Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
- Parent modelling role is more limited: caregivers can continue to eat separately and in different ways;
- The child is also allowed to eat outside family mealtimes, as the request/response element is missing;
- The assumption is that the child is “offered” a food that he or she can handle and does not “request” it as a result of imitating other family members at mealtimes;
- The risk does exist to allow for feeding the child with a poor/unbalanced diet characterized by a lack of essential nutrients, because in the early stages, not all foods rich in these nutrients (e.g., meat and fish) can be easily manipulated or eaten by the child.
1.1. Why This Systematic Review Is Important
1.2. Objectives
1.3. Key Questions
- -
- Can the BLW/BLISS method during CF influence, either positively or negatively, infant weight–length gain?
- -
- Can the BLW/BLISS method during CF influence, either positively or negatively, the development of overweight/obesity?
- -
- Can RF during the CF period (responsive complementary feeding—RCF) influence, either positively or negatively, physical growth?
- -
- Can non-responsive feeding during the CF period (non-responsive complementary feeding—NRCF) influence, either positively or negatively, physical growth?
- -
- Can RCF influence the development of overweight and obesity?
- -
- Can NRCF influence the development of overweight and obesity?
- -
- Do the different caregivers’ feeding practices (CFPs) during the CF period result in different risks of choking?
- -
- Can RCF influence the development of type 2 diabetes mellitus (DM2)?
- -
- Can TCF influence the development of DM2?
- -
- Can RCF influence the development of hypertension?
- -
- Can TCF influence the development of hypertension?
- -
- Can RCF influence the development of dental caries?
- -
- Can TCF influence the development of dental caries?
2. Materials and Methods
2.1. Design of the Studies Included
- -
- Randomized controlled trials (RCTs) and controlled trials (CTs) in which the effect of the caregivers’ feeding styles could be accurately assessed as an experimental intervention;
- -
- Observational studies (cohort studies, longitudinal studies, case-control studies, and cross-sectional studies) in which this effect could be evaluated as an exposure factor while taking into account possible confounding factors.
2.2. Population
2.3. Intervention(s), Exposure(s)
2.4. Comparator(s)/Control
2.5. Inclusion Criteria
- -
- Be addressed to mothers or to the whole family;
- -
- Start before the birth of the child and then continue in the first months or years of life of the child, or start after the birth and continue in the first months or years of life of the child;
- -
- Be associated or not associated with any recommendations on the intake of specific foods rich in macro- and micro-nutrients.
- -
- The presence of objective outcome indicators (measurement of weight (W), length/height (L/H), head circumference (HC), etc.), with the exclusion of studies in which the outcome was made up only of behavioral or psychological indices of the individual elements of the mother/child dyad, or of the dyad itself;
- -
- a ≥ 6 month follow-up at least introducing CF.
2.6. Exclusion Criteria
2.7. Main Outcomes
- -
- General growth parameters assessed in prospective differential terms (different increase in W or L over time) or assessed at a specific time point (with differing frequencies of weights and lengths in the populations being compared: W, L, W/L z-score ratio, BMI, BMI z-score (BMIz);
- -
- Risk of NCDs (overweight/obesity, DM2, and hypertension);
- -
- Risk of choking;
- -
- Risk of dental caries.
2.8. Keywords and Search Strategy
2.9. Measures of Effect
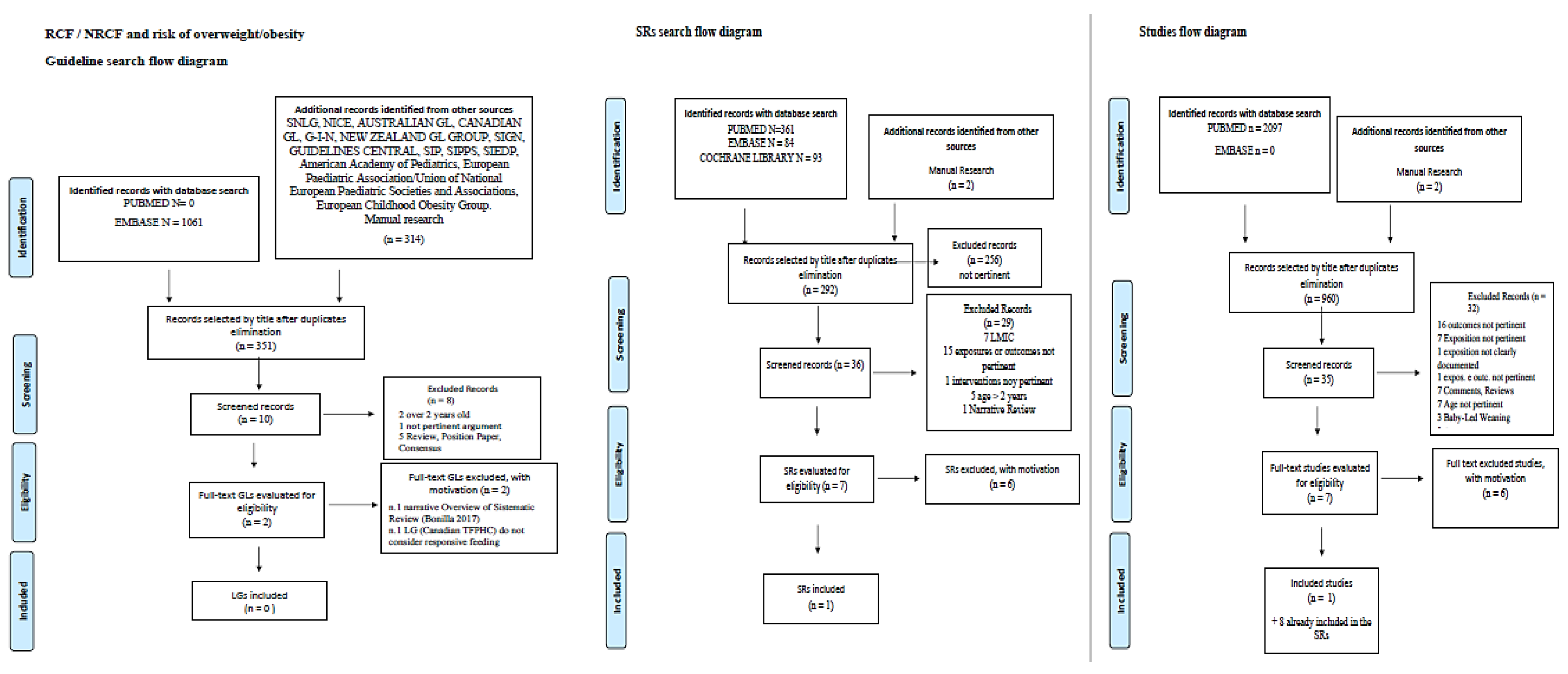
2.10. Studies Selection
2.11. Assessment of Heterogeneity
2.12. Strategy for Data Synthesis
2.13. Publication Bias Assessment
2.14. Software
3. Results
3.1. Can the BLW/BLISS Method during CF Influence, Either Positively or Negatively, Infant Weight–Length Gain?
- -
- Recruitment of the mothers who intended to adopt BLW on a voluntary basis;
- -
- Uncertainty of weight measurement by parents, with unspecified frequency;
- -
- Significant drop-out rate during the period of observation;
- -
- Non-homogeneous intervention and control groups in terms of subject age.
3.2. Can the BLW/BLISS Method during CF Influence, Either Positively or Negatively, the Development of Overweight/Obesity?
3.3. Can RF during the CF Period (Responsive Complementary Feeding—RCF) Influence, Either Positively or Negatively, Physical Growth?
- -
- Can non-responsive feeding during the CF period (non-responsive complementary feeding—NRCF) influence, either positively or negatively, physical growth?
3.4. Can RCF Influence the Development of Overweight and Obesity?
3.5. Do the Different Caregivers’ Feeding Practices (CFPs), BLW, BLISS, RCF, and NRCF, during the CF Period Result in Different Risks of Choking?
3.6. Can RCF Influence the Development of DM2?
- -
- Can TCF influence the development of DM2?
3.7. Can RCF Influence the Development of Hypertension?
3.8. Can RCF Influence the Development of Dental Caries?
- -
- Can TCF influence the development of dental caries?
4. Discussion
4.1. Weight–Length Gain and Development of Overweight and Obesity
4.1.1. BLW/BLISS
4.1.2. RCF/NRCF
- -
- In many studies, the interventions planned started from the first semester of life and were not strictly coinciding with the beginning of CF, thus making it impossible to establish a specific correlation between CFP and the precise time period of CF;
- -
- The RCF instructions given to the caregivers in the intervention groups were part of multi-component interventions with general instructions on childcare; on the contrary, the children in the control groups were given the usual standard of care, based on some good practices that, even if in a non-pre-established way, could contain CFPs similar to those recommended in the intervention groups.
- -
- The monitoring of caregivers’ compliance and consistency in implementing the instructions received was not always ensured and checked [6].
- -
- In very few cases was the same exposure studied during the CF period and in more than one cohort;
- -
- The outcome indicators selected and the timing of the measurements varied a lot within the individual studies;
- -
- Finally, the results for the same indicator were in some cases conflicting or significant in one study and not significant in another.
4.2. CFPs and Risk of Choking
4.3. Risk of DM2, Hypertension, and Dental Caries
5. Quality of Evidence
- -
- BLW or BLISS studies—outcome indicators (e.g., W and L/H) often self-reported, high loss to follow-up, analysis by protocol only, inconsistent results, and single study on some questions;
- -
- RCF studies—uncertainty about the intervention (usually part of a multi-component intervention), borderline loss to follow-up, and single study on some questions;
- -
- For the same reasons, the methodological assessment of the observational studies, which with the GRADE method started at a low level, was also downgraded (very low quality).
6. Agreements and Disagreements with Other Studies or Reviews
7. Limitations of the SR and Potential Bias in the Review Process
8. Implications for Research
- -
- A design including the most important confounding factors, first of all, the diverse eating styles of the families; this will allow the reliability of the results obtained, as well as their ampler transferability;
- -
- A clear definition of the interventions (in terms of CFPs), which should be limited to the sole period of CF (i.e., 6 months to 2 years of age) and be carefully monitored over time to ensure their real and continuous application;
- -
- A meticulous prospective and retrospective documentation of the CFPs, which infants have been exposed to, in case of observational studies;
- -
- Strict criteria to define which categories of infants and families can be enrolled as control groups; this will avoid similar expositions in subjects pertaining to different groups, as well as differences that might influence the results (e.g., different percentages of breastfed infants between the intervention and control groups);
- -
- An appropriate follow-up period of time, possibly of at least three years or more, to collect data on pre-defined outcomes;
- -
- The most limited drop-off possible, even in observational studies;
- -
- A uniform instrumental documentation of specific outcomes, namely the anthropometric ones, that should be collected by qualified healthcare professionals.
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, J.O.; Birch, L.L. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am. J. Clin. Nutr. 1999, 69, 1264–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashdan, E. A sensitive period for learning about food. Hum. Nat. 1994, 5, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Lipsitt, L.P.; Crook, C.; Booth, C.A. The transitional infant: Behavioral development and feeding. Am. J. Clin. Nutr. 1985, 41, 485–496. [Google Scholar] [CrossRef] [PubMed]
- DiSantis, K.I.; Hodges, E.A.; Johnson, S.L.; Fisher, J.O. The role of responsive feeding in overweight during infancy and toddlerhood: A systematic review. Int. J. Obes. 2011, 35, 480–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matvienko-Sikar, K.; Toomey, E.; Delaney, L.; Harrington, J.; Byrne, M.; Kearney, P.M.; Choosing Healthy Eating for Infant Health (CHErIsH) study team. Effects of healthcare professional delivered early feeding interventions on feeding practices and dietary intake: A systematic review. Appetite 2018, 123, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Spill, M.K.; Callahan, E.H.; Shapiro, M.J.; Spahn, J.M.; Wong, Y.P.; Benjamin-Neelon, S.E.; Birch, L.; Black, M.M.; Cook, J.T.; Faith, M.S.; et al. Caregivers’ feeding practices and child weight outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. S7), 990S–1002S. [Google Scholar] [CrossRef]
- Rapley, G.; Murkett, T. Baby-Led Weaning; Vermilion: London, UK, 2008. [Google Scholar]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domello, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; Heath, L.M.; Taylor, R.W. How feasible is Baby Led Weaning as an approach to Infant feeding? A Review of the evidence. Nutrients 2012, 2, 1575–1609. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, E.; Bergamini, M.; Staiano, A.; Banderali, G.; Pendezza, E.; Penagini, F.; Zuccotti, G.V.; Peroni, D.G. Italian Society of Pediatrics. Baby-led weaning: What a systematic review of the literature adds on. Ital. J. Pediatr. 2018, 44, 49. [Google Scholar] [CrossRef] [Green Version]
- Birch, L.L.; Davison, K.K. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr. Clin. N. Am. 2001, 48, 893–907. [Google Scholar] [CrossRef]
- Townsend, E.; Pitchford, N.J. Baby knows best? The impact of weaning style on food preferences and body mass index in early childhood in a case-controlled sample. BMJ Open 2012, 2, e000298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.A.; Mallan, K.M.; Battistutta, D.; Nicholson, J.M.; Perry, R.; Magarey, A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: Outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int. J. Obes. 2012, 36, 1292–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.A.; Mallan, K.M.; Nicholson, J.M.; Battistutta, D.; Magarey, A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics 2013, 132, e109–e118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.A.; Mallan, K.M.; Nicholson, J.M.; Thorpe, K.; Nambiar, S.; Mauch, C.E.; Magarey, A. An early feeding practices intervention for obesity prevention. Pediatrics 2015, 136, e40–e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.; Health, A.-L.M.; Williams, S.M.; Cameron, S.L.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Gibson, R.S.; Taylor, R.W. Baby-Led Introduction to Solids (BLISS) study: A randomised controlled trial of a baby-led approach to complementary feeding. BMC Pediatr. 2015, 15, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Complementary Feeding. Report of Global Consultation, Geneva, 10–13 December 2011. Summary of Guiding Principles. Available online: https://www.who.int/nutrition/publications/ComplementaryFeeding.pdf (accessed on 15 March 2022).
- American Academy of Pediatrics (AAP). Is Your Baby Hungry or Full? Responsive Feeding Explained. Available online: https://www.healthychildren.org/English/ages-stages/baby/feeding-nutrition/Pages/Is-Your-Baby-Hungry-or-Full-Responsive-Feeding-Explained.aspx (accessed on 15 March 2022).
- Adair, L. How could complementary feeding patterns affect the susceptibility to NCD later in life? Nutr. Metab. Cardiovasc. Dis. 2012, 22, 765–769. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Brown, A.; Lee, D.W. Early influences on child satiety-responsiveness: The role of weaning style. Pediatr. Obes. 2015, 10, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Prospero—International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/n°CRD42021259486 (accessed on 15 March 2022).
- Brouwers, M.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing guideline development, reporting, and evaluation in healthcare. Can. Med. Assoc. J. 2010, 182, E839–E842. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3001530/ (accessed on 15 March 2022). [CrossRef] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions [Updated March 2011]; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 March 2022).
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Falck-Ytter, Y.; Vist, G.E.; Liberati, A.; Schünemann, H.J. GRADE Working Group. Going from evidence to recommendations. BMJ 2008, 336, 1049–1051. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Jaeschke, R.; Helfand, M.; Liberati, A.; Vist, G.E.; Schünemann, H.J. GRADE working group. Incorporating considerations of resource use into grading recommendations. BMJ 2008, 336, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Caroli, M.; Vania, A.; Verga, M.C.; Di Mauro, G.; Bergamini, M.; Cuomo, B.; D’Anna, R.; D’Antonio, G.; Dello Iacono, I.; Dessì, A.; et al. Recommendations on Complementary Feeding as a Tool for Prevention of Non-Communicable Diseases (NCDs)—Paper Co-Drafted by the SIPPS, FIMP, SIDOHaD, and SINUPE Joint Working Group. Nutrients 2022, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane, 2021; Available online: www.training.cochrane.org/handbook (accessed on 27 December 2021).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919e. [Google Scholar] [CrossRef] [Green Version]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3; Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- McMaster University (Developed by Evidence Prime). GRADEpro GDT. Hamilton (ON, Can): McMaster University (Developed by Evidence Prime). Available online: https://gradepro.org/ (accessed on 2 June 2019).
- Martinón-Torres, N.; Carreira, N.; Picáns-Leis, R.; Pérez-Ferreirós, A.; Kalén, A.; Leis, R. Baby-Led Weaning: What Role Does It Play in Obesity Risk during the First Years? A Systematic Review. Nutrients 2021, 13, 1009. [Google Scholar] [CrossRef]
- Kahraman, A.; Gümüs, M.; Yaz, S.B.; Basbakkal, Z. Baby-led weaning versus traditional weaning: The assessment of nutritional status in early childhood and maternal feeding practices in Turkey. Early Child Dev. Care 2020, 190, 615–624. [Google Scholar] [CrossRef]
- Taylor, R.W.; Williams, S.M.; Fangupo, L.J.; Wheeler, B.J.; Taylor, B.J.; Daniels, L.; Flemig, E.A.; McArthur, J.; Morison, B.; Erickson, L.W.; et al. Effect of a baby-led approach to complementary feeding on infant growth and overweight: A randomized clinical trial. JAMA Pediatr. 2017, 171, 838–846. [Google Scholar] [CrossRef]
- Dogan, E.; Yilmaz, G.; Caylan, N.; Turgut, M.; Gokcay, G.; Oguz, M.M. Baby-led complementary feeding: Randomized controlled study. Pediatr. Int. 2018, 60, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, S.; Wall, C. How We Eat—Reviews of the Evidence on Food and Eating Behaviours Related to Diet and Body Size. A Report Commissioned by the Ministry of Health. Available online: https://www.health.govt.nz/publication/how-we-eat-reviews-evidence-food-and-eating-behaviours-related-diet-and-body-size (accessed on 16 February 2022).
- Savage, J.S.; Birch, L.L.; Marini, M.; Anzman-Frasca, S.; Paul, I.M. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: A randomized clinical trial. JAMA Pediatr. 2016, 170, 742–749. [Google Scholar] [CrossRef]
- Wright, C.M.; Parkinson, K.N.; Drewett, R.F. How does maternal and child feeding behavior relate to weight gain and failure to thrive? Data from a prospective birth cohort. Pediatrics 2006, 117, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; McNiven, S.; Vosti, S.A.; Kaiser, L.L. Sweetened food purchases and indulgent feeding are associated with increased toddler anthropometry. J. Nutr. Educ. Behav. 2014, 46, 293–298. [Google Scholar] [CrossRef]
- Dinkevich, E.; Leid, L.; Pryor, K.; Wey, Y.; Huberman, H.; Carnell, S. Mothers’ feeding behaviors in infancy: Do they predict child weight trajectories? Obesity 2015, 23, 2470–2476. [Google Scholar] [CrossRef] [Green Version]
- Stifter, C.A.; Moding, K.J. Understanding and measuring parent use of food to soothe infant and toddler distress: A longitudinal study from 6 to 18 months of age. Appetite 2015, 95, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hittner, J.B.; Johnson, C.; Tripicchio, G.; Faith, M.S. Infant emotional distress, maternal restriction at a home meal, and child BMI gain through age 6 years in the Colorado Adoption Project. Eat. Behav. 2016, 21, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.M.; Savage, J.S.; Anzman-Frasca, S.; Marini, M.E.; Beiler, J.S.; Hess, L.B.; Loken, E.; Birch, L.L. Effect of a Responsive Parenting Educational Intervention on Childhood Weight Outcomes at 3 Years of Age: The INSIGHT Randomized Clinical Trial. JAMA 2018, 320, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redsell, S.A.; Edmonds, B.; Swift, J.A.; Siriwardena, A.N.; Weng, S.; Nathan, D.; Galzebook, C. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern. Child. Nutr. 2016, 12, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, I.M.; Savage, J.S.; Anzman, S.L.; Beiler, J.S.; Marini, M.E.; Stokes, J.L.; Birch, L.L. Preventing obesity during infancy: A pilot study. Obesity 2011, 19, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Machuca, H.; Arevalo, S.; Hackley, B.; Applebaum, J.; Mishkin, A.; Heo, M.; Shapiro, A. Well Baby Group Care: Evaluation of a Promising Intervention for Primary Obesity Prevention in Toddlers. Child Obes. 2016, 12, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.L.; Adair, L.S.; Bentley, M.E. Pressuring and restrictive feeding styles influence infant feeding and size among a low-income African-American sample. Obesity 2013, 21, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Rifas-Shiman, S.L.; Sherry, B.; Scanlon, K.; Birch, L.L.; Gillman, M.W.; Taveras, E.M. Does maternal feeding restriction lead to childhood obesity in a prospective cohort study? Arch. Dis. Child. 2011, 96, 265–269. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Ozbeki, T.N.; Appugliese, D.P.; Kaciroti, N.; Corwyn, R.F.; Bradley, R.H. Observed assertive and intrusive maternal feeding behaviors increase child adiposity. Am. J. Clin. Nutr. 2012, 95, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. 2013 Pediatric Nutrition Surveillance System: Growth Indicators by Race/Ethnicity and Age, Children Aged <5 Years. Table 16D. 2012. Available online: www.cdc.gov/pednss/pednss_tables/pdf/national_table16.pdf (accessed on 7 August 2021).
- Farrow, C.; Blissett, J. Does maternal control during feeding moderate early infant weight gain? Pediatrics 2006, 118, e293–e298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministero Della Salute, Direzione Generale Per l’Igiene e la Sicurezza Degli Alimenti e la Nutrizione, Office 5. Guidelines for the Prevention of Choking on Food, June 2017. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2618_allegato.pdf (accessed on 8 August 2021).
- Canadian Pediatric Society. Position Statement. Preventing Choking and Suffocation in Children February 1 2012. Available online: https://www.cps.ca/en/documents/position/preventing-choking-suffocation-children (accessed on 6 August 2021).
- American Academy of Pediatrics (AAP). Policy Statement. Committee on Injury, Violence, and Poison Prevention. Prevention of Choking among Children. Pediatrics 2010, 125, 601–607. [Google Scholar]
- Cameron, S.L.; Taylor, R.W.; Heath, A.L. Parent-led or baby-led? Associations between complementary feeding practices and health-related behaviours in a survey of New Zealand families. BMJ Open 2013, 3, e003946. [Google Scholar] [CrossRef] [Green Version]
- Brown, A. No difference in self-reported frequency of choking between infants introduced to solid foods using a baby-led weaning or traditional spoon-feeding approach. J. Hum. Nutr. Diet. 2018, 31, 496–504. [Google Scholar] [CrossRef]
- Fangupo, L.J.; Heath, A.M.; Williams, S.M.; Erickson Williams, L.W.; Morison, B.J.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Taylor, R.W. A Baby-Led Approach to Eating Solids and Risk of Choking. Pediatrics 2016, 138, e20160772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.X.; Conlon, C.A.; Haszard, J.J.; Beck, K.L.; von Hurst, P.R.; Taylor, R.W.; Heath, A.-L.M. Food fussiness and early feeding characteristics of infants following Baby-Led Weaning and traditional spoon-feeding in New Zealand: An internet survey. Appetite 2018, 130, 110–116. [Google Scholar] [CrossRef]
- Nerenberg, K.A.; Zarnke, K.B.; Leung, A.A.; Dasgupta, K.; Butalia, S.; McBrien, K.; Harris, K.C.; Nakhla, M.; Cloutier, L.; Gelfer, M.; et al. Hypertension Canada’s 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2018, 34, 506–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, S.; Saelens, B.; Levin, L.; Dart, K.; Falciglia, G.; Daniels, S.R. The efficacy of a clinic-based behavioral nutrition intervention emphasizing a DASH-type diet for adolescents with elevated blood pressure. J. Pediatr. 2008, 152, 494–501. [Google Scholar] [CrossRef]
- Van Horn, L.; Vincent, E. The CHILD 1 and DASH diets: Rationale and translational applications. Pediatr. Ann. 2013, 42, 372–374. [Google Scholar] [CrossRef] [Green Version]
- Ministero Della Salute. National Guidelines for Oral Health Promotion and Prevention of Oral Diseases in Childhood. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2073_allegato.pdf (accessed on 15 March 2022).
- Chou, R.; Cantor, A.; Zakher, B.; Mitchell, J.P.; Pappas, M. Prevention of Dental Caries in Children Younger Than 5 Years Old: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 104; AHRQ Publication No. 12-05170-EF-1; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014.
- SIGN 138—Dental Interventions to Prevent Caries in Children. Available online: https://www.scottishdental.org/wp-content/uploads/2014/04/SIGN138.pdf (accessed on 15 March 2022).
- American Academy of Pediatric Dentistry. Policy on Dietary Recommendations for Infants, Children, and Adolescents. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2021; pp. 87–89. [Google Scholar]
- Leong, P.M.; Gussy, M.G.; Barrow, S.Y.; de Silva-Sanigorski, A.; Waters, E. A systematic review of risk factors during first year of life for early childhood caries. Int. J. Paediatr. Dent. 2013, 23, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Pelto, G.; Levitt, E.; Thairu, L. Improving feeding practices: Current patterns, common constraints, and the design of interventions. Presentation at the WHO Global Consultation on Complementary Feeding, Geneva, December 2001. Food Nutr. Bull. 2003, 24, 45–82. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Rifas-Shiman, S.L.; Scanlon, K.S.; Grummer-Strawn, L.M.; Sherry, B.; Gillman, M.W. To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics 2006, 118, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Jansen, E.; Williams, K.E.; Mallan, K.M.; Nicholson, J.M.; Daniels, L.A. Bidirectional associations between mothers’ feeding practices and child eating behaviours. Int. J. Behav. Nutr. Phys. Acta 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.; Jones, S.W.; Rowan, H. Baby-Led Weaning: The Evidence to Date. Curr. Nutr. Rep. 2017, 6, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Özyüksel, G.; Soyer, T.; Üzümcügil, F.; Yalçın, Ş.; Ekinci, S.; Karnak, I.; Çiftçi, A.Ö.; Tanyel, F.C. Foreign Body Aspiration in Infants: Role of Self-Feeding. Pediatr. Aller. Immunol. Pulmonol. 2019, 32, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Baby-led weaning (BLW) This is a complementary feeding practice that promotes the autonomy of the child by offering him/her food that is usually consumed by the family and that the child is able to handle and bring to his/her mouth on his/her own. The child is free to choose what and how much to eat with his/her hands from the food on the table. Baby-led introduction to solids (BLISS) A modified version of BLW that relies on the same basic principles, while recommending that at each meal the child be offered three different types of food: an iron-rich food (red meat or iron-fortified infant cereals), an energy-rich food, and a fiber-rich food such as a fruit or vegetable. Responsive complementary feeding (RCF) The active behavior of the child is prioritized. Food is offered only in response to cues from the child and stops when the child stops asking. Food is offered at the times, in the ways, in the textures, and in the quantities that best suit his or her level of psycho-neuro-motor and physical development as part of what the family eats, provided these foods are suitable for the infant. Non-responsive complementary feeding (NRCF) NRCF is characterized by a lack of reciprocity between the caregiver and child (in terms of request/response) at mealtime. The caregiver can be “overly active” (forcing, insisting, or limiting food intake), “overly passive” (to the point of becoming too tolerant of the qualitative and quantitative choices made by the child), “predominantly functional” (using food for soothing strategies), or even “completely uninvolved” and not at all interested in the child’s request or refusal of food, as in a mechanism of detachment. Traditional complementary feeding (TCF) In this systematic review, TCF refers to a practice in which one meal of milk (either breast milk or formula) is replaced with a solid meal, and then, later on, and over the months, a second meal is also used as a replacement. The foods, in the form of commercial or home-made baby food, are prepared specifically for the infant and initially consist of purees given in line with local eating habits, with gradual adaptation to the use of the spoon. Subsequently, cut-up foods are added. In this practice, family foods are typically introduced at around one year of age. Indicative portions are recommended by pediatricians or by other health professionals. |
| (BLW-BLISS) Compared to (Other Models of CF) in (Healthy Child, Can Influence, Either Positively or Negatively, Infant Weight−Length Gain) | |||||
|---|---|---|---|---|---|
| Patient or Population: (Healthy Child Aged 6–24 Months) Setting: Outpatient Intervention: (BLW-BLISS) Comparator: (Other Models of CF) | |||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Overweight/obesity risk (BLW-observational studies) (follow up: interval 18 to 78 months; evaluated with: BMI−BMIz (% obesity overweight)) | 388 per 1.000 (299 to 485) | 189 per 1.000 | OR 0.37 (0.25 to 0.55) | 969 (3 observational studies) [6,7,8] | ⨁◯◯◯ Very low a,b |
| Overweight/obesity risk (BLISS-RCT) (follow up: medium 24 months; evaluated with: WHO P/L z score/BMIz (% obesity overweight)) | 142 per 1.000 | 17 per 1.000 (0 to 1.000) | RR 0.15 (0.00 to 17.79) | 457 (2 RCTs) [9,10] | ⨁⨁◯◯ Low b,c,d |
| (RCF) Compared to (Other Models of CF) in [Healthy Child, in the Period 6–24 Months], Can Influence (the Development of Overweight and Obesity) | ||||||
|---|---|---|---|---|---|---|
| Patient or Population (Healthy Child Aged 6–24 Months) Setting: Outpatient Intervention: (RCF) Comparator: (Other Models of CF) | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with [RCF] | Risk with [other models of CF] | |||||
| Risk of overweight and obesity after 2 years. RCT (follow up: 3 years; assessed with: % of overweight/obesity children) | 76/1.000 (44 to 132) | 185/1.000 | RR 0.41 (0.24 to 0.71) | 478 (2 RCTs) [16,44] | ⨁⨁⨁◯ Moderate a,b,c | |
| Risk of overweight and obesity after 13 mo. RCT (evaluated with BMIz) | DANIELS 2012. Children in the intervention group had a lower BMIz at 13 months of age than children in the control group: 0.23 ± 0.93 and 0.42 ± 0.85 (p = 0.01), respectively | 698 (1 RCTs) [14] | ⨁⨁◯◯ Low d,e | |||
| (NRCF) Compared to (Other Models of CF) in (Healthy Child, in the Period 6–24 Months), Can Influence, Can Influence (the Development of Overweight and Obesity) | |||
|---|---|---|---|
| Patient or Population (Healthy Child Aged 6–24 Months) Setting: Outpatient Intervention: (NRCF) Comparator: (Other Models of CF) | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| NRCF. Risk of overweight and obesity. Observational (follow up: interval 15 months to 20 months; assessed with:% overweight/obesity. BMIz, ΔBMI, Skinfold.) | No significant association for all comparisons (for documented exposures ≥6 months) | (4 observational studies) [19,45,46,47] | ⨁◯◯◯ Very low a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamini, M.; Simeone, G.; Verga, M.C.; Doria, M.; Cuomo, B.; D’Antonio, G.; Dello Iacono, I.; Di Mauro, G.; Leonardi, L.; Miniello, V.L.; et al. Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2646. https://doi.org/10.3390/nu14132646
Bergamini M, Simeone G, Verga MC, Doria M, Cuomo B, D’Antonio G, Dello Iacono I, Di Mauro G, Leonardi L, Miniello VL, et al. Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(13):2646. https://doi.org/10.3390/nu14132646
Chicago/Turabian StyleBergamini, Marcello, Giovanni Simeone, Maria Carmen Verga, Mattia Doria, Barbara Cuomo, Giuseppe D’Antonio, Iride Dello Iacono, Giuseppe Di Mauro, Lucia Leonardi, Vito Leonardo Miniello, and et al. 2022. "Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis" Nutrients 14, no. 13: 2646. https://doi.org/10.3390/nu14132646
APA StyleBergamini, M., Simeone, G., Verga, M. C., Doria, M., Cuomo, B., D’Antonio, G., Dello Iacono, I., Di Mauro, G., Leonardi, L., Miniello, V. L., Palma, F., Scotese, I., Tezza, G., Caroli, M., & Vania, A. (2022). Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients, 14(13), 2646. https://doi.org/10.3390/nu14132646






