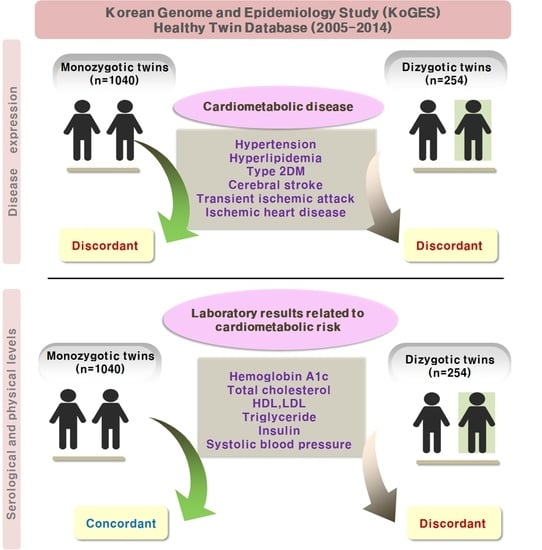
Comparison of the Concordance of Cardiometabolic Diseases and Physical and Laboratory Examination Findings between Monozygotic and Dizygotic Korean Adult Twins: A Cross-Sectional Study Using KoGES HTS Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
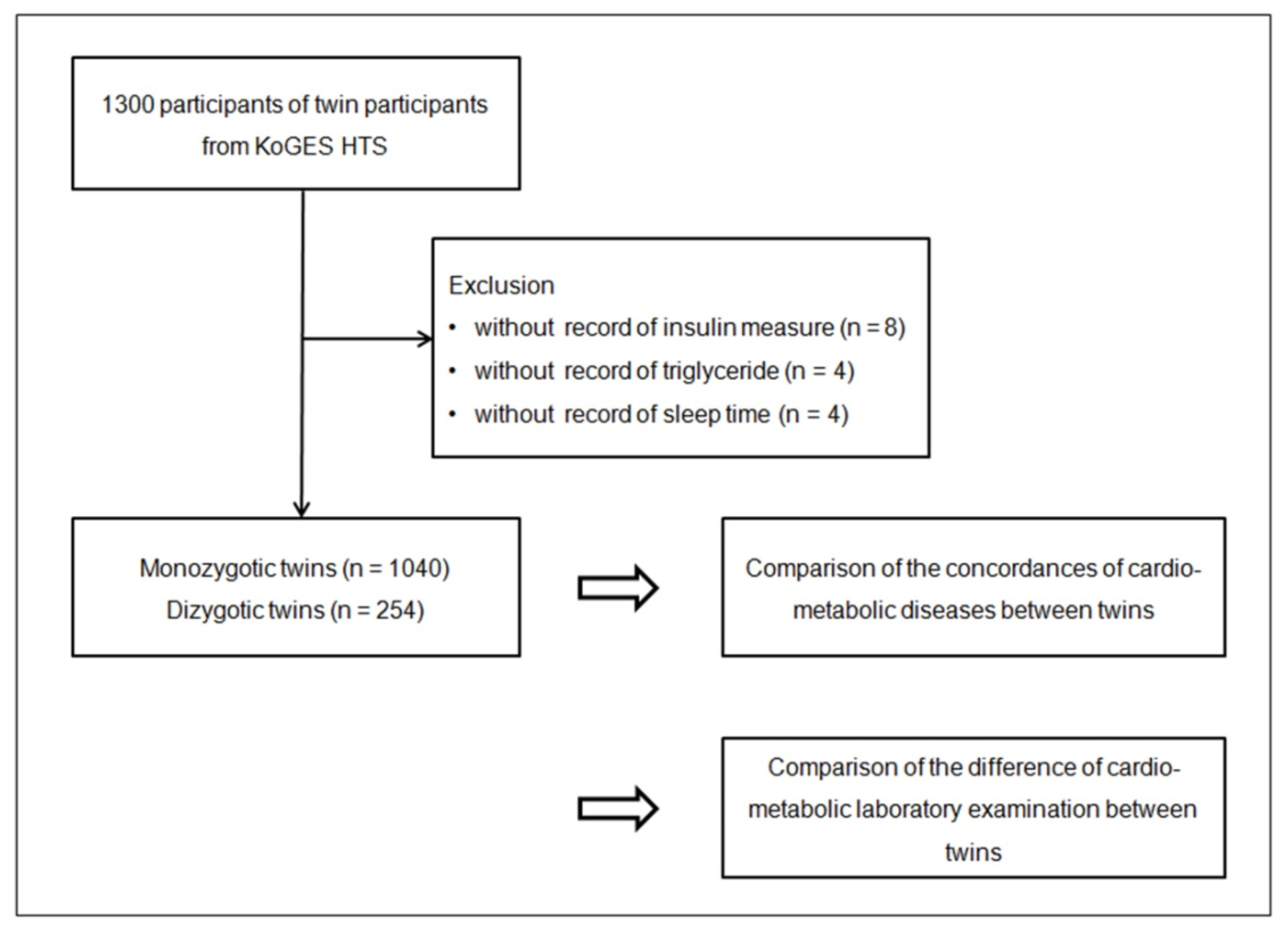
2.2. Participant Selection
2.3. Survey
2.4. Exposure
2.5. Outcome
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinclair, A.J.; Abdelhafiz, A.H. Cardiometabolic disease in the older person: Prediction and prevention for the generalist physician. Cardiovasc. Endocrinol. Metab. 2020, 9, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; VanHeest, J.L. Physical activity mediates a healthier body weight in the presence of obesity. Br. J. Sport. Med. 2000, 34, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Rastogi, S. Cardiometabolic diseases and related complications: Current status and future perspective. BioMed Res. Int. 2013, 2013, 467682. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Matsui, T. Pigment Epithelium-Derived Factor: A Novel Therapeutic Target for Cardiometabolic Diseases and Related Complications. Curr. Med. Chem. 2018, 25, 1480–1500. [Google Scholar] [CrossRef]
- Upadhyaya, B.; Larsen, T.; Barwari, S.; Louwagie, E.J.; Baack, M.L.; Dey, M. Prenatal Exposure to a Maternal High-Fat Diet Affects Histone Modification of Cardiometabolic Genes in Newborn Rats. Nutrients 2017, 9, 407. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study. Nutrients 2017, 9, 145. [Google Scholar] [CrossRef]
- Song, Y.M.; Sung, J.; Lee, K. Genetic and environmental influences on the associations between change in kidney function and changes in cardiometabolic factors in Koreans. Clin. Exp. Nephrol. 2017, 21, 474–480. [Google Scholar] [CrossRef]
- Scott, K.M. Depression, anxiety and incident cardiometabolic diseases. Curr. Opin. Psychiatry 2014, 27, 289–293. [Google Scholar] [CrossRef]
- An, S.; Ahn, C.; Jang, J.; Lee, J.; Kang, D.; Lee, J.K.; Park, S.K. Comparison of the Prevalence of Cardiometabolic Disorders and Comorbidities in Korea and the United States: Analysis of the National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2022, 37, e149. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Han, S.; Rane, P.P.; Fox, K.M.; Qian, Y.; Suh, H.S. Prevalence and incidence of atherosclerotic cardiovascular disease and its risk factors in Korea: A nationwide population-based study. BMC Public Health 2019, 19, 1112. [Google Scholar] [CrossRef]
- Lim, S.; Shin, H.; Song, J.H.; Kwak, S.H.; Kang, S.M.; Ji, W.Y.; Choi, S.H.; Cho, S.I.; Park, K.S.; Lee, H.K.; et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011, 34, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Konta, T.; Takasaki, S.; Abiko, H.; Ishikawa, M.; Takahashi, T.; Ikeda, A.; Ichikawa, K.; Kawata, S.; Kato, T.; et al. The association between microalbuminuria and metabolic syndrome in the general population in Japan: The Takahata study. Intern. Med. 2007, 46, 341–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeh, C.J.; Chang, H.Y.; Pan, W.H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: From NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 292–300. [Google Scholar]
- Xi, B.; He, D.; Hu, Y.; Zhou, D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: The China Health and Nutrition Survey in 2009. Prev. Med. 2013, 57, 867–871. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, S.M.J.; Lee, H.; Baek, J.; Bae, J.H.; Chung, W.J.; Kim, H.C. Korea Heart Disease Fact Sheet 2020: Analysis of Nationwide Data. Korean Circ. J. 2021, 51, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y.; Park, T. New Common and Rare Variants Influencing Metabolic Syndrome and Its Individual Components in a Korean Population. Sci. Rep. 2018, 8, 5701. [Google Scholar] [CrossRef] [PubMed]
- Amare, A.T.; Schubert, K.O.; Klingler-Hoffmann, M.; Cohen-Woods, S.; Baune, B.T. The genetic overlap between mood disorders and cardiometabolic diseases: A systematic review of genome wide and candidate gene studies. Transl. Psychiatry 2017, 7, e1007. [Google Scholar] [CrossRef] [PubMed]
- Marenberg, M.E.; Risch, N.; Berkman, L.F.; Floderus, B.; de Faire, U. Genetic susceptibility to death from coronary heart disease in a study of twins. N. Engl. J. Med. 1994, 330, 1041–1046. [Google Scholar] [CrossRef]
- Hawkes, C.H. Twin studies in diabetes mellitus. Diabet. Med. 1997, 14, 347–352. [Google Scholar] [CrossRef]
- Kaprio, J.; Tuomilehto, J.; Koskenvuo, M.; Romanov, K.; Reunanen, A.; Eriksson, J.; Stengard, J.; Kesaniemi, Y.A. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 1992, 35, 1060–1067. [Google Scholar] [CrossRef]
- Kyvik, K.O.; Green, A.; Beck-Nielsen, H. Concordance rates of insulin dependent diabetes mellitus: A population based study of young Danish twins. BMJ 1995, 311, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.M.; Cherkas, L.F.; Spector, T.D.; MacGregor, A.J. A common genetic factor underlies hypertension and other cardiovascular disorders. BMC Cardiovasc. Disord. 2004, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Pang, Z.; Jiang, W.; Wang, S.; Li, S.; von Bornemann Hjelmborg, J.; Tan, Q. Multivariate modeling of body mass index, pulse pressure, systolic and diastolic blood pressure in Chinese twins. Twin Res. Hum. Genet. 2015, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, H.; Busjahn, A.; Munter, S.; Nagy, Z.; Faulhaber, H.D.; Schuster, H.; Luft, F.C. Heritability analysis of lipids and three gene loci in twins link the macrophage scavenger receptor to HDL cholesterol concentrations. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.L.; Heller, R.F.; Roberts, D.C.; Allen, J.R.; Knapp, J.C.; Steele, P.L.; Silove, D. Twin study of genetic and environmental effects on lipid levels. Genet. Epidemiol. 1988, 5, 323–341. [Google Scholar] [CrossRef]
- Wijayatunga, N.N.; Dhurandhar, E.J. Normal weight obesity and unaddressed cardiometabolic health risk-a narrative review. Int. J. Obes. (Lond.) 2021, 45, 2141–2155. [Google Scholar] [CrossRef]
- Barsotti, S.; Saponaro, C.; Gaggini, M.; Talarico, R.; Bianchini, E.; Di Lascio, N.; Ferrari, C.; Buzzigoli, E.; Mosca, M.; Gastaldelli, A.; et al. Cardiometabolic risk and subclinical vascular damage assessment in idiopathic inflammatory myopathies: A challenge for the clinician. Clin. Exp. Rheumatol. 2019, 37, 1036–1043. [Google Scholar]
- Adriouch, S.; Lelong, H.; Kesse-Guyot, E.; Baudry, J.; Lampure, A.; Galan, P.; Hercberg, S.; Touvier, M.; Fezeu, L.K. Compliance with Nutritional and Lifestyle Recommendations in 13,000 Patients with a Cardiometabolic Disease from the Nutrinet-Sante Study. Nutrients 2017, 9, 546. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suner, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef]
- Jermendy, G.; Horvath, T.; Littvay, L.; Steinbach, R.; Jermendy, A.L.; Tarnoki, A.D.; Tarnoki, D.L.; Metneki, J.; Osztovits, J. Effect of genetic and environmental influences on cardiometabolic risk factors: A twin study. Cardiovasc. Diabetol. 2011, 10, 96. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Necheles, J.; Tsai, H.J.; Wang, G.; Wang, B.; Xing, H.; Li, Z.; Liu, X.; Zang, T.; et al. Genetic and environmental influences on serum lipid tracking: A population-based, longitudinal Chinese twin study. Pediatr. Res. 2010, 68, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Flossmann, E.; Schulz, U.G.; Rothwell, P.M. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke 2004, 35, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, A.P.; Bosma, H.; Gielen, M.; Lindsey, P.J.; Derom, C.; Vlietinck, R.; Loos, R.J.; Zeegers, M.P. Do genetic factors contribute to the relation between education and metabolic risk factors in young adults? A twin study. Eur. J. Public Health 2013, 23, 986–991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Cho, S.I.; Lee, K.; Ha, M.; Choi, E.Y.; Choi, J.S.; Kim, H.; Kim, J.; Hong, K.S.; Kim, Y.; et al. Healthy Twin: A twin-family study of Korea--protocols and current status. Twin Res. Hum. Genet. 2006, 9, 844–848. [Google Scholar] [CrossRef]
- Song, Y.M.; Lee, D.; Lee, M.K.; Lee, K.; Lee, H.J.; Hong, E.J.; Han, B.; Sung, J. Validity of the zygosity questionnaire and characteristics of zygosity-misdiagnosed twin pairs in the Healthy Twin Study of Korea. Twin Res. Hum. Genet. 2010, 13, 223–230. [Google Scholar] [CrossRef]
- Hayakawa, K.; Shimizu, T. Blood pressure discordance and lifestyle: Japanese identical twins reared apart and together. Acta Genet. Med. Gemellol. 1987, 36, 485–491. [Google Scholar] [CrossRef]
- Newman, B.; Selby, J.V.; King, M.C.; Slemenda, C.; Fabsitz, R.; Friedman, G.D. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia 1987, 30, 763–768. [Google Scholar] [CrossRef]
- Kaminsky, Z.A.; Tang, T.; Wang, S.C.; Ptak, C.; Oh, G.H.; Wong, A.H.; Feldcamp, L.A.; Virtanen, C.; Halfvarson, J.; Tysk, C.; et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat. Genet. 2009, 41, 240–245. [Google Scholar] [CrossRef]
- Vogt, G.; Huber, M.; Thiemann, M.; van den Boogaart, G.; Schmitz, O.J.; Schubart, C.D. Production of different phenotypes from the same genotype in the same environment by developmental variation. J. Exp. Biol. 2008, 211, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Rukh, G.; Varga, T.V.; Ali, A.; Kurbasic, A.; Shungin, D.; Ericson, U.; Koivula, R.W.; Chu, A.Y.; Rose, L.M.; et al. Gene x physical activity interactions in obesity: Combined analysis of 111,421 individuals of European ancestry. PLoS Genet. 2013, 9, e1003607. [Google Scholar] [CrossRef] [PubMed]
- Rooks, C.; Faber, T.; Votaw, J.; Veledar, E.; Goldberg, J.; Raggi, P.; Quyyumi, A.A.; Bremner, J.D.; Vaccarino, V. Effects of smoking on coronary microcirculatory function: A twin study. Atherosclerosis 2011, 215, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.J.; Lichtenstein, A.H.; Pittas, A.G.; Roberts, S.B.; Fuss, P.J.; Greenberg, A.S.; McCrory, M.A.; Bouchard, T.J., Jr.; Saltzman, E.; Neale, M.C. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid. Res. 2009, 50, 1917–1926. [Google Scholar] [CrossRef]
- Tambs, K.; Eaves, L.J.; Moum, T.; Holmen, J.; Neale, M.C.; Naess, S.; Lund-Larsen, P.G. Age-specific genetic effects for blood pressure. Hypertension 1993, 22, 789–795. [Google Scholar] [CrossRef]
| Characteristics | Total Participants | ||
|---|---|---|---|
| Monozygotic Twin | Dizygotic Twin | p | |
| Age (years old, n, %) | 0.004 * | ||
| 20–24 | 6 (0.6) | 0 (0.0) | |
| 25–29 | 68 (6.5) | 4 (1.6) | |
| 30–34 | 352 (33.8) | 87 (35.7) | |
| 35–39 | 244 (23.5) | 65 (26.6) | |
| 40–44 | 139 (13.4) | 36 (14.8) | |
| 45–49 | 131 (12.6) | 20 (8.2) | |
| 50–54 | 80 (7.7) | 22 (9.0) | |
| 55–59 | 14 (1.3) | 10 (4.1) | |
| 60–64 | 4 (0.4) | 0 (0.0) | |
| 65+ | 2 (0.2) | 0 (0.0) | |
| Sex (n, %) | 0.024 * | ||
| Males | 386 (37.1) | 110 (45.1) | |
| Females | 654 (62.9) | 134 (54.9) | |
| Income (n, %) | 0.983 | ||
| <2 million (won) | 346 (33.3) | 81 (33.2) | |
| 2 to <3 million (won) | 282 (27.1) | 68 (27.9) | |
| 3 to <4 million (won) | 209 (20.1) | 50 (20.5) | |
| ≥4 million (won) | 203 (19.5) | 45 (18.4) | |
| Education (n, %) | 0.743 | ||
| Under high school | 121 (11.6) | 25 (10.2) | |
| Graduated from High school | 366 (35.2) | 92 (37.7) | |
| Commercial college-Dropped out of college | 123 (11.8) | 32 (13.1) | |
| Graduated from college | 430 (41.3) | 95 (38.9) | |
| Marriage (n, %) | 0.26 | ||
| Unmarried | 240 (23.1) | 50 (20.5) | |
| Married | 733 (70.5) | 173 (70.9) | |
| Divorced or others | 67 (6.4) | 21 (8.6) | |
| Physical Activity | |||
| Hard (hour/1 week, mean, SD) | 3.1 (6.8) | 4.7 (9.7) | 0.015 * |
| Moderate (hour/1 week, mean, SD) | 5.9 (10.5) | 6.2 (10.2) | 0.651 |
| Walk (hour/1 week, mean, SD) | 6.2 (9.6) | 6.8 (10.9) | 0.339 |
| Sit (hour/1 week, mean, SD) | 39.9 (21.9) | 37.9 (20.7) | 0.190 |
| Obesity (n, %) | 0.241 | ||
| Underweight (BMI < 18.5) | 27 (2.6) | 5 (2) | |
| Normal (BMI ≥ 18.5 to <23) | 499 (48) | 113 (46.3) | |
| Overweight (BMI 23 to <25) | 220 (21.2) | 68 (27.9) | |
| Obese I (BMI ≥ 25 to <30) | 262 (25.2) | 52 (21.3) | |
| Obese II (BMI ≥ 30) | 32 (3.1) | 6 (2.5) | |
| Smoking status (n, %) | 0.180 | ||
| Nonsmoker | 680 (65.4) | 145 (59.4) | |
| Past smoker | 108 (10.4) | 33 (13.5) | |
| Current smoker | 252 (24.2) | 66 (27) | |
| Frequency of drinking alcohol (n, %) | 0.249 | ||
| Nondrinker | 301 (28.9) | 64 (26.2) | |
| ≤1 time monthly | 230 (22.1) | 46 (18.9) | |
| 2–4 times monthly | 300 (28.8) | 80 (32.8) | |
| ≥2 times weekly | 209 (20.1) | 54 (22.1) | |
| Sleeping hours (n, %) | 0.370 | ||
| ≤5 h | 53 (5.1) | 16 (6.6) | |
| 6–7 h | 610 (58.7) | 146 (59.8) | |
| 8–9 h | 349 (33.6) | 72 (29.5) | |
| ≥10 h | 28 (2.7) | 10 (4.1) | |
| Cardio-metabolic diseases (categorical) | |||
| Hypertension (n, %) | 95 (9.1) | 22 (9.0) | 1.000 |
| Hyperlipidemia (n, %) | 75 (7.2) | 14 (5.7) | 0.485 |
| Type 2 diabetes mellitus (n, %) | 37 (3.6) | 7 (2.9) | 0.699 |
| Cerebral stroke (n, %) | 6 (0.6) | 1 (0.4) | 1.000 |
| Transient Ischemic Attack (n, %) | 1 (0.1) | 1 (0.4) | 0.344 |
| Ischemic heart disease (n, %) | 15 (1.4) | 4 (1.6) | 1.000 |
| Physical and laboratory examination (continuous) | |||
| Hemoglobin A1c (g/dL, mean, SD) | 13.9 (1.7) | 14 (1.7) | 0.493 |
| Total cholesterol (mg/dL, mean, SD) | 188.1 (33.9) | 184.8 (32.9) | 0.175 |
| HDL (mg/dL, mean, SD) | 51.9 (12.6) | 51.4 (11.9) | 0.523 |
| LDL (mg/dL, mean, SD) | 113.5 (30.5) | 111.3 (30.2) | 0.294 |
| Triglyceride (mg/dL, mean, SD) | 114.9 (78.4) | 113.9 (82) | 0.867 |
| Insulin (uIU/mL, mean, SD) | 7.5 (3.3) | 7.5 (3.9) | 0.994 |
| Fasting blood glucose (mg/dL, mean, SD) | 91.7 (18.8) | 90.7 (12.8) | 0.469 |
| Systolic Blood Pressure (mmHg, mean, SD) | 111.0 (15.6) | 112.4 (14.6) | 0.205 |
| Diastolic Blood Pressure (mmHg, mean, SD) | 72.0 (11.5) | 71.8 (10.9) | 0.747 |
| Coincidence of Diseases | Monozygotic Twin | Dizygotic Twin | Odds Ratios (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Crude | p | Model 1 † | p | Model 2 ‡ | p | |
| Hypertension | ||||||||
| concordant | 934/1040 (89.8) | 208/244 (85.2) | 1.53 (1.02–2.29) | 0.042 * | 1.33 (0.84–2.11) | 0.229 | 1.42 (0.88–2.29) | 0.155 |
| discordant | 106/1040 (10.2) | 36/244 (14.8) | 1 | 1 | 1 | |||
| Hyperlipidemia | ||||||||
| concordant | 956/1040 (91.9) | 216/244 (88.5) | 1.48 (0.94–2.32) | 0.092 | 1.52 (0.92–2.52) | 0.103 | 1.55 (0.93–2.59) | 0.097 |
| discordant | 84/1040 (8.1) | 28/244 (11.5) | 1 | 1 | 1 | |||
| Type 2 diabetes | ||||||||
| concordant | 1002/1040 (96.3) | 230/244 (94.3) | 1.61 (0.86–3.01) | 0.141 | 1.66 (0.82–3.39) | 0.162 | 1.63 (0.79–3.36) | 0.183 |
| discordant | 38/1040 (3.7) | 14/244 (5.7) | 1 | 1 | 1 | |||
| Cerebral stroke | ||||||||
| concordant | 1028/1040 (98.8) | 242/244 (99.2) | 0.71(0.16–3.18) | 0.653 | 0.84 (0.13–5.41) | 0.852 | 0.60 (0.06–6.11) | 0.667 |
| discordant | 12/1040 (1.2) | 2/244 (0.8) | 1 | 1 | 1 | |||
| Transient ischemic attack | ||||||||
| concordant | 1038/1040 (99.8) | 242/244 (99.2) | 4.29 (0.60–30.60) | 0.146 | N/A | 0.977 | N/A | 0.988 |
| discordant | 2/1040 (0.2) | 2/244 (0.8) | 1 | 1 | 1 | |||
| Ischemic heart disease | ||||||||
| concordant | 1014/1040 (97.5) | 240/244 (98.4) | 0.65 (0.23–1.80) | 0.427 | 0.63 (0.19–2.04) | 0.438 | 0.75 (0.22–2.52) | 0.639 |
| discordant | 26/1040 (2.5) | 4/244 (1.6) | 1 | 1 | 1 | |||
| Coincidence of Diseases | Monozygotic Twin | Dizygotic Twin | Odds Ratios (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Crude | p | Model 1 † | p | Model 2 ‡ | p | |
| Hypertension | ||||||||
| Positive-positive | 42/1040 (4) | 4/244 (1.6) | 2.40 (0.85–6.77) | 0.098 | 3.61 (1.16–11.21) | 0.026 * | 2.13 (0.83–5.45) | 0.114 |
| Positive-negative | 106/1040 (10.2) | 36/244 (14.8) | 0.673 (0.45–1.01) | 0.057 | 0.76 (0.48–1.21) | 0.249 | 0.72 (0.45–1.16) | 0.180 |
| Negative-negative | 892/1040 (85.8) | 204/244 (83.6) | 1 | 1 | 1 | |||
| Hyperlipidemia | ||||||||
| Positive-positive | 32/1040 (3.1) | 0/244 (0) | N/A | N/A | N/A | N/A | N/A | N/A |
| Positive-negative | 84/1040 (8.1) | 28/244 (11.5) | 0.70 (0.45–1.10) | 0.124 | 0.71 (0.43–1.15) | 0.164 | 0.69 (0.43–1.10) | 0.121 |
| Negative-negative | 924/1040 (88.8) | 216/244 (88.5) | 1 | 1 | 1 | |||
| Type 2 diabetes | ||||||||
| Positive-positive | 18/1040 (1.7) | 0/244 (0) | N/A | N/A | N/A | N/A | N/A | N/A |
| Positive-negative | 38/1040 (3.7) | 14/244 (5.7) | 0.63 (0.34–1.19) | 0.156 | 0.66 (0.33–1.30) | 0.228 | 0.65 (0.33–1.30) | 0.223 |
| Negative-negative | 984/1040 (94.6) | 230/244 (94.3) | 1 | 1 | 1 | |||
| Cerebral stroke | ||||||||
| Positive-positive | 0/1040 (0) | 0/244 (0) | N/A | N/A | N/A | N/A | N/A | N/A |
| Positive-negative | 12/1040 (1.2) | 2/244 (0.8) | 1.41 (0.31–6.35) | 0.653 | 1.59 (0.33–7.75) | 0.567 | 1.40 (0.28–7.04) | 0.680 |
| Negative-negative | 1028/1040 (98.8) | 242/244 (99.2) | 1 | 1 | 1 | |||
| Transient ischemic attack | ||||||||
| Positive-positive | 0/1040 (0) | 0/244 (0) | N/A | N/A | N/A | N/A | N/A | N/A |
| Positive-negative | 2/1040 (0.2) | 2/244 (0.8) | 0.23 (0.03–1.66) | 0.146 | 0.31 (0.04–2.60) | 0.278 | 0.04 (0.00–2.32) | 0.135 |
| Negative-negative | 1038/1040 (99.8) | 242/244 (99.2) | 1 | 1 | 1 | |||
| Ischemic heart diseases | ||||||||
| Positive-positive | 2/1040 (0.2) | 2/244 (0.8) | 0.24 (0.03–1.68) | 0.149 | 0.01 (0.00–1.50) | 0.074 | 0.62 (0.05–7.70) | 0.710 |
| Positive-negative | 26/1040 (2.5) | 4/244 (1.6) | 1.53 (0.53–4.42) | 0.434 | 1.60 (0.53–4.84) | 0.410 | 1.15 (0.38–3.48) | 0.809 |
| Negative-negative | 1012/1040 (97.3) | 238/244 (97.5) | 1 | 1 | 1 | |||
| Differences in Clinical Examination | Estimated Values of the Absolute Difference between Twins (95% CI) | |||||
|---|---|---|---|---|---|---|
| Crude | p | Model 1 †‡ | p | Model 2 ‡ | p | |
| Differences in Hemoglobin A1c | 0.12 (0.01–0.23) | 0.030 * | 0.14 (0.03–0.25) | 0.011 * | 0.14 (0.03–0.26) | 0.011 * |
| Differences in Total Cholesterol | 7.45 (4.49–10.42) | <0.001 * | 7.41 (4.45–10.37) | <0.001 * | 7.73 (4.82–10.65) | <0.001 * |
| Differences in HDL-Cholesterol | 3.44 (2.50–4.37) | <0.001 * | 3.36 (2.42–4.29) | <0.001 * | 3.38 (2.44–4.32) | <0.001 * |
| Differences in LDL-Cholesterol | 5.50 (2.80–8.20) | <0.001 * | 5.42 (2.73–8.11) | <0.001 * | 5.68 (3.04–8.32) | <0.001 * |
| Differences in Triglyceride | 10.38 (1.80–18.95) | 0.018 * | 9.13 (0.94–17.31) | 0.029 * | 9.84 (1.67–18.02) | 0.018 * |
| Differences in Insulin | 0.91 (0.50–1.31) | <0.001 * | 0.96 (0.55–1.36) | <0.001 * | 0.97 (0.56–1.37) | <0.001 * |
| Differences in Glucose | 0.55 (−1.54–2.65) | 0.604 | 0.14 (−1.94–2.21) | 0.897 | 0.33 (−1.70–2.37) | 0.749 |
| Differences in SBP | 2.40 (1.03–3.77) | 0.001 * | 2.39 (1.01–3.77) | 0.001 * | 2.39 (1.05–3.72) | <0.001 * |
| Differences in DBP | 0.83 (−0.24–1.90) | 0.130 | 0.65 (−0.43–1.73) | 0.241 | 0.90 (−0.17–1.96) | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Kim, S.Y.; Choi, H.G.; Lim, H.; Kim, J.-H.; Kim, J.H.; Cho, S.-J.; Nam, E.S.; Min, K.-W.; Park, H.Y.; et al. Comparison of the Concordance of Cardiometabolic Diseases and Physical and Laboratory Examination Findings between Monozygotic and Dizygotic Korean Adult Twins: A Cross-Sectional Study Using KoGES HTS Data. Nutrients 2022, 14, 4834. https://doi.org/10.3390/nu14224834
Kang HS, Kim SY, Choi HG, Lim H, Kim J-H, Kim JH, Cho S-J, Nam ES, Min K-W, Park HY, et al. Comparison of the Concordance of Cardiometabolic Diseases and Physical and Laboratory Examination Findings between Monozygotic and Dizygotic Korean Adult Twins: A Cross-Sectional Study Using KoGES HTS Data. Nutrients. 2022; 14(22):4834. https://doi.org/10.3390/nu14224834
Chicago/Turabian StyleKang, Ho Suk, So Young Kim, Hyo Geun Choi, Hyun Lim, Joo-Hee Kim, Ji Hee Kim, Seong-Jin Cho, Eun Sook Nam, Kyueng-Whan Min, Ha Young Park, and et al. 2022. "Comparison of the Concordance of Cardiometabolic Diseases and Physical and Laboratory Examination Findings between Monozygotic and Dizygotic Korean Adult Twins: A Cross-Sectional Study Using KoGES HTS Data" Nutrients 14, no. 22: 4834. https://doi.org/10.3390/nu14224834
APA StyleKang, H. S., Kim, S. Y., Choi, H. G., Lim, H., Kim, J.-H., Kim, J. H., Cho, S.-J., Nam, E. S., Min, K.-W., Park, H. Y., Kim, N. Y., Choi, Y., & Kwon, M. J. (2022). Comparison of the Concordance of Cardiometabolic Diseases and Physical and Laboratory Examination Findings between Monozygotic and Dizygotic Korean Adult Twins: A Cross-Sectional Study Using KoGES HTS Data. Nutrients, 14(22), 4834. https://doi.org/10.3390/nu14224834