Interactions between Vitamin D Genetic Risk and Dietary Factors on Metabolic Disease-Related Outcomes in Ghanaian Adults
Abstract
1. Introduction
2. Methodology
2.1. Study Population
2.2. Data Collection
2.3. Biochemical Measurements
3. Assessment of Dietary Intake
SNP Selection, GRS Construction and Genotyping
4. Statistical Analysis
5. Results
5.1. Characteristics of Study Participants
5.2. Genetic Associations between Vitamin D-GRS and Metabolic Traits
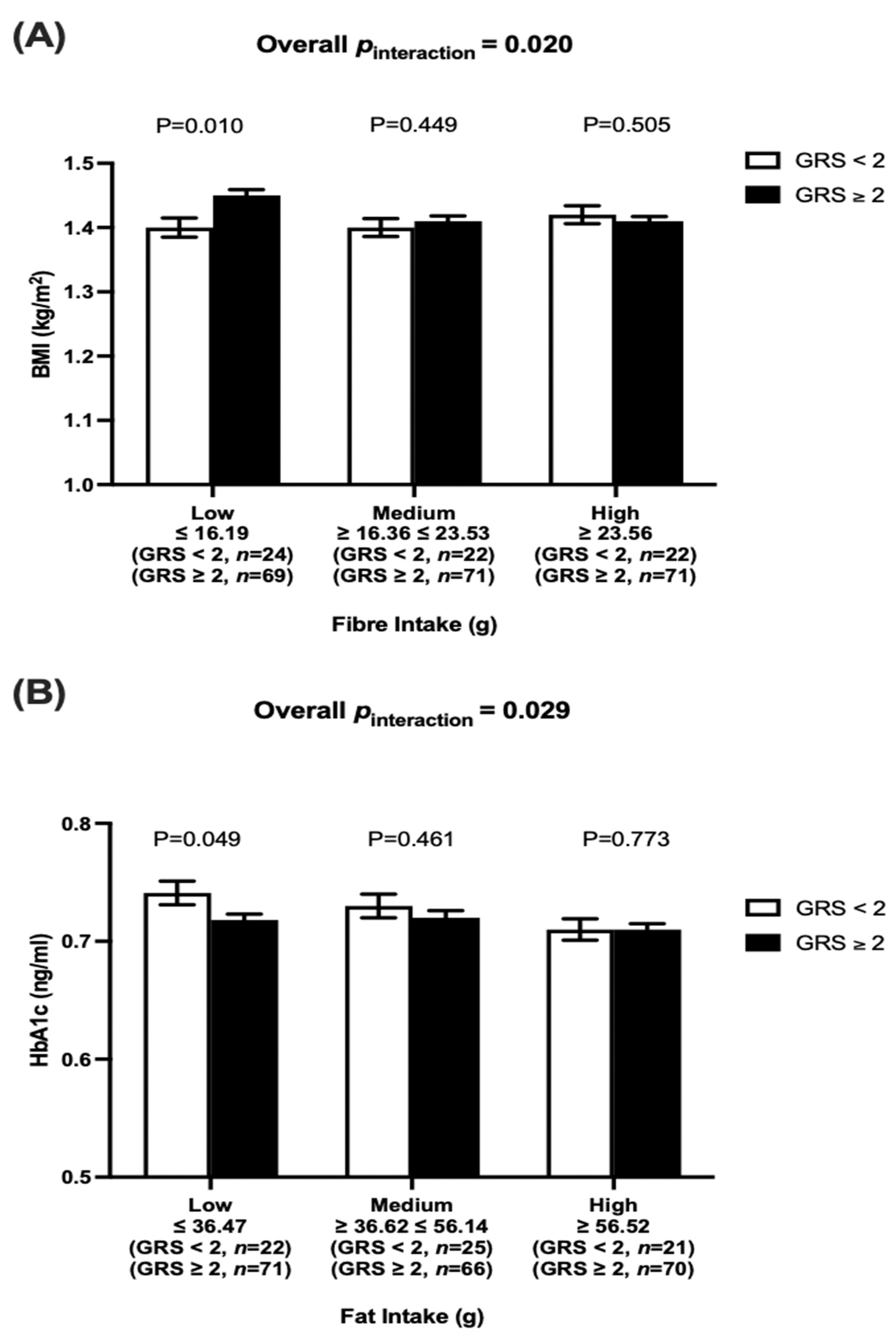
5.3. Interactions between Dietary Factors and Vitamin D-GRS on Metabolic Traits
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSA | Sub-Saharan Africa |
| T2D | type 2 diabetes |
| WC | waist circumference |
| BMI | body mass index |
| HbA1c | glycated haemoglobin |
| VDR | vitamin D receptor |
| DHCR7 | 7-dehydrocholesterol reductase |
| CYP2R1 | 25-hydroxylase |
| CYP24A1 | 24-hydroxylase |
| DBP | vitamin D binding protein |
| GC | group-specific component |
| CASR | calcium sensing receptor |
| GRS | genetic risk score |
| SNP | single nucleotide polymorphism |
| HWE | Hardy Weinberg equilibrium |
| SD | standard deviation |
| IOM | Institute of Medicine |
References
- Adeboye, B.; Bermano, G.; Rolland, C. Obesity and its health impact in Africa: A systematic review. Cardiovasc. J. Afr. 2012, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ajemu, K.F.; Desta, A.A.; Berhe, A.A.; Woldegebriel, A.G.; Bezabih, N.M.; Negash, D.; Wuneh, A.D.; Woldearegay, T.W. Magnitude, components and predictors of metabolic syndrome in Northern Ethiopia: Evidences from regional NCDs STEPS survey, 2016. PLoS ONE 2021, 16, e0253317. [Google Scholar] [CrossRef] [PubMed]
- Mbanya, J.C.; Assah, F.K.; Saji, J.; Atanga, E.N. Obesity and Type 2 Diabetes in Sub-Sahara Africa. Curr. Diabetes Rep. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.P.; Fleming, T.B.S.; Thomson, B.B.A.; Biryukov, S.B.S.; Abbafati, C.P.; Abraham, J.P.M.P.H.; AlBuhairan, F.S.M.; Alemu, Z.A.M.P.H.; Alfonso, R.M.D.; Ali, M.K.M.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Scott, A.; Ejikeme, C.S.; Clottey, E.N.; Thomas, J.G. Obesity in sub-Saharan Africa: Development of an ecological theoretical framework. Health Promot. Int. 2012, 28, 4–16. [Google Scholar] [CrossRef]
- Ziraba, A.K.; Fotso, J.C.; Ochako, R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health 2009, 9, 465. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Agyeman, A.A.; Laar, A.; Boateng, D. Overweight and obesity epidemic in Ghana-a systematic review and meta-analysis. BMC Public Health 2016, 16, 1239. [Google Scholar] [CrossRef]
- Asamoah-Boaheng, M.; Sarfo-Kantanka, O.; Tuffour, A.B.; Eghan, B.; Mbanya, J.C. Prevalence and risk factors for diabetes mellitus among adults in Ghana: A systematic review and meta-analysis. Int. Health 2018, 11, 83–92. [Google Scholar] [CrossRef]
- Danquah, I.; Bedu-Addo, G.; Terpe, K.; Micah, F.; Amoako, Y.A.; Awuku, Y.A.; Dietz, E.; van der Giet, M.; Spranger, J.; Mockenhaupt, F.P. Diabetes mellitus type 2 in urban Ghana: Characteristics and associated factors. BMC Public Health 2012, 12, 210. [Google Scholar] [CrossRef]
- Tuei, V.C.; Maiyoh, G.K.; Ha, C.-E. Type 2 diabetes mellitus and obesity in sub-Saharan Africa. Diabetes/Metab. Res. Rev. 2010, 26, 433–445. [Google Scholar] [CrossRef]
- Polfus, L.M.; Darst, B.F.; Highland, H.; Sheng, X.; Ng MC, Y.; Below, J.E.; Petty, L.; Bien, S.; Sim, X.; Wang, W.; et al. Genetic discovery and risk characterization in type 2 diabetes across diverse populations. HGG Adv. 2021, 2. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018, 233, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Kuan, V.; Martineau, A.R.; Griffiths, C.J.; Hypponen, E.; Walton, R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol. Biol. 2013, 13, 144–153. [Google Scholar] [CrossRef]
- Berry, D.J.; Vimaleswaran, K.S.; Whittaker, J.C.; Hingorani, A.D.; Hypponen, E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS ONE 2012, 7, e37465. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Whittaker, J.C.; Power, C.; Jarvelin, M.R.; Hypponen, E. Genetic association analysis of vitamin D pathway with obesity traits. Int. J. Obes. 2013, 37, 1399–1406. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid. Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Molin, A.; Coudray, N.; Richard, N.; Kottler, M.-L.; Deschenes, G.; Kesler-Roussey, G.; Tiulpakov, A.; Baudoin, R.; Jones, G.; Kaufmann, M.; et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. J. Clin. Endocrinol. Metab. 2015, 100, E1343–E1352. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Jiang, X.; Kiel, D.P.; Kraft, P. The genetics of vitamin D. Bone 2019, 126, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Mangino, M.; Andrews, P.; Moore, J.H.; Spector, T.D.; Power, C.; Jarvelin, M.R.; Hypponen, E. Interaction between allelic variations in vitamin D receptor and retinoid X receptor genes on metabolic traits. BMC Genet. 2014, 15, 37. [Google Scholar]
- Fetahu, I.S.; Hummel, D.M.; Manhardt, T.; Aggarwal, A.; Baumgartner-Parzer, S.; Kállay, E. Regulation of the calcium-sensing receptor expression by 1,25-dihydroxyvitamin D3, interleukin-6, and tumor necrosis factor alpha in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 228–231. [Google Scholar] [CrossRef]
- Krasniqi, E.; Boshnjaku, A.; Wagner, K.H.; Wessner, B. Association between Polymorphisms in Vitamin D Pathway-Related Genes, Vitamin D Status, Muscle Mass and Function: A Systematic Review. Nutrients 2021, 13, 3109. [Google Scholar] [CrossRef] [PubMed]
- Griz, L.H.; Bandeira, F.; Gabbay, M.A.; Dib, S.A.; Carvalho, E.F. Vitamin D and diabetes mellitus: An update 2013. Arq. Bras. Endocrinol. Metabol. 2014, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis: 25(OH) vitamin D and BMI. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; VanderWeele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. GeNuIne (gene–nutrient interactions) Collaboration: Towards implementing multi-ethnic population-based nutrigenetic studies of vitamin B12 and D deficiencies and metabolic diseases. Proc. Nutr. Soc. 2021, 80, 435–445. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. A nutrigenetics approach to study the impact of genetic and lifestyle factors on cardiometabolic traits in various ethnic groups: Findings from the GeNuIne Collaboration. Proc. Nutr. Soc. 2020, 79, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. Gene–nutrient interactions on metabolic diseases: Findings from the GeNuIne Collaboration. Nutr. Bull. 2017, 42, 80–86. [Google Scholar] [CrossRef]
- Surendran, S.; Vimaleswaran, K.S. A nutrigenetic approach to examine the relationship between vitamin B12 status and cardio-metabolic traits in multiple ethnic groups—Findings from the GeNuIne Collaboration. Nutr. Bull. 2021, 46, 185–194. [Google Scholar] [CrossRef]
- Tuncel, G.; Temel, S.G.; Ergoren, M.C. Strong association between VDR FokI (rs2228570) gene variant and serum vitamin D levels in Turkish Cypriots. Mol. Biol. Rep. 2019, 46, 3349–3355. [Google Scholar] [CrossRef]
- Foucan, L.; Vélayoudom-Céphise, F.-L.; Larifla, L.; Armand, C.; Deloumeaux, J.; Fagour, C.; Plumasseau, J.; Portlis, M.-L.; Liu, L.; Bonnet, F.; et al. Polymorphisms in GC and NADSYN1 Genes are associated with vitamin D status and metabolic profile in Non-diabetic adults. BMC Endocr. Disord. 2013, 13, 36. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Xu, X.; Mao, J.; Zhang, M.; Liu, H.; Li, H.; Lei, H.; Han, L.; Gao, M. Vitamin D Deficiency in Uygurs and Kazaks Is Associated with Polymorphisms in CYP2R1 and DHCR7/NADSYN1 Genes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1960–1968. [Google Scholar]
- Zhang, Y.; Wang, X.; Liu, Y.; Qu, H.; Qu, S.; Wang, W.; Ren, L. The GC, CYP2R1 and DHCR7 genes are associated with vitamin D levels in northeastern Han Chinese children. Swiss Med. Wkly. 2012, 142, w13636. [Google Scholar] [CrossRef]
- Elkum, N.; Alkayal, F.; Noronha, F.; Ali, M.M.; Melhem, M.; Al-Arouj, M.; Bennakhi, A.; Behbehani, K.; Alsmadi, O.; Abubaker, J. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS ONE 2014, 9, e113102. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Yongjoo Park, C.; Jo, G.; Yoen Kim, O.; Shin, M.J. Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: Results from the Korea National Health and Nutrition Examination Survey 2011–2012. Endocr. J. 2018, 65, 881–891. [Google Scholar] [CrossRef]
- Hibler, E.A.; Hu, C.; Jurutka, P.W.; Martinez, M.E.; Jacobs, E.T. Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol. Biomark. Prev. 2012, 21, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Batai, K.; Murphy, A.B.; Shah, E.; Ruden, M.; Newsome, J.; Agate, S.; Dixon, M.A.; Chen, H.Y.; Deane, L.A.; Hollowell, C.M.P.; et al. Common vitamin D pathway gene variants reveal contrasting effects on serum vitamin D levels in African Americans and European Americans. Hum. Genet. 2014, 133, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.-X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.; Wang, H.-W.; Recker, R.; Zhao, L.-J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Ge, W.; Koenig, K.L.; Axelsson, T.; Liu, M.; Afanasyeva, Y.; Andersson, A.; Arslan, A.A.; Chen, Y.; Hallmans, G.; et al. Genetic Polymorphisms in Vitamin D Metabolism and Signaling Genes and Risk of Breast Cancer: A Nested Case-Control Study. PLoS ONE 2015, 10, e0140478. [Google Scholar] [CrossRef]
- Cooper, J.D.; Smyth, D.J.; Walker, N.M.; Stevens, H.; Burren, O.S.; Wallace, C.; Greissl, C.; Ramos-Lopez, E.; Hyppönen, E.; Dunger, D.B.; et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011, 60, 1624–1631. [Google Scholar] [CrossRef]
- Davies, J.R.; Chang, Y.M.; Snowden, H.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; Kukalizch, K.; Randerson-Moor, J.; Elliott, F.; et al. The determinants of serum vitamin D levels in participants in a melanoma case-control study living in a temperate climate. Cancer Causes Control 2011, 22, 1471–1482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilbert, R.; Bonilla, C.; Metcalfe, C.; Lewis, S.; Evans, D.M.; Fraser, W.D.; Kemp, J.P.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: A nested case-control study. Cancer Causes Control 2015, 26, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Nissen, J.; Rasmussen, L.B.; Ravn-Haren, G.; Andersen, E.W.; Hansen, B.; Andersen, R.; Mejborn, H.; Madsen, K.H.; Vogel, U. Common variants in CYP2R1 and GC genes predict vitamin D concentrations in healthy Danish children and adults. PLoS ONE 2014, 9, e89907. [Google Scholar] [CrossRef]
- Nissen, J.; Vogel, U.; Ravn-Haren, G.; Andersen, E.W.; Madsen, K.H.; Nexø, B.A.; Andersen, R.; Mejborn, H.; Bjerrum, P.J.; Rasmussen, L.B.; et al. Common variants in CYP2R1 and GC genes are both determinants of serum 25-hydroxyvitamin D concentrations after UVB irradiation and after consumption of vitamin D3–fortified bread and milk during winter in Denmark. Am. J. Clin. Nutr. 2015, 101, 218–227. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Maalmi, H.; Schöttker, B.; Saum, K.-U.; Holleczek, B.; Wang, T.J.; Burwinkel, B.; Brenner, H. Genetic Variants in the Vitamin D Pathway, 25(OH)D Levels, and Mortality in a Large Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 470–477. [Google Scholar] [CrossRef]
- Rees, J.R.; Mott, L.A.; Barry, E.L.; Baron, J.A.; Bostick, R.M.; Figueiredo, J.C.; Bresalier, R.S.; Robertson, D.J.; Peacock, J.L. Lifestyle and Other Factors Explain One-Half of the Variability in the Serum 25-Hydroxyvitamin D Response to Cholecalciferol Supplementation in Healthy Adults. J. Nutr. 2016, 146, 2312–2324. [Google Scholar] [PubMed]
- Slater, N.A.; Rager, M.L.; Havrda, D.E.; Harralson, A.F. Genetic Variation in CYP2R1 and GC Genes Associated with Vitamin D Deficiency Status. J. Pharm. Pract. 2015, 30, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.C.; Moonesinghe, R.; Yang, Q.; Steyerberg, E.W.; van Duijn, C.M.; Khoury, M.J. The impact of genotype frequencies on the clinical validity of genomic profiling for predicting common chronic diseases. Genet. Med. 2007, 9, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Moselakgomo, V.K.; Van Staden, M. Diagnostic accuracy of tri-ponderal mass index and body mass index in estimating overweight and obesity in South African children. Afr. J. Prim. Health Care Fam. Med. 2019, 11, e1–e7. [Google Scholar] [CrossRef]
- Zafra-Tanaka, J.H.; Miranda, J.J.; Gilman, R.H.; Checkley, W.; Smeeth, L.; Bernabe-Ortiz, A. Obesity markers for the prediction of incident type 2 diabetes mellitus in resource-poor settings: The CRONICAS Cohort Study. Diabetes Res. Clin. Pract. 2020, 170, 108494. [Google Scholar] [CrossRef]
- Alaboudi, I.S.; Hassali, M.A.; Shafie, A.A.; Saleem, F. Self-efficacy, self-care behaviours and glycaemic control in type 2 diabetic patients in Riyadh, Saudi Arabia. J. Public Health 2016, 24, 281–290. [Google Scholar] [CrossRef]
- Woo, V.; Shestakova, M.V.; Ørskov, C.; Ceriello, A. Targets and tactics: The relative importance of HbA1c, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int. J. Clin. Pract. 2008, 62, 1935–1942. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary Fiber and Weight Regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Okubo, H.; Takahashi, Y.; Hosoi, Y.; Itabashi, M.; the Freshmen in Dietetic Courses Study II Group. Dietary fiber intake, dietary glycemic index and load, and body mass index: A cross-sectional study of 3931 Japanese women aged 18–20 years. Eur. J. Clin. Nutr. 2007, 61, 986–995. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Barbaresko, J.; Pischke, C.R.; Iser, N.; Beckhaus, J.; Schwingshackl, L.; Schlesinger, S. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. 2020, 17, e1003347. [Google Scholar] [CrossRef] [PubMed]
- Doglikuu, B.-I.D.; Abubakari, A.; Yaseri, M.; Shakibazadeh, E.; Djazayery, A.; Mirzaei, K. Association of household socioeconomic status, neighborhood support system and adherence to dietary recommendation among persons with T2DM, a facility-based cross-sectional study in Ghana. BMC Public Health 2021, 21, 911. [Google Scholar] [CrossRef] [PubMed]
- Beane, K.E.; Redding, M.C.; Wang, X.; Pan, J.H.; Le, B.; Cicalo, C.; Jeon, S.; Kim, Y.J.; Lee, J.H.; Shin, E.-C.; et al. Effects of dietary fibers, micronutrients, and phytonutrients on gut microbiome: A review. Appl. Biol. Chem. 2021, 64, 36. [Google Scholar] [CrossRef]
- Roager, H.M.; Christensen, L.H. Personal diet–microbiota interactions and weight loss. Proc. Nutr. Soc. 2022, 1–12. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Ly, N.P.; Litonjua, A.; Gold, D.R.; Celedón, J.C. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 2011, 127, 1087–1096. [Google Scholar] [CrossRef]
- Sun, J. Dietary vitamin D, vitamin D receptor, and microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 471–474. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Bodhini, D.; Lakshmipriya, N.; Ramya, K.; Anjana, R.M.; Sudha, V.; Lovegrove, J.A.; Kinra, S.; Mohan, V.; Radha, V. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, S.; Nyakotey, D.A.; Dudek, K.; Bawah, A.-M.; Lovegrove, J.A.; Annan, R.A.; Ellahi, B.; Vimaleswaran, K.S. Interaction between Metabolic Genetic Risk Score and Dietary Fatty Acid Intake on Central Obesity in a Ghanaian Population. Nutrients 2020, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Lie, L.; Brown, L.; Forrester, T.E.; Plange-Rhule, J.; Bovet, P.; Lambert, E.V.; Layden, B.T.; Luke, A.; Dugas, L.R. The Association of Dietary Fiber Intake with Cardiometabolic Risk in Four Countries across the Epidemiologic Transition. Nutrients 2018, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Mchiza, Z.J. Obesity and the nutrition transition in Sub-Saharan Africa. Ann. N. Y. Acad. Sci. 2014, 1311, 88–101. [Google Scholar] [CrossRef]
- Mehta, S.; Ruth Dugas, L.; Choo-Kang, C.; Bovet, P.; Forrester, T.; Bedu-Addo, K.; Lambert, E.V.; Plange-Rhule, J.; Riesen, W.; Korte, W.; et al. Consumption of Monounsaturated Fatty Acids Is Associated with Improved Cardiometabolic Outcomes in Four African-Origin Populations Spanning the Epidemiologic Transition. Nutrients 2021, 13, 2442. [Google Scholar] [CrossRef]
- Suara, S.B.; Siassi, F.; Saaka, M.; Rahimiforoushani, A.; Sotoudeh, G. Relationship between dietary carbohydrate quality index and metabolic syndrome among type 2 diabetes mellitus subjects: A case-control study from Ghana. BMC Public Health 2021, 21, 526. [Google Scholar] [CrossRef]
- Buscemi, J.; Pugach, O.; Springfield, S.; Jang, J.; Tussing-Humphreys, L.; Schiffer, L.; Stolley, M.R.; Fitzgibbon, M.L. Associations between fiber intake and Body Mass Index (BMI) among African-American women participating in a randomized weight loss and maintenance trial. Eat. Behav. 2018, 29, 48–53. [Google Scholar] [CrossRef]
- Holdsworth, M.; Pradeilles, R.; Tandoh, A.; Green, M.; Wanjohi, M.; Zotor, F.; Asiki, G.; Klomegah, S.; Abdul-Haq, Z.; Osei-Kwasi, H.; et al. Unhealthy eating practices of city-dwelling Africans in deprived neighbourhoods: Evidence for policy action from Ghana and Kenya. Glob. Food Secur. 2020, 26, 100452. [Google Scholar] [CrossRef]
- Saleh, A.; Amanatidis, S.; Samman, S. The effect of migration on dietary intake, type 2 diabetes and obesity: The Ghanaian Health and Nutrition Analysis in Sydney, Australia (GHANAISA). Ecol. Food Nutr. 2002, 41, 255–270. [Google Scholar] [CrossRef]
- Wolpert, H.A.; Atakov-Castillo, A.; Smith, S.A.; Steil, G.M. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: Implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013, 36, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Grylls, W.K.; McKenzie, J.E.; Horwath, C.C.; Mann, J.I. Lifestyle factors associated with glycaemic control and body mass index in older adults with diabetes. Eur. J. Clin. Nutr. 2003, 57, 1386–1393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smart, C.E.M.; Evans, M.; O’Connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Garonzi, C.; Forsander, G.; Maffeis, C. Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, J.H.; Luan, J.; Luben, R.N.; Rodwell, S.A.; Khaw, K.T.; Ong, K.K.; Wareham, N.J.; Loos, R.J. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am. J. Clin. Nutr. 2010, 91, 184–190. [Google Scholar] [CrossRef]
- Babb de Villiers, C.; Kroese, M.; Moorthie, S. Understanding polygenic models, their development and the potential application of polygenic scores in healthcare. J. Med. Genet. 2020, 57, 725–732. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- National Centre for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 26 June 2022).

| n | Total | n | Men | n | Women | p Value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 279 | 38 ± 10 | 115 | 36 ± 9 | 164 | 40 ± 10 | 0.003 |
| BMI (kg/m2) | 279 | 26.6 ± 4.91 | 115 | 23.6 ± 3.02 | 164 | 28.7 ± 4.92 | <0.001 |
| WC (cm) | 279 | 88.4 ± 12.22 | 115 | 81.8 ± 9.92 | 164 | 93 ± 11.59 | <0.001 |
| WHR | 279 | 1.5 ± 7.24 | 115 | 0.9 ± 0.1 | 164 | 1.9 ± 9.43 | 0.15 |
| BFP (%) | 279 | 32.9 ± 13.55 | 115 | 20.5 ± 10.01 | 164 | 41.6 ± 7.58 | <0.001 |
| Glucose (mg/dl) | 278 | 4.4 ± 0.91 | 115 | 4.3 ± 0.59 | 163 | 4.4 ± 1.09 | 0.33 |
| HbA1c (%) | 275 | 5.3 ± 0.58 | 111 | 5.3 ± 0.5 | 164 | 5.3 ± 0.62 | 0.94 |
| Fasting Insulin (µIU/mL) | 270 | 12.6 ± 14.38 | 109 | 13.1 ± 16.08 | 161 | 12.3 ± 13.15 | 0.62 |
| Total Cholesterol (mg/dL) | 276 | 212.7 ± 58 | 113 | 208.8 ± 41.76 | 163 | 216.6 ± 39.06 | 0.07 |
| HDL-c (mg/dL) | 276 | 69.6 ± 7.70 | 113 | 69.6 ± 7.35 | 163 | 65.7 ± 0.7.73 | 0.12 |
| LDL-c (mg/dL) | 276 | 127.6 ± 41.76 | 113 | 123.7 ± 42.54 | 163 | 131.5 ± 40.99 | 0.06 |
| Serum Triglycerides (mg/dL) | 276 | 87.3 ± 32.78 | 113 | 86.8 ± 29.23 | 163 | 87.7 ± 36.32 | 0.98 |
| Total Energy Intake (kcal) | 279 | 1645 ± 688 | 115 | 1901 ± 714 | 164 | 1465 ± 610 | <0.001 |
| Protein (g) | 279 | 53 ± 23 | 115 | 63 ± 24 | 164 | 46 ± 19 | <0.001 |
| Carbohydrate (g) | 279 | 240 ± 98 | 115 | 281 ± 104 | 164 | 211 ± 81 | <0.001 |
| Fat (g) | 279 | 51 ± 27 | 115 | 57 ± 29 | 164 | 47 ± 24 | 0.001 |
| Saturated fat (g) | 279 | 16 ± 10 | 115 | 18 ± 11 | 164 | 15 ± 9 | 0.006 |
| Monounsaturated fat (g) | 279 | 18 ± 10 | 115 | 20 ± 11 | 164 | 16 ± 9 | 0.002 |
| Polyunsaturated fat (g) | 279 | 9 ± 5 | 115 | 10 ± 6 | 164 | 8 ± 5 | 0.002 |
| Dietary Fibre (g) | 279 | 22 ± 11 | 115 | 25 ± 12 | 164 | 19 ± 10 | <0.001 |
| Carbohydrates (g) | Protein (g) | Fat (g) | Fibre (g) | SFA (g) | PUFA (g) | MUFA (g) | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 0.05 | 0.16 | 0.99 | 0.02 | |||
| WC (cm) | 0.16 | 0.07 | 0.22 | 0.13 | |||
| WHR | 0.72 | 0.76 | 0.85 | 0.87 | |||
| BFP (%) | 1.00 | 0.27 | 0.22 | 0.12 | |||
| Glucose (mg/dL) | 0.98 | 0.83 | 0.88 | 0.52 | |||
| HbA1c (ng/mL) | 0.06 | 0.12 | 0.03 | 0.10 | 0.04 | 0.13 | 0.84 |
| Fasting Insulin (µIU/mL) | 0.35 | 0.68 | 0.43 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alathari, B.E.; Nyakotey, D.A.; Bawah, A.-M.; Lovegrove, J.A.; Annan, R.A.; Ellahi, B.; Vimaleswaran, K.S. Interactions between Vitamin D Genetic Risk and Dietary Factors on Metabolic Disease-Related Outcomes in Ghanaian Adults. Nutrients 2022, 14, 2763. https://doi.org/10.3390/nu14132763
Alathari BE, Nyakotey DA, Bawah A-M, Lovegrove JA, Annan RA, Ellahi B, Vimaleswaran KS. Interactions between Vitamin D Genetic Risk and Dietary Factors on Metabolic Disease-Related Outcomes in Ghanaian Adults. Nutrients. 2022; 14(13):2763. https://doi.org/10.3390/nu14132763
Chicago/Turabian StyleAlathari, Buthaina E., David A. Nyakotey, Abdul-Malik Bawah, Julie A. Lovegrove, Reginald A. Annan, Basma Ellahi, and Karani S. Vimaleswaran. 2022. "Interactions between Vitamin D Genetic Risk and Dietary Factors on Metabolic Disease-Related Outcomes in Ghanaian Adults" Nutrients 14, no. 13: 2763. https://doi.org/10.3390/nu14132763
APA StyleAlathari, B. E., Nyakotey, D. A., Bawah, A.-M., Lovegrove, J. A., Annan, R. A., Ellahi, B., & Vimaleswaran, K. S. (2022). Interactions between Vitamin D Genetic Risk and Dietary Factors on Metabolic Disease-Related Outcomes in Ghanaian Adults. Nutrients, 14(13), 2763. https://doi.org/10.3390/nu14132763







