, E.; Ferri, C.; Fallahi, P. Chemokine (C-X-C Motif) Ligand (CXCL)10 in Autoimmune Diseases. Autoimmun. Rev. 2014, 13, 272–280. [Google Scholar] [CrossRef]
- Gołąb, J.; Jakóbisiak, M.; Lasek, W.; Stokłosa, T. Immunologia, 7th ed.; PWN: Warsaw, Poland, 2017; ISBN 9788301194505. [Google Scholar]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of Short-Term Selenium Supplementation on the Natural Course of Hashimoto’s Thyroiditis: Clinical Results of a Blinded Placebo-Controlled Randomized Prospective Trial. J. Endocrinol. Investig. 2017, 40, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Nanba, T.; Watanabe, M.; Akamizu, T.; Iwatani, Y. The -590CC Genotype in the IL4 Gene as a Strong Predictive Factor for the Development of Hypothyroidism in Hashimoto Disease. Clin. Chem. 2008, 54, 621–623. [Google Scholar] [CrossRef]
- Paul, W.E. History of Interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef]
- Guclu, F.; Ozmen, B.; Kirmaz, C.; Kafesciler, S.O.; Degirmenci, P.B.; Taneli, F.; Hekimsoy, Z. Down-Regulation of the Auto-Aggressive Processes in Patients with Hypothyroid Hashimoto’s Thyroiditis Following Substitutive Treatment with L-Thyroxine. Eur. Cytokine Netw. 2009, 20, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, W.; Gu, R.; Zhang, Y.; Zhang, H.; Tang, K.; Xu, P.; Katirai, F.; Shi, W.; Wang, L.; et al. Th17 Cell Plays a Role in the Pathogenesis of Hashimoto’s Thyroiditis in Patients. Clin. Immunol. 2013, 149, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S.A.; Kammoun-Krichen, M.; Charfeddine, I.; Ayadi, H.; Bougacha-Elleuch, N.; Peraldi-Roux, S. IL-1β and TSH Disturb Thyroid Epithelium Integrity in Autoimmune Thyroid Diseases. Immunobiology 2013, 218, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Lacka, K.; Paradowska-Gorycka, A.; Maciejewski, A.; Kramer, L.; Herman, W.; Lacki, J. Interleukin 1 Beta (IL1beta) Gene Polymorphisms (SNP-511 and SNP + 3953) in Hashimoto’s Thyroiditis among the Polish Population. Exp. Clin. Endocrinol. Diabetes 2014, 122, 544–547. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Dai, F.; Shen, J.; Ren, C.; Zuo, C.; Zhang, Q. Elevated Interleukin-1β in Peripheral Blood Mononuclear Cells Contributes to the Pathogenesis of Autoimmune Thyroid Diseases, Especially of Hashimoto Thyroiditis. Endocr. Res. 2016, 41, 185–192. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but Not Transforming Growth Factor-β Are Essential for the Differentiation of Interleukin 17–Producing Human T Helper Cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Mezosi, E.; Wang, S.H.; Utsugi, S.; Bajnok, L.; Bretz, J.D.; Gauger, P.G.; Thompson, N.W.; Baker, J.R. Interleukin-1β and Tumor Necrosis Factor (TNF)-α Sensitize Human Thyroid Epithelial Cells to TNF-Related Apoptosis-Inducing Ligand-Induced Apoptosis through Increases in Procaspase-7 and Bid, and the Down-Regulation of P44/42 Mitogen-Activated Protein Kinase Activity. J. Clin. Endocrinol. Metab. 2004, 89, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, Ł.; Szczęch, J.; Bieniek, A.; Nowicka-Suszko, D.; Szepietowski, J.C. Increased Interleukin (IL)-17 Serum Levels in Patients with Hidradenitis Suppurativa: Implications for Treatment with Anti-IL-17 Agents. J. Am. Acad. Dermatol. 2017, 76, 670–675. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Zagrodzki, P.; Ochab, E.; Bartyzel, M.; Huk, D. The effectiveness of iodine prophylaxis in Krakow on the example of three age groups of women-a pilot study. Part. I. Urine iodine concentration. Bromatol. Chem. Toksykol. 2008, 41, 323–327. (In Polish) [Google Scholar]
- Lewkowicz, P.; Lewkowicz, N.; Tchórzewski, H. CD4+CD25+ T Regulatory Cells: Their Physiology and Role in Modulating Immune Response. Postepy Hig. Med. Dosw. 2005, 59, 362–370. [Google Scholar]
- Marazuela, M.; García-López, M.A.; Figueroa-Vega, N.; de la Fuente, H.; Alvarado-Sánchez, B.; Monsiváis-Urenda, A.; Sánchez-Madrid, F.; González-Amaro, R. Regulatory T Cells in Human Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2006, 91, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Glick, A.B.; Wodzinski, A.; Fu, P.; Levine, A.D.; Wald, D.N. Impairment of Regulatory T-Cell Function in Autoimmune Thyroid Disease. Thyroid 2013, 23, 871–878. [Google Scholar] [CrossRef]
- Aoki, C.A.; Borchers, A.T.; Li, M.; Flavell, R.A.; Bowlus, C.L.; Ansari, A.A.; Gershwin, M.E. Transforming Growth Factor β (TGF-β) and Autoimmunity. Autoimmun. Rev. 2005, 4, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. TGF-β and Regulatory T Cell in Immunity and Autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar] [CrossRef]
- Marchiori, R.C.; Pereira, L.A.F.; Naujorks, A.A.; Rovaris, D.L.; Meinerz, D.F.; Duarte, M.M.M.F.; Rocha, J.B.T. Improvement of Blood Inflammatory Marker Levels in Patients with Hypothyroidism under Levothyroxine Treatment. BMC Endocr. Disord. 2015, 15, 32. [Google Scholar] [CrossRef]
- Erden, S.; Buyukozturk, S.; Vural, P.; Değirmencioğlu, S. Acute-Phase Reactans in Hashimoto Thyroiditis. Int. Immunopharmacol. 2008, 8, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.I.; Fu, R.; Freeman, M.; Rogers, K.; Helfand, M. C-Reactive Protein as a Risk Factor for Coronary Heart Disease: A Systematic Review and Meta-Analyses for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Baek, S.H.; Jeon, H.K.; Kang, S.-M.; Kim, D.-S.; Kim, W.-S.; Kim, H.S.; Rha, S.W.; Park, J.S.; Seong, I.W.; et al. Correlations between the Level of High-Sensitivity C-Reactive Protein and Cardiovascular Risk Factors in Korean Adults with Cardiovascular Disease or Diabetes Mellitus: The CALLISTO Study. J. Atheroscler. Thromb. 2013, 20, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Thongtang, N.; Diffenderfer, M.R.; Ooi, E.M.M.; Asztalos, B.F.; Dolnikowski, G.G.; Lamon-Fava, S.; Schaefer, E.J. Linkage between C-Reactive Protein and Triglyceride-Rich Lipoprotein Metabolism. Metabolism 2013, 62, 369–375. [Google Scholar] [CrossRef][Green Version]
- Roef, G.L.; Rietzschel, E.R.; van Daele, C.M.; Taes, Y.E.; de Buyzere, M.L.; Gillebert, T.C.; Kaufman, J.-M. Triiodothyronine and Free Thyroxine Levels Are Differentially Associated with Metabolic Profile and Adiposity-Related Cardiovascular Risk Markers in Euthyroid Middle-Aged Subjects. Thyroid 2014, 24, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Paganelli, M.; Morabito, A.; Vedani, P.; Barbieri, M.; Paolisso, G.; Folli, F.; Pontiroli, A.E. Weight Loss Through Gastric Banding: Effects on TSH and Thyroid Hormones in Obese Subjects With Normal Thyroid Function. Obesity 2010, 18, 854–857. [Google Scholar] [CrossRef]
- Araujo, R.L.; Andrade, B.M.; Padrón, A.S.; Gaidhu, M.P.; Perry, R.L.S.; Carvalho, D.P.; Ceddia, R.B. High-Fat Diet Increases Thyrotropin and Oxygen Consumption without Altering Circulating 3,5,3′-Triiodothyronine (T3) and Thyroxine in Rats: The Role of Iodothyronine Deiodinases, Reverse T3 Production, and Whole-Body Fat Oxidation. Endocrinology 2010, 151, 3460–3469. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Wouters, H.J.C.M.; Slagter, S.N.; van Waateringe, R.P.; Muller Kobold, A.C.; van Vliet-Ostaptchouk, J.V.; Links, T.P.; van der Klauw, M.M. Thyroid Function and Metabolic Syndrome in the Population-Based LifeLines Cohort Study. BMC Endocr. Disord. 2017, 17, 65. [Google Scholar] [CrossRef]
- Larsen, P.R.; Zavacki, A.M. Role of the Iodothyronine Deiodinases in the Physiology and Pathophysiology of Thyroid Hormone Action. Eur. Thyroid J. 2012, 1, 232–242. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; Jørgensen, T. Small Differences in Thyroid Function May Be Important for Body Mass Index and the Occurrence of Obesity in the Population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Iacobellis, G.; Cristina Ribaudo, M.; Zappaterreno, A.; Valeria Iannucci, C.; Leonetti, F. Relationship of Thyroid Function with Body Mass Index, Leptin, Insulin Sensitivity and Adiponectin in Euthyroid Obese Women. Clin. Endocrinol. 2005, 62, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Nyrnes, A.; Jorde, R.; Sundsfjord, J. Serum TSH Is Positively Associated with BMI. Int. J. Obes. 2006, 30, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; Berrington de González, A. Body Fatness and Markers of Thyroid Function among U.S. Men and Women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef]
- Bétry, C.; Challan-Belval, M.A.; Bernard, A.; Charrié, A.; Drai, J.; Laville, M.; Thivolet, C.; Disse, E. Increased TSH in Obesity: Evidence for a BMI-Independent Association with Leptin. Diabetes Metab. 2015, 41, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Shaoba, A.; Basu, S.; Mantis, S.; Minutti, C. Serum Thyroid-Stimulating Hormone Levels and Body Mass Index Percentiles in Children with Primary Hypothyroidism on Levothyroxine Replacement. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Siemińska, L.; Wojciechowska, C.; Walczak, K.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Foltyn, W.; Kos-Kudła, B. Associations between metabolic syndrome, serum thyrotropin, and thyroid antibodies status in postmenopausal women, and the role of interleukin-6. Endokrynol. Pol. 2015, 66, 394–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Araujo, R.L.; Carvalho, D.P. Bioenergetic Impact of Tissue-Specific Regulation of Iodothyronine Deiodinases during Nutritional Imbalance. J. Bioenerg. Biomembr. 2011, 43, 59–65. [Google Scholar] [CrossRef]
- Nannipieri, M.; Cecchetti, F.; Anselmino, M.; Camastra, S.; Niccolini, P.; Lamacchia, M.; Rossi, M.; Iervasi, G.; Ferrannini, E. Expression of Thyrotropin and Thyroid Hormone Receptors in Adipose Tissue of Patients with Morbid Obesity and/or Type 2 Diabetes: Effects of Weight Loss. Int. J. Obes. 2009, 33, 1001–1006. [Google Scholar] [CrossRef]
- Asvold, B.O.; Vatten, L.J.; Nilsen, T.I.L.; Bjøro, T. The Association between TSH within the Reference Range and Serum Lipid Concentrations in a Population-Based Study. The HUNT Study. Eur. J. Endocrinol. 2007, 156, 181–186. [Google Scholar] [CrossRef]
- Roos, A.; Bakker, S.J.L.; Links, T.P.; Gans, R.O.B.; Wolffenbuttel, B.H.R. Thyroid Function Is Associated with Components of the Metabolic Syndrome in Euthyroid Subjects. J. Clin. Endocrinol. Metab. 2007, 92, 491–496. [Google Scholar] [CrossRef]
- Shon, H.S.; Jung, E.D.; Kim, S.H.; Lee, J.H. Free T4 Is Negatively Correlated with Body Mass Index in Euthyroid Women. Korean J. Intern. Med. 2008, 23, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.S.; Schwartzberg, P.L.; Kotliarov, Y.; Biancotto, A.; Xie, Z.; Germain, R.N.; Wang, E.; Olnes, M.J.; Narayanan, M.; Golding, H.; et al. Global Analyses of Human Immune Variation Reveal Baseline Predictors of Postvaccination Responses. Cell 2014, 157, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Dooley, J.; Garcia-Perez, J.E.; Lagou, V.; Lee, J.C.; Wouters, C.; Meyts, I.; Goris, A.; Boeckxstaens, G.; Linterman, M.A.; et al. The Cellular Composition of the Human Immune System Is Shaped by Age and Cohabitation. Nat. Immunol. 2016, 17, 461–468. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Initial Values | Final Values | p Value | Normal Values ^ |
|---|---|---|---|---|
| INF-γ (pg/mL) | 6.25 ± 4.98 | 4.47 ± 2.02 | p = 0.04 | (7.0 ± 3.9) a–(15.0 ± 4.0) a [22,23] |
| TNF-α (pg/mL) | 10.66 (2.83; 40.24) # | 9.69 (2.99; 31.33) # | p > 0.05 | (1.25 ± 2.20) a–(12.0 ± 4.0) a [23,24] |
| CXCL10 (pg/mL) | 179.7 ± 104.0 | 191.8 ± 153.8 | p > 0.05 | (25.9 (7.9–95.9)) b–(88.83 ± 28.68) a [25,26] |
| IL-4 (pg/mL) | 1.11 ± 1.07 | 1.07 ± 0.97 | p > 0.05 | (0.55 ± 0.24) a [27] |
| IL-1β (pg/mL) | 8.39 ± 6.62 | 11.46 (5.12; 25.62) # | p = 0.01 | (1.48 ± 0.70) *,a–(3.60 ± 0.20)a [28,29] |
| IL-17 (pg/mL) | 11.31 (3.60; 35.54) # | 8.00 (2.35; 27.23) # | p > 0.05 | (1.8 (1.2–2.3)) b–(4.04 (4.17)) c [9,30] |
| TGF-β (ng/mL) | 7.33 ± 2.60 | 7.12 ± 2.08 | p > 0.05 | (16.31 ± 3.21) a [31] |
| CRP (mg/L) | 0.23 (0.03; 1.87) # | 0.21 (0.03; 1.53) # | p > 0.05 | (0.15 ± 0.13) a–(2.6 ± 4.0) a [32,33] |
| Pairs of Correlated Parameters | Correlation Weights | Spearman’s Rank Correlation Coefficients and Significance Level | |
|---|---|---|---|
| T3 | UI1 | 0.355 | (N.S.) |
| T3 | UI2 | 0.259 | (N.S.) |
| T3 | INF-γ | 0.248 | 0.537 (p = 0.003) |
| T3 | IL-4 | 0.239 | (N.S.) |
| UI1 | UI2 | 0.184 | 0.520 (p = 0.004) |
| UI1 | INF-γ | 0.179 | (N.S.) |
| UI1 | IL-4 | 0.178 | (N.S.) |
| BMI | CRP | 0.169 | 0.500 (p = 0.006) |
| UI2 | INF-γ | 0.169 | (N.S.) |
| TG | CRP | 0.158 | (N.S.) |
| TSH | CRP | 0.153 | (N.S.) |
| UI2 | IL-4 | 0.146 | (N.S.) |
| IL-4 | INF-γ | 0.134 | 0.624 (p = 0.000) |
| UI2 | CRP | 0.113 | (N.S.) |
| TG | BMI | 0.111 | (N.S.) |
| TSH | BMI | 0.104 | (N.S.) |
| TSH | TG | 0.100 | 0.383 (p = 0.040) |
| INF-γ | CRP | 0.098 | 0.465 (p = 0.011) |
| T3 | CRP | 0.094 | (N.S.) |
| UI2 | BMI | 0.090 | (N.S.) |
| HDL | CRP | −0.091 | −0.394 (p = 0.034) |
| HDL | IL-4 | −0.107 | (N.S.) |
| HDL | INF-γ | −0.124 | −0.445 (p = 0.016) |
| HDL | UI2 | −0.137 | (N.S.) |
| T3 | HDL | −0.158 | −0.397 (p = 0.033) |
| Pairs of Correlated Parameters | Correlation Weights | Spearman’s Rank Correlation Coefficients and Significance Level | |
|---|---|---|---|
| TG | BMI | 0.239 | 0.436 (p = 0.018) |
| T3 | CXCL10 | 0.224 | (N.S.) |
| TG | UI2 | 0.190 | (N.S.) |
| GPX3 | TGF-β | 0.181 | 0.632 (p = 0.000) |
| TG | INF-γ | 0.175 | (N.S.) |
| TG | IL-4 | 0.173 | (N.S.) |
| BMI | INF-γ | 0.158 | 0.373 (p = 0.046) |
| UI2 | BMI | 0.148 | (N.S.) |
| UI2 | IL-4 | 0.144 | (N.S.) |
| BMI | IL-4 | 0.133 | 0.379 (p = 0.042) |
| GPX3 | energy intake | 0.132 | (N.S.) |
| energy intake | TGF-β | 0.132 | (N.S.) |
| UI2 | INF-γ | 0.127 | (N.S.) |
| age | BMI | 0.120 | 0.601 (p = 0.001) |
| IL-4 | INF-γ | 0.115 | 0.578 (p = 0.001) |
| fT4 | IL-4 | −0.113 | (N.S.) |
| fT4 | UI2 | −0.125 | (N.S.) |
| fT4 | INF-γ | −0.128 | −0.447 (p = 0.015) |
| CXCL10 | TGF-β | −0.132 | (N.S.) |
| GPX3 | CXCL10 | −0.132 | (N.S.) |
| fT4 | BMI | −0.192 | (N.S.) |
| fT4 | TG | −0.192 | (N.S.) |
| T3 | energy intake | −0.210 | (N.S.) |
| T3 | TGF-β | −0.288 | (N.S.) |
| T3 | GPX3 | −0.288 | (N.S.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryczyk-Kozioł, J.; Prochownik, E.; Błażewska-Gruszczyk, A.; Słowiaczek, M.; Sun, Q.; Schomburg, L.; Ochab, E.; Bartyzel, M.; Zagrodzki, P. Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto’s Thyroiditis. Nutrients 2022, 14, 2869. https://doi.org/10.3390/nu14142869
Kryczyk-Kozioł J, Prochownik E, Błażewska-Gruszczyk A, Słowiaczek M, Sun Q, Schomburg L, Ochab E, Bartyzel M, Zagrodzki P. Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto’s Thyroiditis. Nutrients. 2022; 14(14):2869. https://doi.org/10.3390/nu14142869
Chicago/Turabian StyleKryczyk-Kozioł, Jadwiga, Ewelina Prochownik, Anna Błażewska-Gruszczyk, Marian Słowiaczek, Qian Sun, Lutz Schomburg, Ewa Ochab, Mirosław Bartyzel, and Paweł Zagrodzki. 2022. "Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto’s Thyroiditis" Nutrients 14, no. 14: 2869. https://doi.org/10.3390/nu14142869
APA StyleKryczyk-Kozioł, J., Prochownik, E., Błażewska-Gruszczyk, A., Słowiaczek, M., Sun, Q., Schomburg, L., Ochab, E., Bartyzel, M., & Zagrodzki, P. (2022). Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto’s Thyroiditis. Nutrients, 14(14), 2869. https://doi.org/10.3390/nu14142869







