The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Population
2.2. The Dietary Inflammatory Index (DII®)
2.3. Definition of Early COPD and Exposure History
2.4. Respiratory Diseases, Chronic Respiratory Symptoms and Other Characteristics
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Individuals
3.2. Association between DII and Early COPD
3.3. Multivariable Analysis of the DII and Lung Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Regan, E.A.; Lynch, D.A.; Curran-Everett, D.; Curtis, J.L.; Austin, J.H.; Grenier, P.A.; Kauczor, H.U.; Bailey, W.C.; DeMeo, D.L.; Casaburi, R.H.; et al. Clinical and Radiologic Disease in Smokers with Normal Spirometry. JAMA Intern. Med. 2015, 175, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fagerås, M.; et al. At the Root: Defining and Halting Progression of Early Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabung, F.K.; Liu, L.; Wang, W.; Fung, T.T.; Wu, K.; Smith-Warner, S.A.; Cao, Y.; Hu, F.B.; Ogino, S.; Fuchs, C.S.; et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018, 4, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meteran, H.; Thomsen, S.F.; Miller, M.R.; Hjelmborg, J.; Sigsgaard, T.; Backer, V. Self-reported intake of fruit and vegetables and risk of chronic obstructive pulmonary disease: A nation-wide twin study. Respir. Med. 2018, 144, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Wang, Y.; Jiang, W. Fruit and Vegetable Intake and the Risk of Chronic Obstructive Pulmonary Disease: A Dose-Response Meta-Analysis of Observational Studies. BioMed Res. Int. 2020, 2020, 3783481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Int. J. Epidemiol. 2018, 47, 1897–1909. [Google Scholar] [CrossRef]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: A prospective cohort study of men. Thorax 2017, 72, 500–509. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Lange, P.; Vestbo, J. Importance of Early COPD in Young Adults for Development of Clinical COPD: Findings from the Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook-Mills, J.M.; Averill, S.H.; Lajiness, J.D. Asthma, allergy and vitamin E: Current and future perspectives. Free Radic. Biol. Med. 2022, 179, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Decramer, M.; Mathieu, C.; Korf, H. Vitamin D and chronic obstructive pulmonary disease: Hype or reality? Lancet Respir. Med. 2013, 1, 804–812. [Google Scholar] [CrossRef]
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, D.A.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Greiller, C.L.; Islam, K.; Bhowmik, A.; Timms, P.M.; Rajakulasingam, R.K.; Choudhury, A.B.; et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with Chronic Obstructive Pulmonary Disease in London, UK. J. Steroid Biochem. Mol. Biol. 2018, 175, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Burkes, R.M.; Ceppe, A.S.; Doerschuk, C.M.; Couper, D.; Hoffman, E.A.; Comellas, A.P.; Barr, R.G.; Krishnan, J.A.; Cooper, C.; Labaki, W.W.; et al. Associations Among 25-Hydroxyvitamin D Levels, Lung Function, and Exacerbation Outcomes in COPD: An Analysis of the SPIROMICS Cohort. Chest 2020, 157, 856–865. [Google Scholar] [CrossRef]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 data ementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Hu, G.; Dong, T.; Wang, S.; Jing, H.; Chen, J. Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicine 2019, 45, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathyssen, C.; Serré, J.; Sacreas, A.; Everaerts, S.; Maes, K.; Verleden, S.; Verlinden, L.; Verstuyf, A.; Pilette, C.; Gayan-Ramirez, G.; et al. Vitamin D Modulates the Response of Bronchial Epithelial Cells Exposed to Cigarette Smoke Extract. Nutrients 2019, 11, 2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guénégou, A.; Leynaert, B.; Pin, I.; Le Moël, G.; Zureik, M.; Neukirch, F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax 2006, 61, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keranis, E.; Makris, D.; Rodopoulou, P.; Martinou, H.; Papamakarios, G.; Daniil, Z.; Zintzaras, E.; Gourgoulianis, K.I. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur. Respir. J. 2010, 36, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, H.; Shaheen, S.O.; Ntani, G.; Jameson, K.A.; Syddall, H.E.; Sayer, A.A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Hertfordshire Cohort Study Group. Processed meat consumption and lung function: Modification by antioxidants and smoking. Eur. Respir. J. 2014, 43, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Ducharme-Smith, K.; Mora-Garcia, G.; de Castro Mendes, F.; Ruiz-Diaz, M.S.; Moreira, A.; Villegas, R.; Garcia-Larsen, V. Lung function, COPD and Alternative Healthy Eating Index in US adults. ERJ Open Res. 2021, 7, 00927–02020. [Google Scholar] [CrossRef]
- Ducharme-Smith, K.; Mora-Garcia, G.; de Castro Mendes, F.; Ruiz-Diaz, M.S.; Moreira, A.; Villegas, R.; Garcia-Larsen, V. Association Between Diet-Related Inflammation and COPD: Findings from NHANES III. Front. Nutr. 2021, 8, 732099. [Google Scholar]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef]
- Espírito Santo, C.; Caseiro, C.; Martins, M.J.; Monteiro, R.; Brandão, I. Gut Microbiota, in the Halfway between Nutrition and Lung Function. Nutrients 2021, 13, 1716. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, T.L.; Chen, T.W.; Kuo, Y.L.; Chang, C.J.; Wu, T.R.; Shu, C.C.; Tsai, Y.H.; Swift, S.; Lu, C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 2022, 71, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Daniel, S.; Phillippi, D.; Schneider, L.J.; Nguyen, K.N.; Mirpuri, J.; Lund, A.K. Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet. Part. Fibre Toxicol. 2021, 18, 3. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Root, M.M.; Houser, S.M.; Anderson, J.J.; Dawson, H.R. Healthy Eating Index 2005 and selected macronutrients are correlated with improved lung function in humans. Nutr. Res. 2014, 34, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Cepeda, A.; Dávila-Said, G.; Orea-Tejeda, A.; González-Islas, D.; Elizondo-Montes, M.; Pérez-Cortes, G.; Keirns-Davies, C.; Castillo-Aguilar, L.F.; Verdeja-Vendrell, L.; Peláez-Hernández, V.; et al. Dietary intake of fatty acids and its relationship with FEV1/FVC in patients with chronic obstructive pulmonary disease. Clin. Nutr. ESPEN 2019, 29, 92–96. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [Green Version]
- Pizzini, A.; Lunger, L.; Sonnweber, T.; Weiss, G.; Tancevski, I. The Role of Omega-3 Fatty Acids in the Setting of Coronary Artery Disease and COPD: A Review. Nutrients 2018, 10, 1864. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Thomashow, M.A.; Yip, N.H.; Burkart, K.M.; Lo Cascio, C.M.; Shimbo, D.; Barr, R.G. Randomization to Omega-3 Fatty Acid Supplementation and Endothelial Function in COPD: The COD-Fish Randomized Controlled Trial. Chronic Obstr. Pulm. Dis. 2021, 8, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021, 76, 714–734. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.O.; Lee, S.H.; Choi, J.J.; Kim, D.H.; Choi, J.M.; Kang, M.J.; Oh, Y.M.; Park, Y.J.; Shin, Y.; Lee, S.W. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp. Mol. Med. 2020, 52, 1128–1139. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, S.H.; Chang, J.H.; Lee, H.S.; Kang, E.H.; Lee, S.W. The Impact of Changes in the Intake of Fiber and Antioxidants on the Development of Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 580. [Google Scholar] [CrossRef]
- Robinson, K.; Ma, X.; Liu, Y.; Qiao, S.; Hou, Y.; Zhang, G. Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics. Anim. Nutr. 2018, 4, 160–169. [Google Scholar] [CrossRef]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.; Linden, A.; Wolk, A. Long-term unprocessed and processed red meat consumption and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2019, 58, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Salari-Moghaddam, A.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Processed red meat intake and risk of COPD: A systematic review and dose-response meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 1109–1116. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Cheng, D.T.; Kim, D.K.; Cockayne, D.A.A.; Belousov, A.; Bitter, H.; Cho, M.H.; Duvoix, A.; Edwards, L.D.; Lomas, D.A.; Miller, B.E.; et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.G.; Liu, B.; Kroll, F.; Hanson, C.; Vicencio, A.; Coca, S.; Uribarri, J.; Bose, S. Increased advanced glycation end product and meat consumption is associated with childhood wheeze: Analysis of the National Health and Nutrition Examination Survey. Thorax 2021, 76, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Pan, L.; Cao, W.; Lv, J.; Guo, Y.; Pei, P.; Xia, Q.; Du, H.; Chen, Y.; Yang, L.; et al. Dietary Patterns and Risk of Chronic Obstructive Pulmonary Disease among Chinese Adults: An 11-Year Prospective Study. Nutrients 2022, 14, 996. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Hertfordshire Cohort Study Group. The relationship of dietary patterns with adult lung function and COPD. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigham, E.P.; Steffen, L.M.; London, S.J.; Boyce, D.; Diette, G.B.; Hansel, N.N.; Rice, J.; McCormack, M.C. Diet Pattern and Respiratory Morbidity in the Atherosclerosis Risk in Communities Study. Ann. Am. Thorac. Soc. 2018, 15, 675–682. [Google Scholar] [CrossRef]
- Shin, M.K.; Kwak, S.H.; Park, Y.; Jung, J.Y.; Kim, Y.S.; Kang, Y.A. Association between Dietary Patterns and Chronic Obstructive Pulmonary Disease in Korean Adults: The Korean Genome and Epidemiology Study. Nutrients 2021, 13, 4348. [Google Scholar] [CrossRef]
- Dinparast, F.; Sharifi, A.; Moradi, S.; Alipour, M.; Alipour, B. The associations between dietary pattern of chronic obstructive pulmonary disease patients and depression: A cross-sectional study. BMC Pulm. Med. 2021, 21, 8. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Seligman, H.K.; Schillinger, D. Hunger and socioeconomic disparities in chronic disease. N. Engl. J. Med. 2010, 363, 6–9. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease (2022 Report). Available online: https://goldcopd.org/2022-gold-reports/ (accessed on 15 November 2021).
- Wirth, M.D.; Sevoyan, M.; Hofseth, L.; Shivappa, N.; Hurley, T.G.; Hébert, J.R. The Dietary Inflammatory Index is associated with elevated white blood cell counts in the National Health and Nutrition Examination Survey. Brain Behav. Immun. 2018, 69, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- De Castro Mendes, F.; Ducharme-Smith, K.; Mora-Garcia, G.; Alqahtani, S.A.; Ruiz-Diaz, M.S.; Moreira, A.; Villegas, R.; Garcia-Larsen, V. Household Food Insecurity, Lung Function, and COPD in US Adults. Nutrients 2021, 13, 2098. [Google Scholar] [CrossRef]

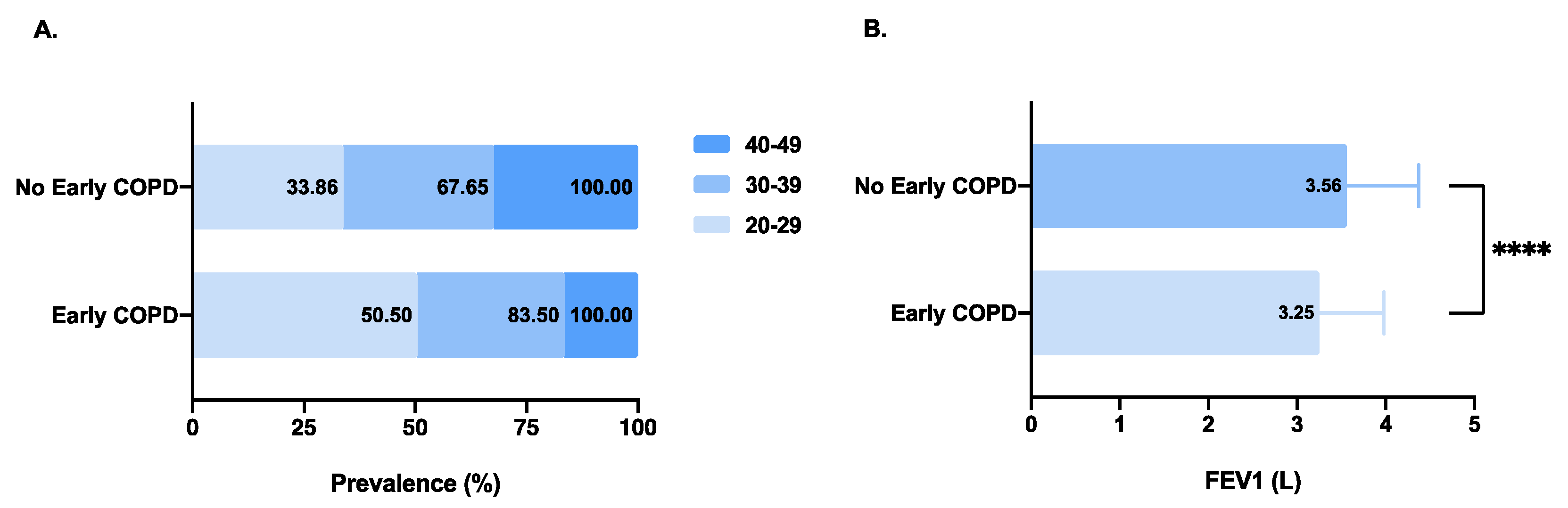

| Characteristics | Early COPD (n = 200) | No Early COPD (n = 3762) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age **** | <0.0001 | ||
| 20–29 | 101 (50.50) | 1274 (33.86) | |
| 30–39 | 66 (33.00) | 1271 (33.79) | |
| 40–49 | 33 (16.50) | 1217 (32.35) | |
| Sex ** | 0.0057 | ||
| Females | 116 (58.00) | 1805 (47.98) | |
| Males | 84 (42.00) | 1957 (52.02) | |
| Race/ethnicity | 0.4984 | ||
| Non-Hispanic white | 96 (48.00) | 1944 (51.67) | |
| Non-Hispanic black | 53 (26.50) | 980 (26.05) | |
| Mexican American | 51 (25.50) | 838 (22.28) | |
| Body-mass index (kg/m²) | 0.7381 | ||
| <18.5 (underweight) | 2 (1.00) | 56 (1.49) | |
| 18.5–24.9 (normal weight) | 67 (33.50) | 1135 (30.18) | |
| 25–29.9 (overweight) | 63 (31.50) | 1213 (32.25) | |
| ≥30 (obese) | 68 (34.00) | 1357 (36.08) | |
| Missing | 0 | 1 | |
| Educational level | 0.7413 | ||
| Primary school and less | 15 (7.54) | 245 (6.52) | |
| Middle and high school | 78 (39.20) | 1421 (37.79) | |
| College and higher | 106 (53.27) | 2094 (55.69) | |
| Missing | 1 | 2 | |
| PIR ** | 0.0053 | ||
| <1.85 | 107 (56.91) | 1624 (46.49) | |
| ≥1.85 | 81 (43.09) | 1869 (53.51) | |
| Missing | 12 | 269 | |
| Health insurance coverage | 0.0588 | ||
| No | 82 (41.00) | 1296 (34.47) | |
| Yes | 118 (59.00) | 2464 (65.53) | |
| Missing | 0 | 2 | |
| Sedentary activity | 0.1867 | ||
| <3 h | 53 (26.50) | 802 (21.35) | |
| 3–6 h | 72 (36.00) | 1367 (36.40) | |
| ≥6 h | 75 (37.50) | 1587 (42.25) | |
| Missing | 0 | 6 | |
| Exposure history | |||
| Smoking status ** | 0.0016 | ||
| Never smoker | 108 (60.34) | 2290 (71.16) | |
| Former smoker | 20 (10.05) | 541 (14.39) | |
| Current smoker | 71 (35.68) | 928 (24.69) | |
| Missing | 1 | 3 | |
| Smoking exposure (pack-years) | 0.1617 | ||
| 0 | 108 (55.96) | 2290 (62.69) | |
| <10 | 54 (27.98) | 888 (24.31) | |
| ≥10 | 31 (16.06) | 475 (13.00) | |
| Missing | 7 | 109 | |
| Passive smoking | 0.4309 | ||
| No | 85 (85.86) | 1628 (82.81) | |
| Yes | 14 (14.14) | 338 (17.19) | |
| Missing | 101 | 1796 | |
| Smokers living in the home *** | 0.0005 | ||
| 0 | 63 (51.64) | 1421 (68.71) | |
| 1–2 | 51 (41.80) | 561 (27.13) | |
| ≥3 | 8 (6.56) | 86 (4.16) | |
| Missing | 78 | 1694 | |
| Mineral dusts ** | 0.0018 | ||
| No | 151 (76.65) | 2393 (65.89) | |
| Yes | 46 (23.35) | 1239 (34.11) | |
| Missing | 3 | 130 | |
| Organic dusts | 0.8919 | ||
| No | 150 (76.14) | 2750 (75.72) | |
| Yes | 47 (23.86) | 882 (24.28) | |
| Missing | 3 | 130 | |
| Fumes from machinery or engines ** | 0.0082 | ||
| No | 161 (81.73) | 2662 (73.21) | |
| Yes | 36 (18.27) | 974 (26.79) | |
| Missing | 3 | 126 | |
| Any other gases, vapors or fumes | 0.4033 | ||
| No | 139 (70.56) | 2461 (67.70) | |
| Yes | 58 (29.44) | 1174 (32.30) | |
| Missing | 3 | 127 | |
| Medical history | |||
| History of emphysema, bronchitis or asthma during childhood * | 0.0441 | ||
| No | 136 (68.00) | 2799 (74.40) | |
| Yes | 64 (32.00) | 963 (25.60) | |
| History of emphysema | 0.1726 | ||
| No | 198 (99.00) | 3747 (99.63) | |
| Yes | 2 (1.00) | 14 (0.37) | |
| Missing | 0 | 1 | |
| History of chronic bronchitis | 0.6110 | ||
| No | 191 (95.98) | 3634 (96.65) | |
| Yes | 8 (4.02) | 126 (3.35) | |
| Missing | 1 | 2 | |
| History of asthma **** | <0.0001 | ||
| No | 147 (73.50) | 3287 (87.44) | |
| Yes | 53 (26.50) | 472 (12.56) | |
| Missing | 0 | 3 | |
| Close relative had asthma | 0.8575 | ||
| No | 155 (78.28) | 2921 (78.82) | |
| Yes | 43 (21.72) | 785 (21.18) | |
| Missing | 2 | 56 | |
| Symptoms (≥40 years old) | |||
| Chronic cough | 0.3452 | ||
| No | 29 (87.88) | 1121 (92.34) | |
| Yes | 4 (12.12) | 93 (7.66) | |
| Missing | 167 | 2548 | |
| Coughing phlegm | 0.4748 | ||
| No | 32 (96.97) | 1142 (93.99) | |
| Yes | 1 (3.03) | 73 (6.01) | |
| Missing | 167 | 2547 | |
| Wheezing **** | <0.0001 | ||
| No | 159 (79.50) | 3386 (90.13) | |
| Yes | 41 (20.50) | 371 (9.87) | |
| Missing | 0 | 5 | |
| Shortness of breath | 0.6677 | ||
| No | 24 (72.73) | 923 (75.97) | |
| Yes | 9 (27.27) | 292 (24.03) | |
| Missing | 167 | 2547 | |
| Lung function | |||
| FEV1, L **** | 3.25 ± 0.73 | 3.56 ± 0.81 | <0.0001 |
| FEV1, % predicted **** | 90.64 ± 11.09 | 99.32 ± 12.07 | <0.0001 |
| FEV1 < 80% predicted-no. (%) | 34 (17.00) | 206 (5.48) | 0.0829 |
| FVC, L **** | 4.48 ± 1.01 | 4.34 ± 1.01 | <0.0001 |
| FVC, % predicted **** | 109.43 ± 17.21 | 103.07 ± 17.31 | <0.0001 |
| FEV1/FVC ** | 0.72 ± 0.02 | 0.82 ± 0.05 | 0.0041 |
| Dietary inflammatory index 1 | |||
| DII **** | 3.82 ± 0.26 | 3.76 ± 0.25 | <0.0001 |
| Early COPD | β (95% CI) 1, p-Value | |||
|---|---|---|---|---|
| Model 1 2 (n = 3962) | Model 2 3 (n = 3437) | |||
| DII | ||||
| Continuous | 2.361 (1.335, 4.174) ** | 0.0031 | 1.903 (1.034, 3.502) * | 0.0386 |
| Quartile 1 | 1.0 (reference) | 1.0 (reference) | ||
| Quartile 2 | 1.108 (0.712, 1.726) | 0.6486 | 0.960 (0.596, 1.547) | 0.8664 |
| Quartile 3 | 1.436 (0.943, 2.186) | 0.0914 | 1.156 (0.732, 1.823) | 0.5345 |
| Quartile 4 | 1.657 (1.100, 2.496) * | 0.0156 | 1.288 (0.824, 2.015) | 0.2667 |
| p-trend | 1.195 (1.051, 1.359) ** | 0.0066 | 1.104 (0.957, 1.273) | 0.1757 |
| DII | Sample Size | Early COPD | p-Value |
|---|---|---|---|
| Age | |||
| 20–29 | 1153 | 1.820 (0.735, 4.503) | 0.1954 |
| 30–39 | 1187 | 2.024 (0.699, 5.855) | 0.1934 |
| 40–49 | 1097 | 0.741 (0.146, 3.754) | 0.7177 |
| Sex | |||
| Females | 1664 | 2.325 (0.999, 5.410) | 0.0502 |
| Males | 1773 | 1.132 (0.435, 2.944) | 0.7988 |
| PIR | |||
| <1.85 | 1576 | 2.350 (1.010, 5.470) * | 0.0474 |
| ≥1.85 | 1861 | 1.077 (0.416, 2.784) | 0.8789 |
| Health insurance coverage | |||
| No | 1136 | 1.878 (0.705, 5.000) | 0.2072 |
| Yes | 2301 | 1.690 (0.742, 3.852) | 0.2117 |
| Sedentary activity | |||
| <3 h | 712 | 2.259 (0.629, 8.110) | 0.2114 |
| 3–6 h | 1256 | 1.223 (0.423, 3.542) | 0.7100 |
| ≥6 h | 1469 | 1.867 (0.678, 5.145) | 0.2272 |
| Smoking exposure (pack-years) | |||
| 0 | 2129 | 1.475 (0.635, 3.429) | 0.3664 |
| <10 | 848 | 3.689 (1.133, 12.012) * | 0.0303 |
| ≥10 | 460 | 0.417 (0.082, 2.126) | 0.2927 |
| Smokers living in the home | |||
| 0 | 1379 | 0.754 (0.245, 2.317) | 0.6219 |
| 1–2 | 551 | 3.550 (0.946, 13.319) | 0.0604 |
| ≥3 | 83 | 0.129 (0.002, 9.767) | 0.3531 |
| Mineral dusts | |||
| No | 2293 | 2.315 (1.117, 4.800) * | 0.0240 |
| Yes | 1144 | 0.731 (0.196, 2.721) | 0.6401 |
| Fumes from machinery or engines | |||
| No | 2529 | 1.800 (0.890, 3.639) | 0.1018 |
| Yes | 908 | 1.416 (0.349, 5.735) | 0.6262 |
| Wheezing | |||
| No | 2973 | 1.298 (0.626, 2.693) | 0.4833 |
| Yes | 464 | 4.016 (1.157, 13.947) * | 0.0286 |
| Asthma | |||
| No | 3069 | 1.702 (0.841, 3.444) | 0.1393 |
| Yes | 368 | 1.796 (0.433, 7.452) | 0.4197 |
| History of emphysema, bronchitis or asthma during childhood | |||
| No | 2549 | 1.180 (0.556, 2.505) | 0.6668 |
| Yes | 893 | 4.277 (1.362, 13.430) * | 0.0128 |
| Lung Function Measures | β | 95% CI | p-Value |
|---|---|---|---|
| All participants | N (missing) = 3440 (522) | ||
| FEV1, L | −0.40 | (−0.48, −0.32) **** | <0.0001 |
| FEV1, % predicted | −4.42 | (−6.08, −2.76) **** | <0.0001 |
| FVC, L | −0.55 | (−0.65, −0.45) **** | <0.0001 |
| FVC, % predicted | −8.86 | (−11.14, −6.58) **** | <0.0001 |
| FEV1/FVC | 0.01 | (0.00, 0.02) ** | 0.0086 |
| Participants with Early COPD | N (missing) = 180 (20) | ||
| FEV1, L | −0.43 | (−0.74, −0.12) ** | 0.0072 |
| FEV1, % predicted | −5.22 | (−11.46, 1.03) | 0.1009 |
| FVC, L | −0.58 | (−1.01, −0.16) ** | 0.0074 |
| FVC, % predicted | −7.83 | (−16.77, 1.12) | 0.0858 |
| FEV1/FVC | −0.003 | (−0.011, 0.005) | 0.4573 |
| Participants without Early COPD | N (missing) = 3260 (502) | ||
| FEV1, L | −0.39 | (−0.47, −0.31) **** | <0.0001 |
| FEV1, % predicted | −4.15 | (−5.85, −2.45) **** | <0.0001 |
| FVC, L | −0.56 | (−0.66, −0.45) **** | <0.0001 |
| FVC, % predicted | −9.05 | (−11.41, −6.7) **** | <0.0001 |
| FEV1/FVC | 0.01 | (0.01, 0.02) *** | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yang, T.; Wang, C. The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey. Nutrients 2022, 14, 2841. https://doi.org/10.3390/nu14142841
Chen C, Yang T, Wang C. The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey. Nutrients. 2022; 14(14):2841. https://doi.org/10.3390/nu14142841
Chicago/Turabian StyleChen, Chen, Ting Yang, and Chen Wang. 2022. "The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey" Nutrients 14, no. 14: 2841. https://doi.org/10.3390/nu14142841
APA StyleChen, C., Yang, T., & Wang, C. (2022). The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey. Nutrients, 14(14), 2841. https://doi.org/10.3390/nu14142841






