Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression
Abstract
:1. Introduction
2. Properties of Paraoxonase
2.1. Paraoxonase Family
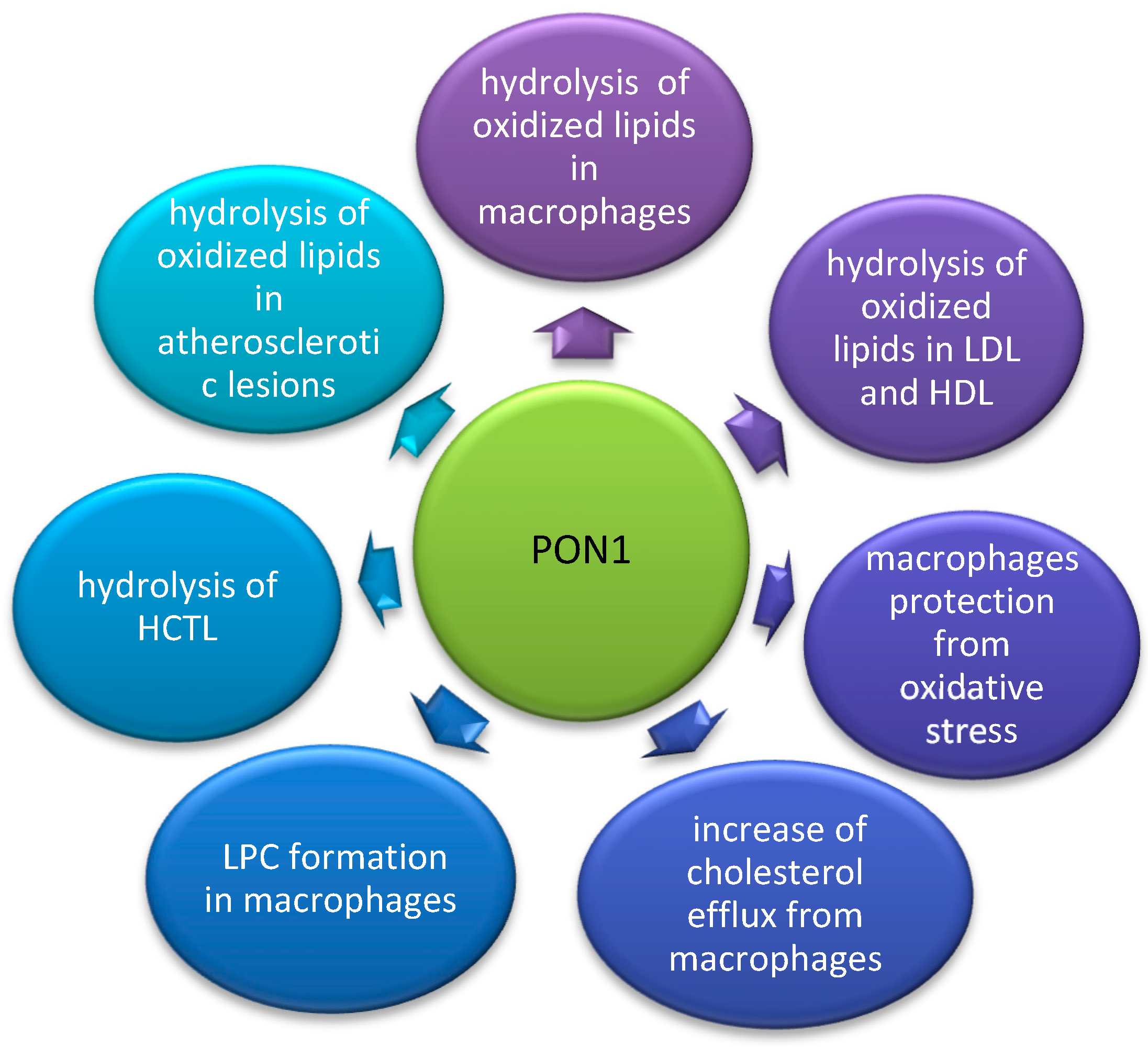
2.2. Anti-Atherosclerotic Effect of PON1
2.3. PON1 Polymorphism
2.4. The Influence of Environmental Factors on PON1 Activity and Concentration
2.5. The Influence of Various Components of Diet on PON1 Activity and Gene Expression
3. Properties of Carotenoids
4. The Influence of Carotenoids on PON1 Activity and Gene Expression
4.1. The Influence of Astaxanthin on PON1 Activity
4.1.1. The Influence of Astaxanthin on PON1 Activity in Animal Studies
4.1.2. The Influence of Astaxanthin on PON1 Activity in Clinical Studies
4.2. The Influence of β-Carotene on PON1 Activity and Gene Expression
The Influence of β-Carotene on PON1 Activity and Gene Expression in In Vitro Studies
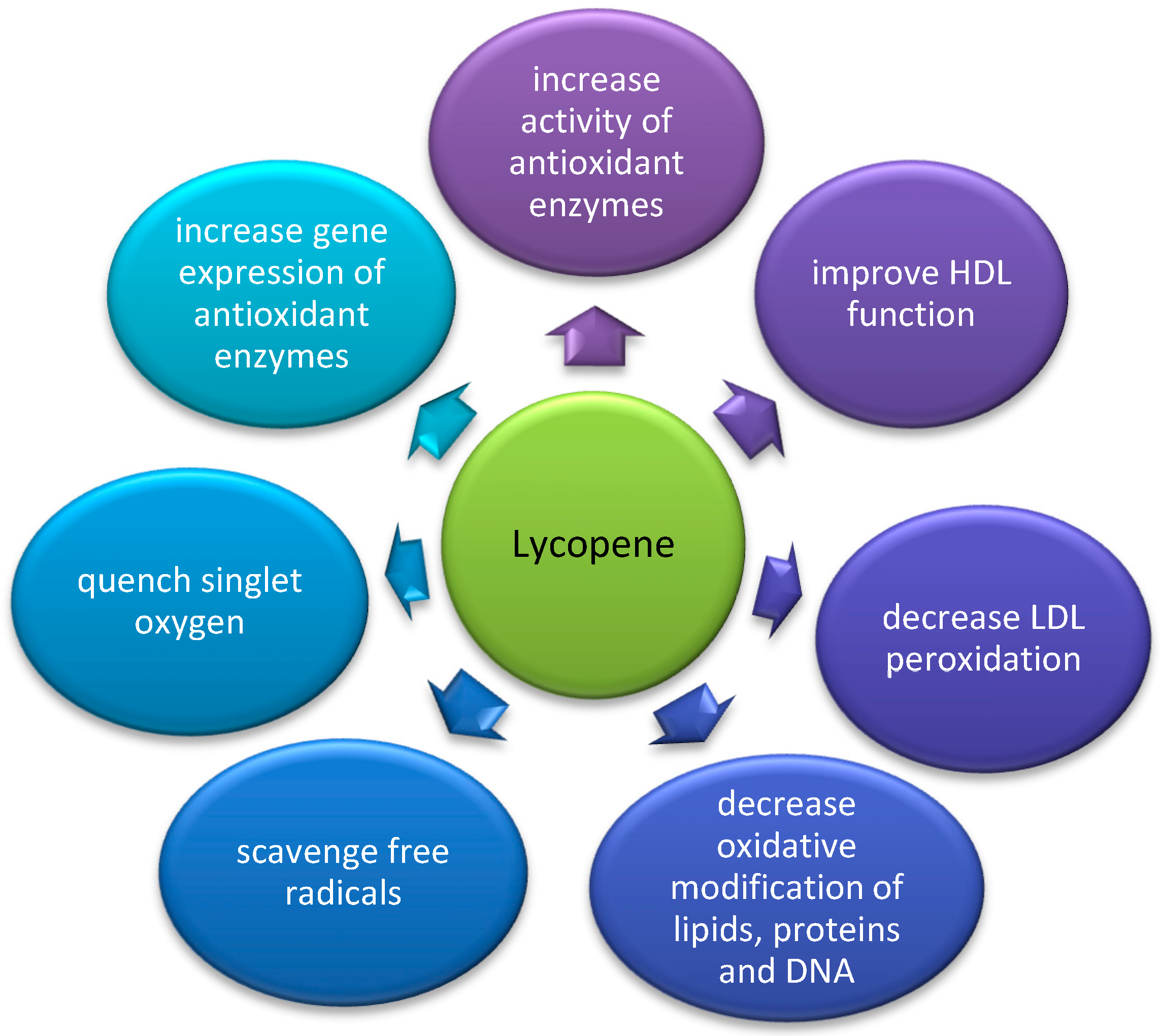
4.3. The Influence of Lycopene on PON1 Activity and Gene Expression
4.3.1. The Influence of Lycopene on PON1 Activity and Gene Expression in Animal Studies
4.3.2. The Influence of Lycopene on PON1 Activity and Gene Expression in Clinical Studies
4.4. The Effect of a Mixture of Carotenoids on PON1 Activity and LDL Oxidation
4.4.1. The Influence of a Mixture of Carotenoids on PON1 Activity in Animal Studies
4.4.2. The Influence of a Mixture of Carotenoids on PON1 Activity in Clinical Studies
4.4.3. Conclusion on the Effect of a Mixture of Carotenoids on PON1 Activity
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisgaier, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; La Du, B. Paraoxonase Active Site Required for Protection against LDL Oxidation Involves Its Free Sulfhydryl Group and Is Different from That Required for Its Arylesterase/Paraoxonase Activities: Selective Action of Human Paraoxonase Allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Perla-Kaján, J.; Jakubowski, H. Paraoxonase 1 Protects against Protein N -homocysteinylation in Humans. FASEB J. 2010, 24, 931–936. [Google Scholar] [CrossRef]
- Osaki, F.; Ikeda, Y.; Suehiro, T.; Ota, K.; Tsuzura, S.; Arii, K.; Kumon, Y.; Hashimoto, K. Roles of Sp1 and Protein Kinase C in Regulation of Human Serum Paraoxonase 1 (PON1) Gene Transcription in HepG2 Cells. Atherosclerosis 2004, 176, 279–287. [Google Scholar] [CrossRef]
- Arii, K.; Suehiro, T.; Ota, K.; Ikeda, Y.; Kumon, Y.; Osaki, F.; Inoue, M.; Inada, S.; Ogami, N.; Takata, H.; et al. Pitavastatin Induces PON1 Expression through P44/42 Mitogen-Activated Protein Kinase Signaling Cascade in Huh7 Cells. Atherosclerosis 2009, 202, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Garige, M.; Gong, M.; Varatharajalu, R.; Lakshman, M.R. Quercetin Up-Regulates Paraoxonase 1 Gene Expression via Sterol Regulatory Element Binding Protein 2 That Translocates from the Endoplasmic Reticulum to the Nucleus Where It Specifically Interacts with Sterol Responsive Element-like Sequence in Paraoxonase 1 Promoter in HuH7 Liver Cells. Metabolism 2010, 59, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Gouédard, C.; Barouki, R.; Morel, Y. Dietary Polyphenols Increase Paraoxonase 1 Gene Expression by an Aryl Hydrocarbon Receptor-Dependent Mechanism. Mol. Cell Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-Gallate Selectively Inhibits Interleukin-1beta-Induced Activation of Mitogen Activated Protein Kinase Subgroup c-Jun N-Terminal Kinase in Human Osteoarthritis Chondrocytes. J. Orthop. Res. 2003, 21, 102–109. [Google Scholar] [CrossRef]
- Précourt, L.P.; Amre, D.; Denis, M.C.; Lavoie, J.C.; Delvin, E.; Seidman, E.; Levy, E. The Three-Gene Paraoxonase Family: Physiologic Roles, Actions and Regulation. Atherosclerosis 2011, 214, 20–36. [Google Scholar] [CrossRef]
- Harel, M.; Aharoni, A.; Gaidukov, L.; Brumshtein, B.; Khersonsky, O.; Meged, R.; Dvir, H.; Ravelli, R.B.G.; McCarthy, A.; Toker, L.; et al. Structure and Evolution of the Serum Paraoxonase Family of Detoxifying and Anti-Atherosclerotic Enzymes. Nat. Struct. Mol. Biol. 2004, 11, 412–419. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids-Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Human Serum Paraoxonase. Gen. Pharmacol. 1998, 31, 329–336. [Google Scholar] [CrossRef]
- Demir, Y.; Beydemir, Ş. Purification, Refolding, and Characterization of Recombinant Human Paraoxonase-1. Turk. J. Chem. 2015, 39, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, B.; Volkova, N.; Aviram, M. Paraoxonase 1 (PON1) Is Present in Postprandial Chylomicrons. Atherosclerosis 2005, 180, 55–61. [Google Scholar] [CrossRef]
- Deakin, S.; Leviev, I.; Gomaraschi, M.; Calabresi, L.; Franceschini, G.; James, R.W. Enzymatically Active Paraoxonase-1 Is Located at the External Membrane of Producing Cells and Released by a High Affinity, Saturable, Desorption Mechanism. J. Biol. Chem. 2002, 277, 4301–4308. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Mackness, M. Anti-Inflammatory Properties of Paraoxonase-1 in Atherosclerosis. Adv. Exp. Med. Biol. 2010, 660, 143–151. [Google Scholar] [CrossRef]
- Primo-Parmo, S.L.; Sorenson, R.C.; Teiber, J.; La Du, B.N. The Human Serum Paraoxonase/Arylesterase Gene (PON1) Is One Member of a Multigene Family. Genomics 1996, 33, 498–507. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shinohara, M.; Sakoh, C.; Kataoka, M.; Shimizu, S. Lactone-Ring-Cleaving Enzyme: Genetic Analysis, Novel RNA Editing, and Evolutionary Implications. Proc. Natl. Acad. Sci. USA 1998, 95, 12787–12792. [Google Scholar] [CrossRef] [Green Version]
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What Are Their Functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.J.; Wadleigh, D.J.; Gangopadhyay, A.; Hama, S.; Grijalva, V.R.; Navab, M.; Fogelman, A.M.; Reddy, S.T. Paraoxonase-2 Is a Ubiquitously Expressed Protein with Antioxidant Properties and Is Capable of Preventing Cell-Mediated Oxidative Modification of Low Density Lipoprotein. J. Biol. Chem. 2001, 276, 44444–44449. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, R.C.; Bisgaier, C.L.; Aviram, M.; Hsu, C.; Billecke, S.; La Du, B.N. Human Serum Paraoxonase/Arylesterase’s Retained Hydrophobic N-Terminal Leader Sequence Associates with HDLs by Binding Phospholipids: Apolipoprotein A-I Stabilizes Activity. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2214–2225. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.T.; Devarajan, A.; Bourquard, N.; Shih, D.; Fogelman, A.M. Is It Just Paraoxonase 1 or Are Other Members of the Paraoxonase Gene Family Implicated in Atherosclerosis? Curr. Opin. Lipidol. 2008, 19, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, C.J.; Lamichhane, S.; Connolly, J.A.; Soehnlen, S.M.; Khalaf, F.K.; Malhotra, D.; Haller, S.T.; Isailovic, D.; Kennedy, D.J. A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action Including the Role of PON3 in Health and Disease. Antioxidants 2022, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Gaidukov, L.; Tawfik, D.S. High Affinity, Stability, and Lactonase Activity of Serum Paraoxonase PON1 Anchored on HDL with ApoA-I. Biochemistry 2005, 44, 11843–11854. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.D.; Berliner, J.A.; Hama, S.Y.; La Du, B.N.; Faull, K.F.; Fogelman, A.M.; Navab, M. Protective Effect of High Density Lipoprotein Associated Paraoxonase. Inhibition of the Biological Activity of Minimally Oxidized Low Density Lipoprotein. J. Clin. Investig. 1995, 96, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase Inhibits High-Density Lipoprotein Oxidation and Preserves Its Functions: A Possible Peroxidative Role for Paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Himbergen, T.M.; Van Tits, L.J.H.; Hectors, M.P.C.; De Graaf, J.; Roest, M.; Stalenhoef, A.F.H. Paraoxonase-1 and Linoleic Acid Oxidation in Familial Hypercholesterolemia. Biochem. Biophys. Res. Commun. 2005, 333, 787–793. [Google Scholar] [CrossRef]
- Rodrigo, L.; Mackness, B.; Durrington, P.N.; Hernandez, A.; Mackness, M.I. Hydrolysis of Platelet-Activating Factor by Human Serum Paraoxonase. Biochem. J. 2001, 354, 1–7. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M. Low Paraoxonase Activity Predicts Coronary Events in the Caerphilly Prospective Study. Circulation 2003, 107, 2775–2779. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of Paraoxonase 1 (PON1) Gene Polymorphisms and Functional Activity with Systemic Oxidative Stress and Cardiovascular Risk. J. Am. Med. Assoc. 2008, 299, 1265–1276. [Google Scholar] [CrossRef] [Green Version]
- Maturu, V.N.; Gupta, N.; Singh, G.; Gill, K.; Sharma, Y.P.; Singh, S. Serum Paraoxonase (PON1) Activity in North-West Indian Punjabi’s with Acute Myocardial Infarction. Indian J. Clin. Biochem. 2013, 28, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Tward, A.; Xia, Y.R.; Wang, X.P.; Shi, Y.S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased Atherosclerotic Lesion Formation in Human Serum Paraoxonase Transgenic Mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice Lacking Serum Paraoxonase Are Susceptible to Organophosphate Toxicity and Atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef]
- Mackness, B.; Quarck, R.; Verreth, W.; Mackness, M.; Holvoet, P. Human Paraoxonase-1 Overexpression Inhibits Atherosclerosis in a Mouse Model of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1545–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M.; Hardak, E.; Vaya, J.; Mahmood, S.; Milo, S.; Hoffman, A.; Billicke, S.; Draganov, D.; Rosenblat, M. Human Serum Paraoxonases (PON1) Q and R Selectively Decrease Lipid Peroxides in Human Coronary and Carotid Atherosclerotic Lesions: PON1 Esterase and Peroxidase-like Activities. Circulation 2000, 101, 2510–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M.; Rosenblat, M. Paraoxonases 1, 2, and 3, Oxidative Stress, and Macrophage Foam Cell Formation during Atherosclerosis Development. Free Radic. Biol. Med. 2004, 37, 1304–1316. [Google Scholar] [CrossRef]
- Sentí, M.; Tomás, M.; Fitó, M.; Weinbrenner, T.; Covas, M.I.; Sala, J.; Masiá, R.; Marrugat, J. Antioxidant Paraoxonase 1 Activity in the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5422–5426. [Google Scholar] [CrossRef] [Green Version]
- Efrat, M.; Aviram, M. Macrophage Paraoxonase 1 (PON1) Binding Sites. Biochem. Biophys. Res. Commun. 2008, 376, 105–110. [Google Scholar] [CrossRef]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Human Serum Paraoxonase 1 Decreases Macrophage Cholesterol Biosynthesis: Possible Role for Its Phospholipase-A2-like Activity and Lysophosphatidylcholine Formation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Rosenblat, M.; Oren, R.; Aviram, M. Lysophosphatidylcholine (LPC) Attenuates Macrophage-Mediated Oxidation of LDL. Biochem. Biophys. Res. Commun. 2006, 344, 1271–1277. [Google Scholar] [CrossRef]
- Guns, P.J.; Van Assche, T.; Verreth, W.; Fransen, P.; Mackness, B.; Mackness, M.; Holvoet, P.; Bult, H. Paraoxonase 1 Gene Transfer Lowers Vascular Oxidative Stress and Improves Vasomotor Function in Apolipoprotein E-Deficient Mice with Pre-Existing Atherosclerosis. Br. J. Pharmacol. 2008, 153, 508–516. [Google Scholar] [CrossRef]
- Richter, R.J.; Furlong, C.E. Determination of Paraoxonase (PON1) Status Requires More than Genotyping. Pharmacogenetics 1999, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Humbert, R.; Adler, D.A.; Disteche, C.M.; Hassett, C.; Omiecinski, C.J.; Furlong, C.E. The Molecular Basis of the Human Serum Paraoxonase Activity Polymorphism. Nat. Genet. 1993, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase Status in Coronary Heart Disease: Are Activity and Concentration More Important than Genotype? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackness, M.I.; Arrol, S.; Mackness, B.; Durrington, P.N. Alloenzymes of Paraoxonase and Effectiveness of High-Density Lipoproteins in Protecting Low-Density Lipoprotein against Lipid Peroxidation. Lancet 1997, 349, 851–852. [Google Scholar] [CrossRef]
- Brophy, V.H.; Jampsa, R.L.; Clendenning, J.B.; McKinstry, L.A.; Jarvik, G.P.; Furlong, C.E. Effects of 5′ Regulatory-Region Polymorphisms on Paraoxonase-Gene (PON1) Expression. Am. J. Hum. Genet. 2001, 68, 1428–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deakin, S.P.; James, R.W. Genetic and Environmental Factors Modulating Serum Concentrations and Activities of the Antioxidant Enzyme Paraoxonase-1. Clin. Sci. 2004, 107, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Mackness, M.; Mackness, B. Human Paraoxonase-1 (PON1): Gene Structure and Expression, Promiscuous Activities and Multiple Physiological Roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Deakin, S.; Leviev, I.; Guernier, S.; James, R.W. Simvastatin Modulates Expression of the PON1 Gene and Increases Serum Paraoxonase: A Role for Sterol Regulatory Element-Binding Protein-2. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2083–2089. [Google Scholar] [CrossRef] [Green Version]
- Zargari, M.; Sharafeddin, F.; Mahrooz, A.; Alizadeh, A.; Masoumi, P. The Common Variant Q192R at the Paraoxonase 1 (PON1) Gene and Its Activity Are Responsible for a Portion of the Altered Antioxidant Status in Type 2 Diabetes. Exp. Biol. Med. 2016, 241, 1489–1496. [Google Scholar] [CrossRef]
- Mackness, B.; Mackness, M.I.; Arrol, S.; Turkie, W.; Durrington, P.N. Effect of the Human Serum Paraoxonase 55 and 192 Genetic Polymorphisms on the Protection by High Density Lipoprotein against Low Density Lipoprotein Oxidative Modification. FEBS Lett. 1998, 423, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.G.; Richter, R.J.; Keifer, M.; Broomfield, C.A.; Sowalla, J.; Furlong, C.E. The Effect of the Human Serum Paraoxonase Polymorphism Is Reversed with Diazoxon, Soman and Sarin. Nat. Genet. 1996, 14, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Gaidukov, L.; Rosenblat, M.; Aviram, M.; Tawfik, D.S. The 192R/Q Polymorphs of Serum Paraoxonase PON1 Differ in HDL Binding, Lipolactonase Stimulation, and Cholesterol Efflux. J. Lipid Res. 2006, 47, 2492–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leviev, I.; Deakin, S.; James, R.W. Decreased Stability of the M54 Isoform of Paraoxonase as a Contributory Factor to Variations in Human Serum Paraoxonase Concentrations. J. Lipid Res. 2001, 42, 528–535. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chuang, L.M.; Chu, A.; Lu, J.; Hu, J.; Fernando, S. Association of Paraoxonase 1 Polymorphism with Beta-Cell Function: A Case of Molecular Heterosis. Pancreas 2004, 28, e96–e103. [Google Scholar] [CrossRef]
- Mendonça, M.I.; Dos Reis, R.P.; Freitas, A.I.; Sousa, A.C.; Pereira, A.; Faria, P.; Gomes, S.; Silva, B.; Santos, N.; Serrão, M.; et al. Human Paraoxonase Gene Polymorphisms and Coronary Artery Disease Risk. Rev. Port. Cardiol. 2008, 27, 1539–1555. [Google Scholar]
- Vaisi-Raygani, A.; Ghaneialvar, H.; Rahimi, Z.; Tavilani, H.; Pourmotabbed, T.; Shakiba, E.; Vaisi-Raygani, A.; Kiani, A.; Aminian, M.; Alibakhshi, R.; et al. Paraoxonase Arg 192 Allele Is an Independent Risk Factor for Three-Vessel Stenosis of Coronary Artery Disease. Mol. Biol. Rep. 2011, 38, 5421–5428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ou, J.; Cai, P.; Niu, B.; Li, J. Association between the PON1 Q192R Polymorphism and Coronary Heart Disease in Chinese: A Meta-Analysis. Medicine 2018, 97, e11151. [Google Scholar] [CrossRef]
- Leviev, I.; Poirier, O.; Nicaud, V.; Evans, A.; Kee, F.; Arveiler, D.; Morrisson, C.; Cambien, F.; James, R.W. High Expressor Paraoxonase PON1 Gene Promoter Polymorphisms Are Associated with Reduced Risk of Vascular Disease in Younger Coronary Patients. Atherosclerosis 2002, 161, 463–467. [Google Scholar] [CrossRef]
- Mirdamadi, H.Z.; Sztanek, F.; Derdak, Z.; Seres, I.; Harangi, M.; Paragh, G. The Human Paraoxonase-1 Phenotype Modifies the Effect of Statins on Paraoxonase Activity and Lipid Parameters. Br. J. Clin. Pharmacol. 2008, 66, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Harangi, M.; Mirdamadi, H.Z.; Seres, I.; Sztanek, F.; Molnár, M.; Kassai, A.; Derdák, Z.; Illyés, L.; Paragh, G. Atorvastatin Effect on the Distribution of High-Density Lipoprotein Subfractions and Human Paraoxonase Activity. Transl. Res. 2009, 153, 190–198. [Google Scholar] [CrossRef]
- Dullaart, R.P.F.; De Vries, R.; Voorbij, H.A.M.; Sluiter, W.J.; Van Tol, A. Serum Paraoxonase-I Activity Is Unaffected by Short-Term Administration of Simvastatin, Bezafibrate, and Their Combination in Type 2 Diabetes Mellitus. Eur. J. Clin. Investig. 2009, 39, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Paragh, G.; Seres, I.; Harangi, M.; Erdei, A.; Audikovszky, M.; Debreczeni, L.; Kovácsay, A.; Illyés, L.; Pados, G. Ciprofibrate Increases Paraoxonase Activity in Patients with Metabolic Syndrome. Br. J. Clin. Pharmacol. 2006, 61, 694–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S. Atorvastatin and Gemfibrozil Metabolites, but Not the Parent Drugs, Are Potent Antioxidants against Lipoprotein Oxidation. Atherosclerosis 1998, 138, 271–280. [Google Scholar] [CrossRef]
- Paragh, G.; Seres, I.; Harangi, M.; Balogh, Z.; Illyés, L.; Boda, J.; Szilvássy, Z.; Kovács, P. The Effect of Micronised Fenofibrate on Paraoxonase Activity in Patients with Coronary Heart Disease. Diabetes Metab. 2003, 29, 613–618. [Google Scholar] [CrossRef]
- Haj Mouhamed, D.; Ezzaher, A.; Mechri, A.; Neffati, F.; Omezzine, A.; Bouslama, A.; Gaha, L.; Douki, W.; Najjar, M.F. Effect of Cigarette Smoking on Paraoxonase 1 Activity According to PON1 L55M and PON1 Q192R Gene Polymorphisms. Environ. Health Prev. Med. 2012, 17, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Otocka-Kmiecik, A.; Lewandowski, M.; Stolarek, R.; Szkudlarek, U.; Nowak, D.; Orlowska-Majdak, M. Effect of Single Bout of Maximal Excercise on Plasma Antioxidant Status and Paraoxonase Activity in Young Sportsmen. Redox Rep. 2010, 15, 275–281. [Google Scholar] [CrossRef]
- Otocka-Kmiecik, A.; Bortnik, K.; Szkudlarek, U.; Nowak, D.; Orłowska-Majdak, M. Effect of Exercise on Plasma Paraoxonase1 Activity in Rugby Players: Dependance on Training Experience. Redox Rep. 2013, 18, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Otocka-Kmiecik, A.; Lewandowski, M.; Szkudlarek, U.; Nowak, D.; Orlowska-Majdak, M. Aerobic Training Modulates the Effects of Exercise-Induced Oxidative Stress on PON1 Activity: A Preliminary Study. Sci. World J. 2014, 2014, 230271. [Google Scholar] [CrossRef] [Green Version]
- Arslan, C.; Gulcu, F.; Gursu, M.F. Effects of Oxidative Stress Caused by Acute and Regular Exercise on Levels of Some Serum Metabolites and the Activities of Paraoxonase and Arylesterase. Biol. Sport 2005, 22, 375–383. [Google Scholar]
- Martinovic, J.; Dopsaj, V.; Dopsaj, M.J.; Kotur-Stevuljevic, J.; Vujovic, A.; Stefanovic, A.; Nesic, G. Long-Term Effects of Oxidative Stress in Volleyball Players. Int. J. Sports Med. 2009, 30, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Atli, M. Serum Paraoxonase Activity and Lipid Hydroperoxide Levels in Adult Football Players after Three Days Football Tournament. Afr. Health Sci. 2013, 13, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Otocka-Kmiecik, A.; Orłowska-Majdak, M.; Stawski, R.; Szkudlarek, U.; Kosielski, P.; Padula, G.; Gałczyński, S.; Nowak, D. Repetitions of Strenuous Exercise Consistently Increase Paraoxonase 1 Concentration and Activity in Plasma of Average-Trained Men. Oxid. Med. Cell. Longev. 2021, 2021, 2775025. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann. N. Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Noll, C.; Hamelet, J.; Matulewicz, E.; Paul, J.L.; Delabar, J.M.; Janel, N. Effects of Red Wine Polyphenolic Compounds on Paraoxonase-1 and Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 in Hyperhomocysteinemic Mice. J. Nutr. Biochem. 2009, 20, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.; Marmillot, P.; Gong, M.; Palmer, D.A.; Seeff, L.B.; Strader, D.B.; Lakshman, M.R. Light, but Not Heavy Alcohol Drinking, Stimulates Paraoxonase by Upregulating Liver MRNA in Rats and Humans. Metabolism 2003, 52, 1287–1294. [Google Scholar] [CrossRef]
- Mahrooz, A.; Mackness, M.; Bagheri, A.; Ghaffari-Cherati, M.; Masoumi, P. The Epigenetic Regulation of Paraoxonase 1 (PON1) as an Important Enzyme in HDL Function: The Missing Link between Environmental and Genetic Regulation. Clin. Biochem. 2019, 73, 1–10. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Mansego, M.L.; Sánchez-Muniz, F.J.; Angeles Zulet, M.; Alfredo Martinez, J. Arylesterase Activity Is Associated with Antioxidant Intake and Paraoxonase-1 (PON1) Gene Methylation in Metabolic Syndrome Patients Following an Energy Restricted Diet. EXCLI J. 2014, 13, 416–426. [Google Scholar] [PubMed]
- Aoi, W.; Ichikawa, H.; Mune, K.; Tanimura, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Muscle-Enriched MicroRNA MiR-486 Decreases in Circulation in Response to Exercise in Young Men. Front. Physiol. 2013, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [Green Version]
- Efrat, M.; Rosenblat, M.; Mahmood, S.; Vaya, J.; Aviram, M. Di-Oleoyl Phosphatidylcholine (PC-18:1) Stimulates Paraoxonase 1 (PON1) Enzymatic and Biological Activities: In Vitro and in Vivo Studies. Atherosclerosis 2009, 202, 461–469. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Almagor, Y.; Aviram, M. Antiatherogenicity of Extra Virgin Olive Oil and Its Enrichment with Green Tea Polyphenols in the Atherosclerotic Apolipoprotein-E-Deficient Mice: Enhanced Macrophage Cholesterol Efflux. J. Nutr. Biochem. 2008, 19, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Gabás-Rivera, C.; Barranquero, C.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary Squalene Increases High Density Lipoprotein-Cholesterol and Paraoxonase 1 and Decreases Oxidative Stress in Mice. PLoS ONE 2014, 9, e104224. [Google Scholar] [CrossRef] [PubMed]
- Calla, M.S.; Lynch, S.M. Vitamin C Preserves the Cardio-Protective Paraoxonase Activity of High-Density Lipoprotein during Oxidant Stress. Arch. Biochem. Biophys. 2006, 452, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kunes, J.P.; Cordero-Koning, K.S.; Lee, L.H.; Lynch, S.M. Vitamin C Attenuates Hypochlorite-Mediated Loss of Paraoxonase-1 Activity from Human Plasma. Nutr. Res. 2009, 29, 114–122. [Google Scholar] [CrossRef]
- Rafraf, M.; Bazyun, B.; Sarabchian, M.A.; Safaeiyan, A.; Gargari, B.P. Vitamin E Improves Serum Paraoxonase-1 Activity and Some Metabolic Factors in Patients with Type 2 Diabetes: No Effects on Nitrite/Nitrate Levels. J. Am. Coll. Nutr. 2016, 35, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kleemola, P.; Freese, R.; Jauhiainen, M.; Pahlman, R.; Alfthan, G.; Mutanen, M. Dietary Determinants of Serum Paraoxonase Activity in Healthy Humans. Atherosclerosis 2002, 160, 425–432. [Google Scholar] [CrossRef]
- Otocka-Kmiecik, A.; Krol, A. The Role of Vitamin C in Two Distinct Physiological States: Physical Activity and Sleep. Nutrients 2020, 12, 3908. [Google Scholar] [CrossRef]
- Daniels, J.A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A Randomised Controlled Trial of Increasing Fruit and Vegetable Intake and How This Influences the Carotenoid Concentration and Activities of PON-1 and LCAT in HDL from Subjects with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects Their Phenolic and Vitamin C Composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Lixandru, D.; Mohora, M.; Coman, A.; Stoian, I.; Van Gils, C.; Aerts, P.; Manuel-Y-Keenoy, B. Diet and Paraoxonase 1 Enzymatic Activity in Diabetic Foot Patients from Romania and Belgium: Favorable Association of High Flavonoid Dietary Intake with Arylesterase Activity. Ann. Nutr. Metab. 2010, 56, 294–301. [Google Scholar] [CrossRef]
- Rosenblat, M.; Aviram, M. Paraoxonases Role in the Prevention of Cardiovascular Diseases. Biofactors 2009, 35, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hayek, T.; Raz, A.; Coleman, R.; Dornfeld, L.; Vaya, J.; Aviram, M. Pomegranate Juice Supplementation to Atherosclerotic Mice Reduces Macrophage Lipid Peroxidation, Cellular Cholesterol Accumulation and Development of Atherosclerosis. J. Nutr. 2001, 131, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate Juice Flavonoids Inhibit Low-Density Lipoprotein Oxidation and Cardiovascular Diseases: Studies in Atherosclerotic Mice and in Humans. Drugs Exp. Clin. Res. 2002, 28, 49–62. [Google Scholar] [PubMed]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate Juice Consumption Reduces Oxidative Stress, Atherogenic Modifications to LDL, and Platelet Aggregation: Studies in Humans and in Atherosclerotic Apolipoprotein E–Deficient Mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Romain, C.; González-Barrio, R.; Cristol, J.P.; Teissèdre, P.L.; Crozier, A.; Rouanet, J.M. Raspberry Juice Consumption, Oxidative Stress and Reduction of Atherosclerosis Risk Factors in Hypercholesterolemic Golden Syrian Hamsters. Food Funct. 2011, 2, 400–405. [Google Scholar] [CrossRef]
- Kujawska, M.; Ignatowicz, E.; Ewertowska, M.; Markowski, J.; Jodynis-Liebert, J. Cloudy Apple Juice Protects against Chemical-Induced Oxidative Stress in Rat. Eur. J. Nutr. 2011, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dalgård, C.; Christiansen, L.; Jonung, T.; Mackness, M.I.; De Maat, M.P.M.; Hørder, M. No Influence of Increased Intake of Orange and Blackcurrant Juices and Dietary Amounts of Vitamin E on Paraoxonase-1 Activity in Patients with Peripheral Arterial Disease. Eur. J. Nutr. 2007, 46, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Del Bo, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of Polyphenols and Polyphenol-Rich Foods in the Modulation of PON1 Activity and Expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Garige, M.; Varatharajalu, R.; Marmillot, P.; Gottipatti, C.; Leckey, L.C.; Lakshman, R.M. Quercetin Up-Regulates Paraoxonase 1 Gene Expression with Concomitant Protection against LDL Oxidation. Biochem. Biophys. Res. Commun. 2009, 379, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of Yerba Mate and Green Tea on Paraoxonase and Leptin Levels in Patients Affected by Overweight or Obesity and Dyslipidemia: A Randomized Clinical Trial. Nutr. J. 2019, 18, 5. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–35. ISBN 978-3-319-54528-8. [Google Scholar]
- Kotake-Nara, E.; Nagao, A. Absorption and Metabolism of Xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High Plasma Levels of Alpha- and Beta-Carotene Are Associated with a Lower Risk of Atherosclerosis: Results from the Bruneck Study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef]
- Koh, W.P.; Yuan, J.M.; Wang, R.; Lee, Y.P.; Lee, B.L.; Yu, M.C.; Ong, C.N. Plasma Carotenoids and Risk of Acute Myocardial Infarction in the Singapore Chinese Health Study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 685–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, S.L.; Ojeda, L.S.; Sexton, G.; Weidner, G.; Connor, W.E. Diets Lower in Folic Acid and Carotenoids Are Associated with the Coronary Disease Epidemic in Central and Eastern Europe. J. Am. Diet. Assoc. 2004, 104, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zou, Z.Y.; Huang, Y.M.; Xiao, X.; Ma, L.; Lin, X.M. Serum Carotenoids in Relation to Risk Factors for Development of Atherosclerosis. Clin. Biochem. 2012, 45, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Tsuboi, Y.; Maeda, K.; Ishihara, Y.; Suzuki, K. Analysis of Repeated Measurements of Serum Carotenoid Levels and All-Cause and Cause-Specific Mortality in Japan. JAMA Netw. Open 2021, 4, e2113369. [Google Scholar] [CrossRef]
- Nakamura, M.; Sugiura, M.; Aoki, N. High Beta-Carotene and Beta-Cryptoxanthin Are Associated with Low Pulse Wave Velocity. Atherosclerosis 2006, 184, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic Aspects of Carotenoid Health Benefits-Where Are We Now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef]
- Augusti, P.R.; Quatrin, A.; Somacal, S.; Conterato, G.M.M.; Sobieski, R.; Ruviaro, A.R.; Maurer, L.H.; Duarte, M.M.F.; Roehrs, M.; Emanuelli, T. Astaxanthin Prevents Changes in the Activities of Thioredoxin Reductase and Paraoxonase in Hypercholesterolemic Rabbits. J. Clin. Biochem. Nutr. 2012, 51, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegin, S.Ç.; Yur, F.; Ceylan, E. Effect of Lycopene Application in Rats with Experimental Diabetes Using Lipoprotein, Paraoxonase and Cytokines. J. Membr. Biol. 2013, 246, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kükürt, A.; Karapehlivan, M. Protective Effect of Astaxanthin on Experimental Ovarian Damage in Rats. J. Biochem. Mol. Toxicol. 2022, 36, e22966. [Google Scholar] [CrossRef] [PubMed]
- Sultan Alvi, S.; Ansari, I.A.; Khan, I.; Iqbal, J.; Khan, M.S. Potential Role of Lycopene in Targeting Proprotein Convertase Subtilisin/Kexin Type-9 to Combat Hypercholesterolemia. Free Radic. Biol. Med. 2017, 108, 394–403. [Google Scholar] [CrossRef]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes. Metab. Syndr. Obes. 2020, 13, 3117–3135. [Google Scholar] [CrossRef]
- Assis, R.; Arcaro, C.; Gutierres, V.; Oliveira, J.; Costa, P.; Baviera, A.; Brunetti, I. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef] [Green Version]
- Somacal, S.; Figueiredo, C.G.; Quatrin, A.; Ruviaro, A.R.; Conte, L.; Augusti, P.R.; Roehrs, M.; Denardin, I.T.; Kasten, J.; da Veiga, M.L.; et al. The Antiatherogenic Effect of Bixin in Hypercholesterolemic Rabbits Is Associated to the Improvement of Lipid Profile and to Its Antioxidant and Anti-Inflammatory Effects. Mol. Cell. Biochem. 2015, 403, 243–253. [Google Scholar] [CrossRef]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phyther. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene Intervention Reduces Inflammation and Improves HDL Functionality in Moderately Overweight Middle-Aged Individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High Intakes of Vegetables, Berries, and Apples Combined with a High Intake of Linoleic or Oleic Acid Only Slightly Affect Markers of Lipid Peroxidation and Lipoprotein Metabolism in Healthy Subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Blum, S.; Aviram, M.; Ben-Amotz, A.; Levy, Y. Effect of a Mediterranean Meal on Postprandial Carotenoids, Paraoxonase Activity and C-Reactive Protein Levels. Ann. Nutr. Metab. 2006, 50, 20–24. [Google Scholar] [CrossRef]
- DiMarco, D.M.; Norris, G.H.; Millar, C.L.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs per Day Is Associated with Changes in HDL Function and Increased Plasma Antioxidants in Healthy, Young Adults. J. Nutr. 2017, 147, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Chee, T.L.; Rowley, K.; Jenkins, A.J.; O’Dea, K.; Itsiopoulos, C.; Stoney, R.M.; Su, Q.; Giles, G.G.; Best, J.D. Paraoxonase Activity in Greek Migrants and Anglo-Celtic Persons in the Melbourne Collaborative Cohort Study: Relationship to Dietary Markers. Eur. J. Nutr. 2005, 44, 223–230. [Google Scholar] [CrossRef]
- Cohen, J.; Jenkins, A.J.; Karschimkus, C.; Qing, S.; Lee, C.T.; O’Dea, K.; Best, J.D.; Rowley, K.G. Paraoxonase and Other Coronary Risk Factors in a Community-Based Cohort. Redox Rep. 2002, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, N.; Camps, J.; Fernández-Ballart, J.; Arija, V.; Murphy, M.M.; Ceruelo, S.; Biarnés, E.; Vilella, E.; Tous, M.; Joven, J. Regulation of Serum Paraoxonase Activity by Genetic, Nutritional, and Lifestyle Factors in the General Population. Clin. Chem. 2003, 49, 1491–1497. [Google Scholar] [CrossRef] [Green Version]
- Bub, A.; Barth, S.; Watzl, B.; Briviba, K.; Herbert, B.M.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1-192) Polymorphism Is Associated with Reduced Lipid Peroxidation in R-Allele-Carrier but Not in QQ Homozygous Elderly Subjects on a Tomato-Rich Diet. Eur. J. Nutr. 2002, 41, 237–243. [Google Scholar] [CrossRef]
- Bub, A.; Barth, S.W.; Watzl, B.; Briviba, K.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1-192) Polymorphism Is Associated with Reduced Lipid Peroxidation in Healthy Young Men on a Low-Carotenoid Diet Supplemented with Tomato Juice. Br. J. Nutr. 2005, 93, 291–297. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, E.S.; El-Sohemy, A.; Rao, A.V.; Rao, L.G. Paraoxonase 1 Polymorphisms 172T→A and 584A→G Modify the Association between Serum Concentrations of the Antioxidant Lycopene and Bone Turnover Markers and Oxidative Stress Parameters in Women 25-70 Years of Age. J. Nutrigenet. Nutr. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Rajasingh, H.; Øyehaug, L.; Våge, D.I.; Omholt, S.W. Carotenoid Dynamics in Atlantic Salmon. BMC Biol. 2006, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of Oxidative Injury of Biological Membranes by Astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- McNulty, H.; Jacob, R.F.; Mason, R.P. Biologic Activity of Carotenoids Related to Distinct Membrane Physicochemical Interactions. Am. J. Cardiol. 2008, 101, S20–S29. [Google Scholar] [CrossRef]
- Jaouad, L.; De Guise, C.; Berrougui, H.; Cloutier, M.; Isabelle, M.; Fulop, T.; Payette, H.; Khalil, A. Age-Related Decrease in High-Density Lipoproteins Antioxidant Activity Is Due to an Alteration in the PON1’s Free Sulfhydryl Groups. Atherosclerosis 2006, 185, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, R.C.; Primo-Parmo, S.L.; Kuo, C.L.; Adkins, S.; Lockridge, O.; La Du, B.N. Reconsideration of the Catalytic Center and Mechanism of Mammalian Paraoxonase/Arylesterase. Proc. Natl. Acad. Sci. USA 1995, 92, 7187–7191. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, K.; Tanaka, N.; Matsufuji, H.; Chino, M. β-Carotene Reverses the IL-1β-Mediated Reduction in Paraoxonase-1 Expression via Induction of the CaMKKII Pathway in Human Endothelial Cells. Microvasc. Res. 2012, 84, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Loppnow, H.; Buerke, M.; Werdan, K.; Rose-John, S. Contribution of Vascular Cell-Derived Cytokines to Innate and Inflammatory Pathways in Atherogenesis. J. Cell. Mol. Med. 2011, 15, 484–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumon, Y.; Suehiro, T.; Ikeda, Y.; Hashimoto, K. Human Paraoxonase-1 Gene Expression by HepG2 Cells Is Downregulated by Interleukin-1beta and Tumor Necrosis Factor-Alpha, but Is Upregulated by Interleukin-6. Life Sci. 2003, 73, 2807–2815. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Anti-Inflammatory Mechanisms in the Vascular Wall. Circ. Res. 2001, 88, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: The Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Martin, K.R.; Wu, D.; Meydani, M. The Effect of Carotenoids on the Expression of Cell Surface Adhesion Molecules and Binding of Monocytes to Human Aortic Endothelial Cells. Atherosclerosis 2000, 150, 265–274. [Google Scholar] [CrossRef]
- Heber, D.; Lu, Q.Y. Overview of Mechanisms of Action of Lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Silaste, M.L.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A.; Hörkkö, S. Tomato Juice Decreases LDL Cholesterol Levels and Increases LDL Resistance to Oxidation. Br. J. Nutr. 2007, 98, 1251–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human Serum Paraoxonase (PON 1) Is Inactivated by Oxidized Low Density Lipoprotein and Preserved by Antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Pereira, B.L.B.; Reis, P.P.; Severino, F.E.; Felix, T.F.; Braz, M.G.; Nogueira, F.R.; Silva, R.A.C.; Cardoso, A.C.; Lourenço, M.A.M.; Figueiredo, A.M.; et al. Tomato (Lycopersicon Esculentum) or Lycopene Supplementation Attenuates Ventricular Remodeling after Myocardial Infarction through Different Mechanistic Pathways. J. Nutr. Biochem. 2017, 46, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Lycopene as a Natural Protector against Gamma-Radiation Induced DNA Damage, Lipid Peroxidation and Antioxidant Status in Primary Culture of Isolated Rat Hepatocytes in Vitro. Biochim. Biophys. Acta 2007, 1770, 659–665. [Google Scholar] [CrossRef]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical Antioxidants Modulate Mammalian Cellular Epigenome: Implications in Health and Disease. Antioxid. Redox Signal. 2012, 17, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Vanderkraats, N.D.; Hiken, J.F.; Decker, K.F.; Edwards, J.R. Discovering High-Resolution Patterns of Differential DNA Methylation That Correlate with Gene Expression Changes. Nucleic Acids Res. 2013, 41, 6816–6827. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato Lycopene and Low Density Lipoprotein Oxidation: A Human Dietary Intervention Study. Lipids 1998, 33, 981–984. [Google Scholar] [CrossRef]
- Tsakiris, S.; Karikas, G.A.; Parthimos, T.; Tsakiris, T.; Bakogiannis, C.; Schulpis, K.H. Alpha-Tocopherol Supplementation Prevents the Exercise-Induced Reduction of Serum Paraoxonase 1/Arylesterase Activities in Healthy Individuals. Eur. J. Clin. Nutr. 2009, 63, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Poh, R.; Muniandy, S. Paraoxonase 1 Activity as a Predictor of Cardiovascular Disease in Type 2 Diabetes. Southestern Asian J. Trop. Med. Public Health 2010, 41, 1231–1246. [Google Scholar]
- Dugas, T.R.; Morel, D.W.; Harrison, E.H. Dietary Supplementation with Beta-Carotene, but Not with Lycopene, Inhibits Endothelial Cell-Mediated Oxidation of Low-Density Lipoprotein. Free Radic. Biol. Med. 1999, 26, 1238–1244. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Rosenblat, M.; Aviram, M. Lycopene Synergistically Inhibits LDL Oxidation in Combination with Vitamin E, Glabridin, Rosmarinic Acid, Carnosic Acid, or Garlic. Antioxid. Redox Signal. 2000, 2, 491–506. [Google Scholar] [CrossRef]
- Linseisen, J.; Hoffmann, J.; Riedl, J.; Wolfram, G. Effect of a Single Oral Dose of Antioxidant Mixture (Vitamin E, Carotenoids) on the Formation of Cholesterol Oxidation Products after Ex Vivo LDL Oxidation in Humans. Eur. J. Med. Res. 1998, 3, 5–12. [Google Scholar]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of Paraoxonase 1 (PON1) as an Antioxidant and Antiatherogenic Enzyme in the Cardiovascular Complications of Type 2 Diabetes: Genotypic and Phenotypic Evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; García-Heredia, A.I.; Solà, R.; Camps, J.; López de la Hazas, M.C.; Farràs, M.; Pedret, A.; Catalán, Ú.; Rubió, L.; Motilva, M.J.; et al. Phenol-Enriched Olive Oils Modify Paraoxonase-Related Variables: A Randomized, Crossover, Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 1600932. [Google Scholar] [CrossRef]
- Tomás, M.; Sentí, M.; Elosua, R.; Vila, J.; Sala, J.; Masià, R.; Marrugat, J. Interaction between the Gln-Arg 192 Variants of the Paraoxonase Gene and Oleic Acid Intake as a Determinant of High-Density Lipoprotein Cholesterol and Paraoxonase Activity. Eur. J. Pharmacol. 2001, 432, 121–128. [Google Scholar] [CrossRef]
- Mackness, M.I.; Mackness, B.; Durrington, P.N.; Fogelman, A.M.; Berliner, J.; Lusis, A.J.; Navab, M.; Shih, D.; Fonarow, G.C. Paraoxonase and Coronary Heart Disease. Curr. Opin. Lipidol. 1998, 9, 319–324. [Google Scholar] [CrossRef]
- Gamboa, R.; Zamora, J.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Cardoso, G.; Posadas-Romero, C.; Vargas-Alarcón, G. Distribution of Paraoxonase PON1 Gene Polymorphisms in Mexican Populations. Its Role in the Lipid Profile. Exp. Mol. Pathol. 2006, 80, 85–90. [Google Scholar] [CrossRef]
- Imai, Y.; Morita, H.; Kurihara, H.; Sugiyama, T.; Kato, N.; Ebihara, A.; Hamada, C.; Kurihara, Y.; Shindo, T.; Oh-Hashi, Y.; et al. Evidence for Association between Paraoxonase Gene Polymorphisms and Atherosclerotic Diseases. Atherosclerosis 2000, 149, 435–442. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Z.; Huang, J.; Su, S.; Yu, Q.; Zhao, J.; Hui, R.; Yao, Z.; Shen, Y.; Qiang, B.; et al. Extensive Association Analysis between Polymorphisms of PON Gene Cluster with Coronary Heart Disease in Chinese Han Population. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Gardemann, A.; Philipp, M.; Heß, K.; Katz, N.; Tillmanns, H.; Haberbosch, W. The Paraoxonase Leu-Met54 and Gln-Arg191 Gene Polymorphisms Are Not Associated with the Risk of Coronary Heart Disease. Atherosclerosis 2000, 152, 421–431. [Google Scholar] [CrossRef]
- Cohen, E.; Aviram, M.; Khatib, S.; Artoul, F.; Rabin, A.; Mannheim, D.; Karmeli, R.; Salamon, T.; Vaya, J. Human Carotid Plaque Phosphatidylcholine Specifically Interacts with Paraoxonase 1, Increases Its Activity, and Enhances Its Uptake by Macrophage at the Expense of Its Binding to HDL. Free Radic. Biol. Med. 2014, 76, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Helbecque, N.; Cottel, D.; Meirhaeghe, A.; Dallongeville, P.; Arveiler, D.; Wagner, A.; Ruidavets, J.B.; Ferrieres, J. Paraoxonase (Gln192-Arg) Polymorphism in French Type 2 Diabetics. Atherosclerosis 1999, 147, 415–416. [Google Scholar] [CrossRef] [PubMed]
| The PON1 Region | The Affected Site | Effect of the Polymorphism | Ref. | |
|---|---|---|---|---|
| Promoter region | −108C/T polymorphism (rs705379) | The center of consensus binding site for Sp1 | Effect on gene expression and serum activity: -Weaker binding of Sp1 in the presence of the T allele than the C allele -Modulation of Sp1 binding affects SREBP2, which upregulates PON1 in the presence of statins | [46,47,48] |
| −162A/G polymorphism (rs705381) | The potential NF-1 binding site | Effect on gene expression and serum activity | [46,47] | |
| Coding region | PON1-Q192R (rs662) | Active site | Direct effect on catalytic activity: The 192R allozyme is -more efficient in hydrolyzing paraoxon and chlorpyrifos-oxon, homocysteine thiolactone, higher affinity to HDL binding | [49,50,51,52] |
| -less efficient in hydrolyzing diazoxon, sarin, and soman, lower protection against LDL oxidation. | [33,46,49,50,51] | |||
| -no effect on hydrolyzation efficiency of phenylacetate | [49,50] | |||
| PON1-L55M (rs854560) (Possible linkage disequilibrium with the −108 promoter region polymorphism) | The protein structure | Effect on plasma PON1 protein concentration: 55L allozyme has: -higher stability, less susceptible to proteolysis | [50,53] | |
| -key role in the packing of the protein | [9] | |||
| Effect on PON1 activity: Location of L55M polymorphism in the neighborhood of two crucial amino acids (Glu52 and Asp53), which are required for PON1 activity | [54] | |||
| Chemical Structure | Dietary Source |
|---|---|
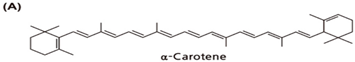 | Carrots, squash, pumpkin, palm fruit |
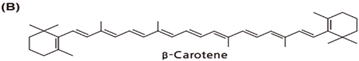 | Apricot, carrots, spinach, green collard, cantaloupe, beet, broccoli, tomato, palm fruit, squash, green leafy, mango |
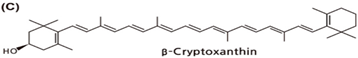 | Tangerine, papaya, orange, loquat, tree tomato, persimmon |
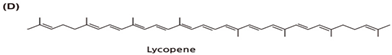 | Tomatoes and tomato-based foods (85%), watermelon, pink guava, pink grapefruit, papaya, apricot, Asian gac |
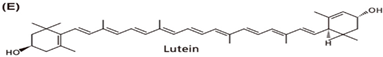 | Spinach, green collard, beet, broccoli, green peas, leafy green, corn, corn products, squash, egg yolks |
 | Corn, corn products, squash, egg yolks |
| Animal Studies | ||||
|---|---|---|---|---|
| Study Objective | Study Protocol | Studied Group | Results | Ref. |
| The effect of asx on PON | Supplementation with 50, 100 and 500 mg/100 g b.w. of asx for 60 days | Hypercholesterolemic rabbits | Restoration of PON by all asx doses | [112] |
| The effect of asx on PON and ovarian damage | Supplementation with 80 mg/kg b.w. of asx for 14 days | 32 female rats in 4 equal groups: control, induced ovarian damage, treated with asx, induced ovarian damage treated with asx | Increase in PON and reduction of ovarian damage | [114] |
| The effect of lycopene on ARE | Administration of different doses (5, 10 and 50 mg/kg b.w./day) of lycopene for 30 days | Hyperlipidemic rats | Improvement in ARE | [115] |
| The effect of lycopene on PON | Administration of lycopene for 28 days and comparison of PON between groups | Non-diabetic rats (7 in the control group and 7 in the lycopene group) | Increase in PON | [113] |
| STZ-induced diabetic rats (7 in the diabetes group and 7 in the diabetes-lycopene group) | Restoration of PON | |||
| The effects of lycopene or metformin, alone or in combination, on PON | Treatment for 35 days. Assessment of PON in plasma before and after treatment | STZ-induced diabetic rats | Increase in PON | [116] |
| The effect of treatment with yogurt enriched with lycopene, bixin, lycopene + curcumin, bixin + curcumin on PON | Administration of antioxidants individually or as mixtures for 50 days. Assessment of antioxidants and PON in plasma before, at 10 days, and at 50 days of treatment | STZ-induced diabetic rats | Increase in PON | [117] |
| The effect of bixin on PON reduced by hypocholesterolemia | 60 days of hypercholesterolemic diet alone or with bixin (10, 30, or 100 mg/kg b.w.) or simvastatin (15 mg/kg b.w.) vs. regular chow (control) | 42 hypercholesterolemic rabbits divided into 7 groups | Partial prevention of serum PON decrease | [118] |
| Clinical Studies | ||||
|---|---|---|---|---|
| Study Objective | Study Protocol | Studied Group | Results | Ref. |
| The effects of asx on PON1 activities | Collection of blood samples before, 45, and 90 days after supplementation, while regular soccer training. | 40 young elite soccer players in two groups (21 asx vs. 19 placebo) | Increase in PON. Interaction effect of asx and training on PON. Increase in PON1 activity towards diazoxon after 90 days in the asx group, and no difference in the placebo group. | [119] |
| The effect of lycopene on ARE | Treatment with 70 mg lycopene/week. Collection of serum before and after a 12-week intervention | 54 moderately overweight middle-aged subjects randomized into 3 groups (lycopene, lycopene-rich diet, and control) | Increase in ARE in serum and HDL2&3 | [120] |
| The effect of a lycopene-rich diet (224–350 mg lycopene/week) on ARE | ||||
| Assessment of relationships between the ARE with the methylation levels of the PON1 gene transcriptional regulatory region and lycopene | Measurement of ARE and lycopene in plasma, and PON1 transcriptional regulatory region methylation before and after a 6-month energy-restricted dietary weight-loss intervention. | 47 obese subjects (46.8% women; 47 ± 10 y.o.; BMI 36.2 ± 3.8 kg/m2) with metabolic syndrome | Positive correlation with ARE | [77] |
| Increase in PON1 gene expression by inhibition of PON1 gene methylation | ||||
| The effects of high and low intakes of vegetables, berries, and apples (containing lutein, β-cryptoxanthin, α-carotene, β-carotene) on PON | Consumption of 1 of 4 controlled isoenergetic diets for 6 weeks containing either 815 or 170 g of vegetables, berries, and apples. Assessment of PON and carotenoids in plasma before and after the diet. | Healthy men and women (n = 77; 19–52 y.o.) vs. 19 healthy control subjects | Decrease in PON in all groups; increase in carotenoids in groups on high fruit and vegetable diets in comparison to baseline | [121] |
| The influence of Mediterranean meal (monounsaturated 61% of fat and antioxidants) vs. Western meal on (saturated 57% of fat) on ARE and carotenoids | Consumption of meals after a 12-h fast, first the Mediterranean meal and after a week of the Western meal. Determination of 0, 2, 4, 7 h postprandial ARE and total carotenoids level in plasma | 8 healthy males | Increase in postprandial ARE and total carotenoids only after Mediterranean-like meal | [122] |
| The impact of consuming 0–3 eggs/d on zeaxanthin, lutein, and ARE | 14 wk crossover intervention. Subjects underwent a 2 wk washout (0 eggs/d) followed by sequentially increasing intake of 1, 2, and 3 eggs/d for 4 weeks each. After each period, fasting blood was collected for measurements. | 38 healthy men and women (18–30 y.o., BMI 18.5–29.9 kg/m2) | Compared with the intake of 0 eggs/d, intake of 2–3 eggs/d promoted a 20–31% increase in plasma lutein and zeaxanthin. Compared with the intake of 1–2 eggs/d, intake of 3 eggs/d resulted in an additional 9–16% increase in serum ARE | [123] |
| The effect of increased fruit and vegetable consumption on carotenoid content (α-carotene, β-cryptoxanthin, lutein, lycopene) and ARE in subjects with T2D | 1- or ≥ 6-portion/day of fruits and vegetable diet for 8 weeks. Collection of fasting serum pre- and post-intervention | 80 obese (BMI > 30 kg/m2) subjects (40–70 y.o.) with T2D | Increase in ARE in serum and HDL3, no change in ARE in HDL2 | [88] |
| β-cryptoxanthin correlation with ARE | Positive correlation between change in HDL3 β-cryptoxanthin with change in ARE in HDL3 | |||
| Determination of the relationship of PON and ARE with β-carotene, lycopene, lutein, and zeaxanthin | Measurement of PON and ARE and carotenoids concentration in serum of subjects on habitual diet | 127 Greek subjects (men and women; diabetic and non-diabetic equally distributed) | Positive correlation of carotenoids with PON in subjects with the R-allele of PON1–192 | [124] |
| 128 Anglo-Celtic subjects (men and women; diabetic and non-diabetic equally distributed) | No correlation of carotenoids with PON | |||
| Determination of the relationship of total carotenoids with PON and ARE | 20 months of diet and exercise intervention. Measurements were taken at baseline and follow-up. | 60 Australian Aboriginal subjects (20 men and 40 women; 16–85 y.o.), 38% had T2D | Carotenoids and PON1 activities increased. At baseline: positive correlation with PON and ARE. At follow-up: no correlation of change in PON1 activities with the change of carotenoids. | [125] |
| Determination of the relationship of individual carotenoids (β-carotene, β-cryptoxanthin lycopene, lutein plus zeaxanthin) with PON and ARE | At baseline: Positive correlation of all individual carotenoids with ARE Positive correlation of lycopene with PON | |||
| Determination of relationship of β-carotene and PON in habitual diet | Assessment of habitual diet by 3-day estimated food record | 388 subjects (194 women and 194 men; 18–75 y.o.) | No correlation of β-carotene with PON | [126] |
| Determination of the relationship of β-carotene and PON in habitual diet | Assessment of habitual diet by 3-day estimated food record | 95 healthy young Finnish volunteers (24 male and 71 females) | Inverse correlation of β-carotene with PON | [86] |
| The effect of tomato juice consumption (rich in β-carotene, and lycopene) on ARE depending on PON1-192 polymorphism | Consumption of 330 mL/day of tomato juice for 8 weeks | 50 elderly subjects in 2 groups (control (mineral water) or intervention group (tomato juice)) | Antioxidant status improvement and LDL-oxidation decrease only in R-allele carriers. Increase in ARE in intervention group and control. | [127] |
| The effect of tomato juice consumption (rich in β-carotene, and lycopene) on PON1 activities depending on PON1-192 polymorphism | Consumption of 330 mL/day of juice for 2 weeks after 2 weeks of low-carotenoid intake. | 20 young healthy non-smoking subjects were randomized into 2 groups (consuming either tomato juice or carrot juice) | Lipid peroxidation decrease only in R-allele carriers. No effect on PON1 activities | [128] |
| The effect of carrot juice (rich in β-carotene and α-carotene) on PON1 activities depending on PON1-192 polymorphism | No effect on lipid peroxidation regardless of PON1-192 genotype. No effect on PON1 activities | |||
| Modification of the association between serum concentration of lycopene and oxidative stress markers and bone turnover markers by PON1 polymorphism | Measurement of lycopene, oxidative stress markers, and bone turnover markers in serum | 107 women (25–70 y.o.) | PON1 L55M polymorphisms modify the association between lycopene and NTx. The Q192R polymorphism modifies the association between lycopene and BAP. In a subject with RR genotype, lycopene was associated with TBARS. | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otocka-Kmiecik, A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients 2022, 14, 2842. https://doi.org/10.3390/nu14142842
Otocka-Kmiecik A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients. 2022; 14(14):2842. https://doi.org/10.3390/nu14142842
Chicago/Turabian StyleOtocka-Kmiecik, Aneta. 2022. "Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression" Nutrients 14, no. 14: 2842. https://doi.org/10.3390/nu14142842
APA StyleOtocka-Kmiecik, A. (2022). Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients, 14(14), 2842. https://doi.org/10.3390/nu14142842






