Abstract
Faecal microbiota transplantation (FMT) has attracted increasing attention as an intervention in many clinical conditions, including autoimmune, enteroendocrine, gastroenterological, and neurological diseases. For years, FMT has been an effective second-line treatment for Clostridium difficile infection (CDI) with beneficial outcomes. FMT is also promising in improving bowel diseases, such as ulcerative colitis (UC). Pre-clinical and clinical studies suggest that this microbiota-based intervention may influence the development and progression of chronic kidney disease (CKD) via modifying a dysregulated gut–kidney axis. Despite the high morbidity and mortality due to CKD, there are limited options for treatment until end-stage kidney disease occurs, which results in death, dialysis, or kidney transplantation. This imposes a significant financial and health burden on the individual, their families and careers, and the health system. Recent studies have suggested that strategies to reverse gut dysbiosis using FMT are a promising therapy in CKD. This review summarises the preclinical and clinical evidence and postulates the potential therapeutic effect of FMT in the management of CKD.
1. Introduction
Chronic kidney disease (CKD) affects approximately 10% of adults worldwide []. It has been identified as a significant health issue, which has been driven by the epidemic trend of diabetes, hypertension, and the dynamics of the population aging process []. CKD is a continuum of chronic changes in the kidney, including chronic inflammation, tubulointerstitial fibrosis, glomerulosclerosis, and vascular rarefaction. Intensive research is ongoing to unravel the pathogenesis of CKD, which should provide more opportunity to slow CKD progression by non-pharmacologic and novel pharmacologic interventions. Blockade of the renin–angiotensin–aldosterone systems has been shown to slow the progression of proteinuric kidney disease [,]. More recently, type 2 sodium–glucose linked transporter (SGLT2) inhibitors in cardiovascular and renal specific outcomes trials have been shown to further limit CKD progression to end-stage kidney disease in diabetic and, in the DAPA-CKD trial, non-diabetic patients [,]. The only effective treatment for end-stage kidney disease is kidney replacement therapy, including dialysis and kidney transplantation. As CKD advances, associated mortality, loss of life quality, and increased hospitalization lead to a dramatically ascending financial burden on the public healthcare system, communities, patients, and families []. The stage and progression of CKD may change the absorption, distribution, metabolism/transport, and excretion of medications [,,]. Hence, novel treatments have been urgently needed to prevent and treat CKD. Over the past decade, mounting studies have identified the role of the gut microbiota in a wide range of inflammation-associated clinical conditions, including Clostridium difficile infection (CDI) induced diarrhea [], irritable bowel disease (IBD) [], obesity, and diabetes []. These studies have led to the hypothesis that targeting the gut microbiota may be a potential strategy to manage CKD, which is increasingly regarded as an inflammatory disease [].
The gut microbiota is the broad spectrum of microorganisms accommodated in the gastrointestinal (GI) tract, consisting of bacteria, viruses, archaea, protists, and fungi. A healthy human GI tract harbors more than 1014 bacteria, which encode 100 times more genetic information than the human genome []. With the availability of cheaper and faster biotechnologies, such as high-throughput next-generation sequencing, features of the gut microbiome, including composition, abundance, diversity, and functions, have been explored []. The current understanding of the gut microbiome community is primarily based on the taxonomic characteristics at genus levels due to relatively lower sequencing costs. Applying the bacterial 16S ribosomal RNA (16S rRNA) gene sequencing is a successful strategy for genus identification and characterization, which is independent of labour-intensive culture-based techniques []. Alternatively, gut microbiota can be analysed at species levels via metataxonomics, which is a deeper sequencing strategy and has more power to discover those bacteria with less abundance []. In healthy adults, most of the gut ecosystem is primarily dominated by Firmicutes and Bacteroides, with a relatively low population of Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobia, etc. []. In addition, the homeostatic gut microbiome community serves the host via a wide range of physiological activities, including digesting food [,], preventing pathogens [], maintaining the function and integrity of the intestinal epithelial layer [,], and modulating the host immune system [,,]. With the assistance of advanced bioinformatics and machine learning artificial intelligence, strong links have been identified between the gut microbiota and many human diseases [], such as diabetes [], irritable bowel disease [], and autism []. Increasing studies have identified microbiota-derived metabolites as key molecular mediators between the microbiota and host, mainly short-chain fatty acids (SCFAs) [], bile acids [], branched-chain amino acids [], trimethylamine N-oxide [], tryptophan, and indole derivatives [].
Appreciation of the importance of the gut microbiome for human health and disease derives from clinical practices affecting gut bacteria, such as antibiotic use. Disturbance of healthy gut microbiota, also termed gut dysbiosis, contributes to the pathogenesis of many clinical diseases, such as metabolic disorders [], inflammatory bowel disease [], neurodegenerative diseases [], and CKD [,]. Recently, gut dysbiosis has been suggested to play a pathogenic role in CKD via modulation of the immune system, impairment of gut barrier integrity, and gut microbiota-derived metabolites []. Gut bacteria-derived metabolites, such as short-chain fatty acids (SCFAs), uremic toxins, endotoxins, etc., have also shown a close association with different severities of CKD, indicating a potential important role of gut metabolites in the progression of CKD [].
As a potent microbiome-based invention, faecal microbiota transplantation (FMT) transfers the gut microbial community from healthy donors to recipients to restore gut microbiota homeostasis. FMT is an established therapy for recurrent CDI, with a treatment efficacy above 85% []. Moreover, accumulating studies have shown the benefit of FMT for several diseases associated with dysregulated gut microbiota, including autism, cancer, and type 2 diabetes [,,,,]. Although studies using FMT to prevent or treat CKD are still limited, preliminary evidence of the efficacy of FMT indicates that the application of FMT may offer a promising alternative for the treatment of CKD.
This review will demonstrate gut dysbiosis occurring in CKD and summarise its predominant pathogenic mechanisms that contribute to progressive kidney dysfunction, including the regulation of the immune system, gut microbiota-derived metabolites, renin–angiotensin system, and gut barrier. The application of FMT in CKD as a potential therapeutic option will also be discussed.
2. Evidence of Gut Microbiota Dysbiosis in CKD
Gut dysbiosis is both a quantitative and qualitative alteration in the composition and metabolic profiles of the gut microbiome. Increasing evidence has confirmed gut dysbiosis occurs at the onset of CKD, including in the development of CKD and progression to end-stage kidney disease (ESKD) [,,]. Recent pre-clinical and clinical studies exploring gut microbiota features in CKD are summarized in Table 1, focusing on the relative abundance of primary bacterial groups, such as Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia compared to controls.

Table 1.
Recent pre-clinical and clinical studies on the gut microbial composition in CKD.
2.1. Animal Studies
In the diabetic kidney disease (DKD) mouse model, the microbiome analysis of operational taxonomic units (OTUs) identified an increased abundance of Firmicutes, Proteobacteria, and Verrucomicrobia but a significantly lower presence of Bacteriodetes in the DKD group compared to the healthy controls []. Similarly, in an adenine-induced CKD rat model, there was an enrichment of Firmicutes but a lower abundance of Bacteroides in the CKD group compared to the normal rats. Additionally, a reduced amount of Ruminococcaceae UCG-014, Prevotellaceae_NK3B31_group, Ruminococcus 1, Lachnospiraceae UCG-001, and Clostridium leptum group bacteria have been found in the adenine-induced CKD rat or mouse models, respectively [].
2.2. Human Studies
In a recent systematic review incorporating twenty-five studies with 1436 CKD patients and 918 healthy adults, Zhao et al. found that the gut microbiome in CKD patients had an increased abundance of phylums Fusobacteria and Proteobacteria, genera Streptococcus, Desulfovibrio, and Escherichia_Shigella, and a reduced presence of genera Faecalibacterium, Roseburia, Pyramidobacter, Prevotella_9, and Prevotellaceae_UCG-001. Moreover, elevated levels of bacterial metabolites trimethylamine n-oxide (TMAO) and p-cresol sulfate (PCS) and lower production of SCFAs were found in CKD patients []. A study on 29 Chinese patients with immunoglobulin A nephropathy (IgAN) also showed a reduced amount of Clostridia, Eubacterium, and Alistipes compared with healthy controls, indicating the pathogenic role of depletion of SCFA-producing bacteria in systemic inflammation and IgAN progression []. In patients with IgAN, a higher abundance of class Coriobacteriia and genera Legionella, Enhydrobacter, and Parabacteroides was present in the blood, and genera Bacteroides, Escherichia-Shigella, and Ruminococcus in stool in comparison with healthy controls []. Additionally, a clinical study in ESKD patients identified six taxa, including Akkermansia, Dialester, Enterococcus, Rominicoccus, Blautia, and Bacteroides, that contributed to the elevated levels of uraemic toxins []. Patients with DKD had a higher abundance of Verrucomicrobia, Proteobacteria, and a lower amount of Bacteroidetes at the phylum level, with Escherichia-Shigella, Enter Klebsiella, Streptococcus, Akkermansia, and lactobacillus increased at the genus level. In particular, SCFA-producing bacteria, such as Blautia and Faecalibacterium, were decreased in DKD patients compared to healthy controls []. Kim et al. analysed the gut microbiome from 103 patients with CKD (stage 1 to 5) and 46 healthy controls. The results showed an increased abundance of Oscillibacter and Alistipes and a decreased abundance of Veillonella, Lachnospira, and Dialister in CKD patients compared to the healthy controls []. Another clinical study that enrolled 14 healthy controls and 138 CKD patients indicated a lower proportion of SCFA-producing bacteria, including Bifidobacterium and Streptococcus, in CKD patients. Interestingly, kidney function loss was positively related to a higher proportion of Enterobacteriaceae and E. coli [].
3. The Role of the Gut Microbiota in CKD
In CKD, the gut–kidney axis is potently affected by the gut microbiota in a bidirectional manner []. CKD increases the retention of metabolic waste products, gut bacteria-derived metabolites (i.e., uremic toxins) with concomitant medications, poor nutrition, and comorbidities contributing to the altered gut microbiota. Conversely, gut dysbiosis contributes to progressive CKD mainly via maladaptive immune responses, microbial metabolites that are harmful to the kidneys, activation of the RAS, and a disruption of the gut barrier, allowing gut toxins and metabolites to enter the systemic circulation. Thus, a vicious cyclical interaction occurs between the gut and kidney, amplifying both organs’ injury.
3.1. Immunomodulatory Mechanisms of Gut Microbiota in CKD
It is postulated that gut dysbiosis promotes CKD by modulating the host’s immunity, which depends on the activation of the host’s innate and adaptive immune responses []. Previous studies have shown that host defence systems, such as innate and adaptive immunity, could coevolve, be trained, and tolerated reciprocally with the prevailing gut microbiota []. This intricate and symbiotic interaction between microbiota and host immune systems significantly affects the health status of animals and humans.
Gut microbiota dysbiosis contributes to the impaired gut barrier, excessive production of uremic toxins, and translocation of the gut microbiome, leading to the abnormal activation of the immune cells []. The active immune cells, such as neutrophils, macrophages, dendritic cells, and lymphocytes, then infiltrate kidneys and promote the pro- or anti-inflammatory reactions []. Wang et al. found that neutrophils and macrophages were recruited and activated as part of the first-line immune response in innate immunity against kidney disease induced by adenine in mice []. Moreover, Funken et al. identified that the γδ-T cells as potent mediators in ischemia-reperfusion injury (IRI) contributed to the increased influx of neutrophils and pro-inflammatory cytokines, which were not found in δ-T cell deficiency mice []. IRI mouse model induced by bilateral renal pedicle clamping resulted in altered gut bacterial dysbiosis resulting in significant decreases in Lactobacilli, Ruminococacceae, and an increase in Enterobacteriaceae. Depleting gut bacteria by oral antibiotics or FMT derived from sham-operated mice prevented mice from developing IRI, which was confirmed to be associated with reduced Th 17, Th 1 activation, and the expansion of M2 macrophage and Treg []. Germ-free animal models have shown a significant increase in the population of T helper 17 (TH17) cells but a deficit in the immune-mediating regulatory T (Treg) cells, leading to susceptibility to colitis after FMT from IBD patients []. Notably, a bacterial polysaccharide from segmented filamentous Bacteroides fragilis could ‘educate’ the host immune system to be tolerant to residing microbes, primarily by modulating CD4+ Tregs to secrete IL-10, which was dependent on Toll-like receptor (TLR) in mice []. TH17 cells are present in high density in the gut, and their presence and activation rely on the adjacent gut microbiota. Krebs and his team directly identified that microbiota-induced TH17 cells migrated from the gut and accumulated in kidneys, which was mediated by the chemokine C-C motif chemokine ligand 20 (CCL20) and its receptor C-C Motif Chemokine Receptor 6 (CCR6) in autoimmune kidney disease [].
Individual compartmentalized gut-associated lymphoid tissues (GALTs) inhabit the interface between the intestinal lymphatic and blood systems, allowing mature immune cells constant access to the epithelial layers and lamina propria. In humans, the GALT interplay with the gut microbiota to exert immune responses and tolerance []. However, a recent study strongly supported that GALT is a significant induction site of IgAN [].
3.2. Gut Microbial Metabolites and CKD
Due to the relative richness of proteolytic bacteria, gut bacterial metabolites are profoundly altered in CKD, resulting in reduced local and systemic SCFAs and increased uremic toxins [].
SCFAs are aliphatic carboxylic acids with fewer than six carbon (c) atoms produced by bacteria inhabiting the colon after fermentation of dietary fibre or protein catabolism. In humans, the major SCFAs are acetate, propionate, and butyrate, which are produced by prebiotic fermentation via specific gut bacteria, such as Blautia, Bacteroides, Butyricoccus, Bifidobacterium, Prevotella, Megasphaera, and Butyrivibrio [] in the intestine and colon []. In previous studies, metabolites derived from the gut microbiota via fermentation of dietary fibre have been shown to directly influence the composition and function of the host immune system [,,]. SCFAs activate G-protein-coupled receptors (GPR), including GPR43, GPR109A, GPR41, and O1f78, etc., thereby attenuating reactive oxygen species (ROS), inflammation [], and mitigating fibrosis in the kidney []. SCFAs preferably activate GPRs; for example, acetate activates GPR43, propionate activates GPR41, while GPR43 and butyrate activate GPR41 [].
In a cohort clinical study, Wang et al. measured the levels of SCFAs in 127 patients with CKD and 63 healthy controls. The results showed that the serum SCFAs levels, especially butyrate, were significantly decreased in CKD patients compared with healthy controls, and supplementation of butyrate might slow the progression of CKD []. An investigation that enrolled 105 children and adolescents with CKD found a difference in plasma acetate between children with and without hypertension, suggesting its preventive role for hypertension in children with CKD. In addition, reduced plasma butyrate was found in the advanced and stable blood pressure groups at the 1-year follow up, which may potentially be caused by the decline in eGFR []. In addition, Chai et al. identified substantially lower production of SCFAs, including acetic acid, propionic acid, butyric acid, and iso-butyric acid, in IgAN patients compared with those in the control group []. Furthermore, a recent clinical study recruiting 30 patients with DKD confirmed that SCFAs were reduced in serum and stool compared to 30 normal controls []. In line with those clinical findings, the decreased plasma levels of SCFAs and impaired renal function were observed in a rat model of CKD induced by dietary adenine, which was attenuated by gum acacia []. In a DKD mouse model induced by streptozotocin (STZ), Li et al. reported that a high-fibre diet significantly reduced albuminuria, kidney hypertrophy, glomerular injury, tubulointerstitial fibrosis, and interstitial macrophage infiltration compared with healthy controls. They further confirmed that those benefits were associated with improved gut dysbiosis, increased SCFA-producing bacteria, and boosted SCFAs production. Supplementation with SCFA further confirmed that SCFA is responsible for the renoprotective effect of dietary fibre, which was mediated through the activation of GPR43 or GPR109A [].
The accumulation of serum uremic toxins due to gut microbiota dysregulation is strongly associated with oxidative stress and systemic inflammation, which is increasingly recognized to play a pivotal role in the onset and progression of CKD []. Protein-bound uremic toxins (PBUTs) are produced by gut bacteria by the metabolism of aromatic amino acids and are not efficiently removed by dialysis. The most investigated PBUTs derived from the gut microbiota are indoxyl sulfate (IS) and para-cresyl sulfate (pCS). IS is synthesized from dietary tryptophan, and pCS is metabolized from dietary phenylalanine and tyrosine via gut bacteria. IS and pCS were negatively correlated to the estimated glomerular filtration rate through all stages of CKD among children and adolescents []. Similarly, another clinical study recruiting 342 patients with CKD measured total and free serum IS and pCS by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and found a positive correlation between the analyte and CKD progressions []. Both PBUTs can trigger and exacerbate tubulointerstitial fibrosis and glomerular sclerosis, impairing kidney function in vivo [,].
In addition to IS and pCS, trimethylamine-N-oxide (TMAO) has been recognized as a gut microbiota-derived uremic retention product associated with higher mortality and risk of cardiovascular events [,]. TMAO is produced from the metabolism of quaternary amines such as betaine, L-carnitine, and phosphatidylcholine, which are precursors of trimethylamine []. Food sources of choline and carnitine, such as cheese and red meat, are metabolized into trimethylamine mainly by gut microorganisms such as Firmicutes and Proteobacteria []. Elevated serum TMAO was identified as positively related to mortality in 521 patients with CKD studies over a 5-year follow-up []. Furthermore, an observational prospective cohort study of peritoneal dialysis (PD) patients (n = 105) demonstrated that elevated plasma TMAO induces peritoneal inflammation and increases the risk of peritonitis in patients undergoing PD [].
3.3. Renin–Angiotensin System (RAS) and CKD
The renin–angiotensin system (RAS) has been recently confirmed as a critical link between gut microbiota and CKD []. RAS inhibitors are prescribed to patients to decrease proteinuria and defer CKD progression []. However, uremic toxins, including IS, pCS, and TMAO, can activate the RAS [,], leading to increased intraglomerular pressure and excessive local release of pro-inflammatory factors []. Sun et al. reported that IS and pCS could induce kidney fibrosis via activating the RAS/pro-inflammatory transforming growth factor-β1 (TGF-β1) axis in half-nephrectomized-induced mouse CKD models []. Similarly, Jiang et al. found that TMAO augmented angiotensin II-induced vasoconstriction and promoted angiotensin II-induced hypertension through the PERK/ROS/CaMKII/PLCβ3 axis []. Consistent with this, another recent study in the rat DKD model confirmed that gut microbiota disorders contributed to kidney injuries via activating the RAS, as evidenced by significantly increased expressions of angiotensin II, angiotensin-converting enzyme and the angiotensin II type 1 receptor in DKD groups compared to the healthy controls []. Accumulating studies have explored the potential therapeutic strategy for treating CKD by suppressing the gut dysbiosis-induced activation of the RAS. For example, Chen et al. demonstrated that Alisol B 23-acetate, isolated from Alisma Orientale, can attenuate CKD progression via modulating the RAS and gut–kidney axis in 5/6 nephrectomized and unilateral ureteral obstructed rat models [].
3.4. Disrupted Gut Barrier and CKD
Healthy gut microbiota homeostasis may assist with the development of host immunity, while a disturbed gut microbiome hampers and damages it []. Gut dysbiosis may manipulate host immunity/inflammation directly through its interplay with the adjacent epithelial barrier of the gastrointestinal (GI) tract [] and the mucus layer secreted by mucosal epithelia, the mucosa, and resident immune cells []. Vaziri et al. identified the critical role of the intestinal barrier in a rat model of 5/6 nephrectomy-induced CKD. They demonstrated that uremia impaired the integrity of the intestinal barrier, as evidenced by the reduction in the key proteins expressed in the colonic mucosa, including claudins-1, occludin, and zonula occludens-1 (ZO-1). They postulated that the impaired intestinal barrier function due to the disintegration of the colonic tight junction might be a key contributor to the systemic inflammation in CKD []. Similarly, Yang et al. confirmed that gut microbiota dysbiosis in CKD was positively associated with the severity of the impaired gut barrier and abnormal intestinal immunity in the mucosal layer in 5/6 nephrectomized mice []. Decreased expression of tight junction proteins, gradual loss of colonocytes, and gut dysbiosis were also positively correlated with the impaired gut barrier in the mouse model of IRI []. Ji et al. reported that the treatment with rhubarb (a traditional Chinese herbal medicine) improved gut microbiota dysbiosis, suppressed systemic inflammation, and attenuated kidney fibrosis in 5/6 nephrectomized rats through restoring the intestinal barrier. These positive outcomes suggest that the intestinal barrier is an essential target for CKD treatment [].
CKD can further impair gut barrier function due to decreased SCFAs production and the accumulation of uremic toxins, which contributes to inflammation, oxidative stress and further damages the gut epithelial monolayer. A reduction in SCFAs and excessive production of uremic toxins lead to a downward spiral of worsening CKD in association with worsening gut function [,]. CKD then promotes malnutrition, which further impairs the gut epithelial layer due to fluid retention, metabolic acidosis, and uric acid accumulation []. Microbiota depletion with multiple broad-spectrum antibiotics, including ampicillin, neomycin sulfate, metronidazole, and vancomycin, often used in patients with CKD, can further impair gut barrier function []. Conversely, supplementation with the SCFA sodium butyrate to db/db mice significantly increased gut epithelial integrity with increased expression of intercellular adhesion molecules, such as ZO-1 [].
4. Faecal Microbiota Transplantation (FMT)
FMT potently ‘rebuilds’ the gut microflora’s dynamic homeostasis, structure, and diversity by transferring healthy gut microbiota into recipients. The significant relationship between gut microbiota dysregulation and many clinical conditions has enabled FMT to treat multiple clinical conditions, such as autoimmune [], neurodegenerative [], CDI [], and metabolic disorders [,,]. The most successful clinical application of FMT is currently to treat CDI with up to a 90% success rate and with a good safety profile [], as no short- or long-term adverse effects have been described []. In addition, FMT can be delivered to the recipients’ GI tract in several ways, including capsules, naso-enteric tube, and colonoscopy [].
FMT plays a vital role in shaping and modulating the immune system, significantly impacting the peripheral immune system and inflammatory responses. Burrello et al. demonstrated that FMT could attenuate inflammatory responses in the colon and restore gut homeostasis via activating different immunoregulatory signalling pathways. Consequently, FMT increased IL-10 secretion from innate and adaptive immune cells and inhibited the activity of monocytes, dendritic cells, and macrophages to acquire, process, and present antigens to T cells inhabiting the intestine []. In another colitis mouse model induced by dextran sodium sulfate (DSS), the abundance of colonic CD4+, CD8+, and invariant natural killer T (iNKT) cells were significantly reduced in the FMT group compared to the non-FMT and control group. In addition, colonic expression levels of crucial cytokine genes Il6, Il7, Ifng, Tnf, and Il1b were downregulated significantly compared with the non-FMT group [].
FMT has been used as a “brute-force” therapeutic strategy to restore or improve gut homeostasis mediated through the regulation of gut bacteria-derived metabolites. In mouse models with atopic dermatitis (AD), gut microbiota dysbiosis was restored to the donor state together with elevated concentrations of SCFAs []. FMT also corrected the gut bacterial imbalance in traumatic brain injury rat models and reduced gut bacteria-synthesized uremic toxins, such as TMAO []. In a traumatic brain injury rat model, FMT could reduce the concentration of TMAO in the ipsilateral brain and serum. In contrast, the TMAO level was lowered in the faeces via restoring gut microbiota dysbiosis []. In addition, FMT strongly reduced systemic endotoxemia in a sepsis rat model, leading to beneficial effects on improving gut barrier functions [].
Although a wide variety of the gut microbiota-based approaches, such as probiotics and prebiotics, have shown positive outcomes in managing CKD, a considerable number of studies generated controversial results [,,]. Hence, FMT, which restores the overall gut microbiome community, might offer a promising alternative to protect kidneys from injury. It is worth exploring mechanisms accounting for those benefits from FMT in various clinical and pre-clinical contexts.
5. FMT Studies in CKD
Accumulating evidence has highlighted the pathogenic role of gut microbiota dysbiosis in the setting and progression of CKD [,,], which has led researchers to investigate animal studies of FMT in CKD further, either alone or as adjunctive therapy (Table 2).

Table 2.
Experimental and clinical studies of FMT in CKD.
FMT from patients, donors or CKD rodent models has been used to investigate the correlation between gut dysbiosis and CKD progression. For example, Wang et al. administered FMT from patients with ESKD or healthy donors to germ-free CKD mice or antibiotic-treated CKD rats. They found that the gut microbiome in the recipient mice was modulated and exhibited the features of the ESKD patients or control donors. The gut microbiota from patients with ESKD increased the production of uraemic toxins, aggravated interstitial fibrosis, and oxidative stress, which further confirmed the causative relationship between the aberrant gut microbiota and kidney disease development []. Consistently, similar findings were observed in FMT experiments in STZ-induced DKD mice. Higher levels of TMAO and lipopolysaccharide (LPS), different microbiota constituents, and more significant kidney damage were found in the group receiving FMT from mice with severe proteinuria compared to those receiving FMT from mice with mild proteinuria, indicating that the differences in the gut microbiome may contribute to kidney disease severity [].
In addition, FMT has been a helpful tool to demonstrate that the modulation of the intestinal microbiota may account for one of the key mechanisms mediating the renal protective effect of therapeutic interventions for CKD, as demonstrated in various FMT studies. By transplanting the resveratrol-modified faecal microbial, Cai et al. confirmed that resveratrol, a stilbene polyphenolic compound, exerted its renal protective effect by improving the faecal microbial community. They reported that FMT from healthy resveratrol-treated control mice restored the gut microbiome and regulated intestinal permeability accompanied by an inhibited inflammatory response and improved renal function []. Similarly, in the mechanistic study for Astragalus membranaceus and Salvia miltiorrhiza (AS), the administration of FMT from the AS-treated group attenuated cyclosporin A-induced kidney damage and fatty acid metabolism through modulation of the “gut–kidney axis.” Those benefits were positively correlated with an improved intestinal barrier, restored intestinal flora structure, increased abundance of bacteria producing butyric acid and lactic acid, and improvement in the miRNA–mRNA interaction profiles related to Butanoate and Tryptophan metabolism [].
FMT, using a homeostatic gut microbiome collected from healthy donors, has been investigated as a potential therapeutic strategy for managing CKD. In a mouse model of adenine-induced CKD, FMT significantly reduced the production of uremic toxins derived from the intestinal cresol pathway with a profound improvement of gut microbiota disturbance, as evidenced by the increased alpha diversity. These studies suggested that FMT is an effective intervention for gut microbiota []. Hu et al. recently applied FMT from the healthy rats into rats induced to diabetes with STZ. The results showed that transplantation of microbiota from healthy rats potently ameliorated tubulointerstitial injury in the diabetic group associated with restoring dysregulated cholesterol homeostasis with decreased serum triglycerides and less lipid accumulation in the kidneys of diabetic rats, which was mediated through the activation of GPR43 []. Recently, Lu et al. reported that antibiotic treatment mediated gut microbiota depletion and treatment with FMT from healthy control rats effectively increased podocyte insulin sensitivity and alleviated glomerular injury in diabetic rats. This was associated with downregulation of GPR43 expression, suggesting an essential role of gut microbiota-modulated GPR43 in STZ-induced diabetic kidney disease []. Most recently, Bastos, R.M. et al. further confirmed the safety and efficacy of FMT as an effective non-pharmacological approach in BTBRob/ob DKD mice [].
Interestingly, instead of transplantation of the whole gut microbiome, Zheng et al. constructed a bacterial micro-ecosystem (BME) with a small bacterial consortium encapsulated in calcium alginate microspheres coated with a layer of polydopamine nanofilm. The administration of a microencapsulated bacterial cocktail consisting of three different bacteria, including Escherichia.coli, Bacillus subtilis, and Lactobacllus acidophilus, showed benefits without any evident adverse effects by eliminating nitrogenous waste products in multiple animal models of kidney failure, including murine AKI, murine chronic kidney failure, and porcine kidney failure models. Importantly, this study provides a direction for microbiome-based therapies using a bioengineering strategy [].
FMT has also been applied in clinical case studies to improve kidney function (Table 2). In a clinical case report, FMT extracted from a healthy male eligible donor was administered endoscopically to treat a patient with membranous nephropathy. After two treatments, FMT ameliorated the associated nephrotic syndrome and improved kidney function with increased total serum protein and albumin levels as well as decreased serum creatinine and 24 h urine protein []. Two patients with IgAN received FMT regularly via transendoscopic enteral tubing for 6–7 months. FMT treatment decreased 24 h urinary protein, increased serum albumin, and restored gut microbiota in both patients []. Given that FMT could potently alter gut microbiota’s overall composition and function, as reviewed above, FMT may benefit CKD via several classic mechanisms (Figure 1).
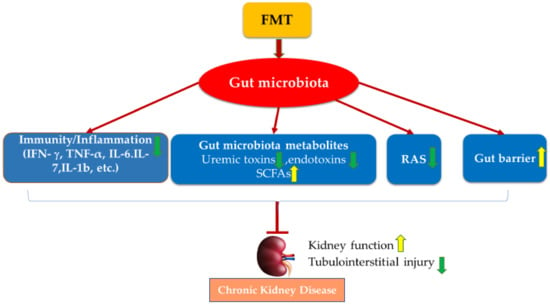
Figure 1.
Potential mechanisms of FMT on managing CKD. FMT improves kidney function and protects against kidney injury via the modulation of gut microbiota dysbiosis, mediated by restoring hosts’ immunity, regulating gut microbiota metabolites, inactivating RAS, and improving gut epithelial barrier integrity.
6. Conclusions and Future Studies
This review summarises the role of the gut microbiome in the pathogenesis of CKD, the primary mechanisms of FMT as a novel microbiota-based intervention and, most importantly, an update in animal and clinical studies on managing CKD using FMT. The predominant effects of FMT on CKD include modulating immune responses, gut microbiota metabolites, the RAS axis, and gut barrier function. Although FMT has yielded clinical benefits in several diseases, its utilization in extended clinical practice may be limited due to numerous factors, such as the definition of the healthy donor, screening procedures, sample preparation, storage condition, dose–response, methods of delivery, settings, and progression of different diseases. Clinical studies using FMT in patients with CKD are primarily small-scale and underpowered, as they were generally designed as pilot studies for safety reasons.
It is well recognized that “one stool does not fit all”. Hence, donor selection and administration methods in specific diseases are dominant determinants of FMT success rates. Therefore, decoding FMT strategies have to rely on the advantage of artificial intelligence, advanced bioinformatic techniques, and machine learning algorithms, such as multi-omics correlation analysis. A set of parameters for the gut microbiota, plasma, uraemic toxins, faecal metabolites, and kidney function need to be linked and systemically analysed, which will strongly support researchers in understanding the role of FMT and standardizing procedures and protocol evolution in the future.
Considering the potential pathogenic factors derived from overall gut microbiome transplantation, novel FMT consisting of several specific bacterial strains extracted from healthy stool might be an alternative intervention with much lower risks. For example, FMT could be further optimized in alternative ways, such as bacterial consortia and BME, to reduce potential pathogenic factors before application in patients to improve or slow CKD progression. Bacterial consortia are derived from culturing selected gut bacteria from the donor faecal samples, allowing for mass production, increased consistency, and thorough bacterial community characterization, including eliminating pathogenic microorganisms. Biomedical engineering techniques could also contribute to FMT development.
To conclude, standardization of FMT procedures in CKD treatment is urgently needed, both clinically and commercially. Clinical trials should evaluate the impact of FMT on hard clinical endpoints in CKD with the assistance of sophisticated artificial intelligence and up-to-date bioinformatics interpretation before successfully moving FMT therapy for CKD into clinical practice.
Author Contributions
Conceptualization, J.B., A.L., B.B., X.-M.C., C.H. and C.A.P.; writing—original draft preparation, J.B. and C.H.; review and editing, J.B., A.L., B.B., X.-M.C., C.H. and C.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Haileamlak, A. Chronic Kidney Disease is on the Rise. Ethiop. J. Health Sci. 2018, 28, 681–682. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Perazella, M.A.; Nolin, T.D. Adverse Drug Effects in Patients with CKD: Primum Non Nocere. Clin. J. Am. Soc. Nephrol. 2020, 15, 1075–1077. [Google Scholar] [CrossRef]
- Roux-Marson, C.; Baranski, J.B.; Fafin, C.; Exterman, G.; Vigneau, C.; Couchoud, C.; Moranne, O. Investigators. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr. 2020, 20, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vondracek, S.F.; Teitelbaum, I.; Kiser, T.H. Principles of Kidney Pharmacotherapy for the Nephrologist: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, M.; Tsintarakis, E. Insights into the role of human gut microbiota in Clostridioides difficile infection. Microorganisms 2020, 8, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Liu, C.W.; Ho, Y.H.; Huang, C.K.; Hung, C.S.; Smith, B.H.; Lin, J.C. Gut Microbiota Composition and Its Metabolites in Different Stages of Chronic Kidney Disease. J. Clin. Med. 2021, 10, 3881. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullish, B.H.; McDonald, J.A.K.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Kapila, D.; Petrof, E.O.; Joyce, S.A.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Liskiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wronski, M.; Baba-Kubis, A.; Skonieczna-Zydecka, K.; Marlicz, W.; Bienkowski, P.; Misera, A.; Pelka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110076. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Liu, W.H.; Zheng, H.; Feng, H.; Zhao, W.; Hung, W.L.; Li, H. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food Funct. 2022, 13, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Zhu, X.; Li, W.L.; Mak, J.W.Y.; Wong, S.H.; Zhu, S.T.; Guo, S.L.; Chan, F.K.L.; Zhang, S.T.; Ng, S.C. Modulation of gut microbiota protects against viral respiratory tract infections: A systematic review of animal and clinical studies. Eur. J. Nutr. 2021, 60, 4151–4174. [Google Scholar] [CrossRef]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: A systematic review. Diabetes Res. Clin. Pract. 2021, 173, 108689. [Google Scholar] [CrossRef]
- Aldars-Garcia, L.; Chaparro, M.; Gisbert, J.P. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Wan, Y.; Zuo, T.; Xu, Z.; Zhang, F.; Zhan, H.; Chan, D.; Leung, T.F.; Yeoh, Y.K.; Chan, F.K.L.; Chan, R.; et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut 2022, 71, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Vaziri, N.D. Gut microbial short-chain fatty acids and the risk of diabetes. Nat. Rev. Nephrol. 2019, 15, 389–390. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Qian, Y.; Xu, S.; Mo, C.; Yan, Z.; Yang, X.; Xiao, Q. Plasma branched-chain and aromatic amino acids correlate with the gut microbiota and severity of Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Diet Quality and Protein-Bound Uraemic Toxins: Investigation of Novel Risk Factors and the Role of Microbiome in Chronic Kidney Disease. J. Ren. Nutr. 2021. [Google Scholar] [CrossRef]
- Atzeni, A.; Galie, S.; Muralidharan, J.; Babio, N.; Tinahones, F.J.; Vioque, J.; Corella, D.; Castaner, O.; Vidal, J.; Moreno-Indias, I.; et al. Gut Microbiota Profile and Changes in Body Weight in Elderly Subjects with Overweight/Obesity and Metabolic Syndrome. Microorganisms 2021, 9, 346. [Google Scholar] [CrossRef]
- Collij, V.; Klaassen, M.A.Y.; Weersma, R.K.; Vila, A.V. Gut microbiota in inflammatory bowel diseases: Moving from basic science to clinical applications. Hum. Genet. 2021, 140, 703–708. [Google Scholar] [CrossRef]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Yu, S.M.; He, J.C. Happy gut, happy kidneys? Restoration of gut microbiome ameliorates acute and chronic kidney disease. Cell Metab. 2021, 33, 1901–1903. [Google Scholar] [CrossRef]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.W.; Pollock, C.A.; Saad, S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef]
- Knauf, F.; Brewer, J.R.; Flavell, R.A. Immunity, microbiota and kidney disease. Nat. Rev. Nephrol. 2019, 15, 263–274. [Google Scholar] [CrossRef]
- Wu, I.W.; Gao, S.S.; Chou, H.C.; Yang, H.Y.; Chang, L.C.; Kuo, Y.L.; Dinh, M.C.V.; Chung, W.H.; Yang, C.W.; Lai, H.C.; et al. Integrative metagenomic and metabolomic analyses reveal severity-specific signatures of gut microbiota in chronic kidney disease. Theranostics 2020, 10, 5398–5411. [Google Scholar] [CrossRef] [PubMed]
- Cold, F.; Baunwall, S.M.D.; Dahlerup, J.F.; Petersen, A.M.; Hvas, C.L.; Hansen, L.H. Systematic review with meta-analysis: Encapsulated faecal microbiota transplantation—Evidence for clinical efficacy. Ther. Adv. Gastroenterol. 2021, 14, 17562848211041004. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Zitvogel, L. Fecal microbiota transplantation: Can it circumvent resistance to PD-1 blockade in melanoma? Signal. Transduct. Target. Ther. 2021, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M. The Microbiota and Kidney Transplantation: Influence on the Graft. Urology 2021, 9, 95–105. [Google Scholar] [CrossRef]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef]
- Mafra, D.; Kalantar-Zadeh, K.; Moore, L.W. New Tricks for Old Friends: Treating Gut Microbiota of Patients With CKD. J. Ren. Nutr. 2021, 31, 433–437. [Google Scholar] [CrossRef]
- Mikusic, N.L.R.; Kouyoumdzian, N.M.; Choi, M.R. Gut microbiota and chronic kidney disease: Evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflüg. Arch.-Eur. J. Physiol. 2020, 472, 303–320. [Google Scholar] [CrossRef]
- Hu, X.; Ouyang, S.; Xie, Y.; Gong, Z.; Du, J. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 2020, 132, 495–505. [Google Scholar] [CrossRef]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci 2020, 21, 1986. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.B.; Nigwekar, S.U.; Kalim, S.; Lelouvier, B.; Servant, F.; Dalal, M.; Krinsky, S.; Fasano, A.; Tolkoff-Rubin, N.; Allegretti, A.S. The Gut and Blood Microbiome in IgA Nephropathy and Healthy Controls. Kidney360 2021, 2, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Yang, L.; Wan, Y.; Liu, C.; Jiang, S.; Shang, E.X.; Duan, J.A. Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1174, 122728. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Luo, Q.; Cai, K.; Wang, K.; Xu, B. Reduced fecal short-chain fatty acids levels and the relationship with gut microbiota in IgA nephropathy. BMC Nephrol. 2021, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, W.; Guo, R.; Cui, W.; Zhang, F.; Geng, Y.; Liu, C.; Shang, J.; Xiao, J.; Wen, X. Gut microbiome alterations predict diabetic kidney disease in general population. Res. Square 2020. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, R.; Guo, L.; Wang, Y.; Liu, W.J.; Ai, S.; Woon, T.H.; Wang, Z.; Zhai, Y.; Wang, Z.; et al. Qing-Re-Xiao-Zheng Formula Modulates Gut Microbiota and Inhibits Inflammation in Mice With Diabetic Kidney Disease. Front. Med. 2021, 8, 719950. [Google Scholar] [CrossRef]
- Joossens, M.; Faust, K.; Gryp, T.; Nguyen, A.T.L.; Wang, J.; Eloot, S.; Schepers, E.; Dhondt, A.; Pletinck, A.; Vieira-Silva, S.; et al. Gut microbiota dynamics and uraemic toxins: One size does not fit all. Gut 2019, 68, 2257–2260. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.-E.; Park, J.I.; Cho, H.; Kwak, M.-J.; Kim, B.-Y.; Yang, S.H.; Lee, J.P.; Kim, D.K.; Joo, K.W. The association between gut microbiota and uremia of chronic kidney disease. Microorganisms 2020, 8, 907. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Aftzoglou, K.; Voidarou, C.; Konstantinidis, T.; Chifiriuc, M.C.; Thodis, E.; Bezirtzoglou, E. Microbiome, Immunosenescence, and Chronic Kidney Disease. Front. Med. 2021, 8, 661203. [Google Scholar] [CrossRef]
- Carmody, R.N.; Sarkar, A.; Reese, A.T. Gut microbiota through an evolutionary lens. Science 2021, 372, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021, 2021, 5516035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Chen, C.; Lu, J.; Yu, J.; Xu, X.; Peng, Y.; Zhang, S.; Jiang, S.; Guo, J.; et al. Targeting the gut microbial metabolic pathway with small molecules decreases uremic toxin production. Gut Microbes 2020, 12, 1823800. [Google Scholar] [CrossRef]
- Funken, D.; Yu, Y.; Feng, X.; Imvised, T.; Gueler, F.; Prinz, I.; Madadi-Sanjani, O.; Ure, B.M.; Kuebler, J.F.; Klemann, C. Lack of gamma delta T cells ameliorates inflammatory response after acute intestinal ischemia reperfusion in mice. Sci. Rep. 2021, 11, 18628. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.G.; Oh, S.W.; Cho, W.Y.; Im, S.H.; Jo, S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Britton, G.J.; Contijoch, E.J.; Spindler, M.P.; Aggarwala, V.; Dogan, B.; Bongers, G.; San Mateo, L.; Baltus, A.; Das, A.; Gevers, D.; et al. Defined microbiota transplant restores Th17/RORgammat(+) regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc. Natl. Acad. Sci. USA 2020, 117, 21536–21545. [Google Scholar] [CrossRef] [PubMed]
- Erturk-Hasdemir, D.; Oh, S.F.; Okan, N.A.; Stefanetti, G.; Gazzaniga, F.S.; Seeberger, P.H.; Plevy, S.E.; Kasper, D.L. Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. USA 2019, 116, 26157–26166. [Google Scholar] [CrossRef]
- Krebs, C.F.; Paust, H.J.; Krohn, S.; Koyro, T.; Brix, S.R.; Riedel, J.H.; Bartsch, P.; Wiech, T.; Meyer-Schwesinger, C.; Huang, J.; et al. Autoimmune Renal Disease Is Exacerbated by S1P-Receptor-1-Dependent Intestinal Th17 Cell Migration to the Kidney. Immunity 2016, 45, 1078–1092. [Google Scholar] [CrossRef] [Green Version]
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Kano, T.; Suzuki, H.; Makita, Y.; Fukao, Y.; Suzuki, Y. Nasal-associated lymphoid tissue is the major induction site for nephritogenic IgA in murine IgA nephropathy. Kidney Int. 2021, 100, 364–376. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Manneras-Holm, L.; Stahlman, M.; Olsson, L.M.; Serino, M.; Planas-Felix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Jia, A.; Wang, Y.; Bi, Y.; Liu, G. The crosstalk between gut bacteria and host immunity in intestinal inflammation. J. Cell Physiol. 2021, 236, 2239–2254. [Google Scholar] [CrossRef]
- Esgalhado, M.; Kemp, J.A.; Damasceno, N.R.; Fouque, D.; Mafra, D. Short-chain fatty acids: A link between prebiotics and microbiota in chronic kidney disease. Futur. Microbiol. 2017, 12, 1413–1425. [Google Scholar] [CrossRef]
- Zaidman, N.A.; Pluznick, J.L. Understudied G Protein-Coupled Receptors in the Kidney. Nephron 2022, 146, 278–281. [Google Scholar] [CrossRef]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Lu, P.C.; Hsu, C.N.; Lin, I.C.; Lo, M.H.; Yang, M.Y.; Tain, Y.L. The Association Between Changes in Plasma Short-Chain Fatty Acid Concentrations and Hypertension in Children With Chronic Kidney Disease. Front. Pediatr. 2020, 8, 613641. [Google Scholar] [CrossRef]
- Zhong, C.; Dai, Z.; Chai, L.; Wu, L.; Li, J.; Guo, W.; Zhang, J.; Zhang, Q.; Xue, C.; Lin, H.; et al. The change of gut microbiota-derived short-chain fatty acids in diabetic kidney disease. J. Clin. Lab. Anal. 2021, 35, e24062. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Sohail, M.U.; Al-Jamal, O.; Shoair, B.M.; Al-Baniali, A.Y.; Bouabidi, S.; Nasr, S.; Bawadi, H. The Effects of Gum Acacia on the Composition of the Gut Microbiome and Plasma Levels of Short-Chain Fatty Acids in a Rat Model of Chronic Kidney Disease. Front. Pharmacol. 2020, 11, 569402. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxid. Med. Cell Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
- Holle, J.; Querfeld, U.; Kirchner, M.; Anninos, A.; Okun, J.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; Bulut, I.K.; et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr. Nephrol. 2019, 34, 2571–2582. [Google Scholar] [CrossRef]
- Lin, C.N.; Wu, I.W.; Huang, Y.F.; Peng, S.Y.; Huang, Y.C.; Ning, H.C. Measuring serum total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease using UPLC-MS/MS. J. Food Drug Anal. 2019, 27, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Rysz, J.; Franczyk, B.; Lawinski, J.; Olszewski, R.; Cialkowska-Rysz, A.; Gluba-Brzozka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Cheng, T.H.; Ma, M.C.; Liao, M.T.; Zheng, C.M.; Lu, K.C.; Liao, C.H.; Hou, Y.C.; Liu, W.C.; Lu, C.L. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins 2020, 12, 684. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Salve Coutinho-Wolino, K.; de F. Cardozo, L.F.M.; de Oliveira Leal, V.; Mafra, D.; Stockler-Pinto, M.B. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, F.; Tang, H.; Zhang, X.; Hu, J.; Zhong, X.; Gong, N.; Lai, Y.; Zhou, M.; Tian, J.; et al. Gut microbial metabolite TMAO increases peritoneal inflammation and peritonitis risk in peritoneal dialysis patients. Transl. Res. 2022, 240, 50–63. [Google Scholar] [CrossRef]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef]
- Cernaro, V.; Loddo, S.; Macaione, V.; Ferlazzo, V.T.; Cigala, R.M.; Crea, F.; De Stefano, C.; Genovese, A.R.R.; Gembillo, G.; Bolignano, D.; et al. RAS inhibition modulates kynurenine levels in a CKD population with and without type 2 diabetes mellitus. Int. Urol. Nephrol. 2020, 52, 1125–1133. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Mak, R.H. Progression of chronic kidney disease in children—Role of glomerular hemodynamics and interstitial fibrosis. Curr. Opin. Pediatr. 2018, 30, 220–227. [Google Scholar] [CrossRef]
- Lu, C.C.; Hu, Z.B.; Wang, R.; Hong, Z.H.; Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J. Gut microbiota dysbiosis-induced activation of the intrarenal renin–angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol. Sin. 2020, 41, 1111–1118. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.C.; Chen, Y.Y.; Chen, L.; Wang, Y.N.; Vaziri, N.D.; Miao, H.; Zhao, Y.Y. Alisol B 23-acetate attenuates CKD progression by regulating the renin-angiotensin system and gut-kidney axis. Ther. Adv. Chronic Dis. 2020, 11, 2040622320920025. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, H.E.; Rodriguez-Pineiro, A.M.; Schutte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Backhed, F.; Hansson, G.C.; Johansson, M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. [Google Scholar] [CrossRef]
- Ji, C.; Deng, Y.; Yang, A.; Lu, Z.; Chen, Y.; Liu, X.; Han, L.; Zou, C. Rhubarb Enema Improved Colon Mucosal Barrier Injury in 5/6 Nephrectomy Rats May Associate with Gut Microbiota Modification. Front. Pharmacol. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Kanbay, M.; Onal, E.M.; Afsar, B.; Dagel, T.; Yerlikaya, A.; Covic, A.; Vaziri, N.D. The crosstalk of gut microbiota and chronic kidney disease: Role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int. Urol. Nephrol. 2018, 50, 1453–1466. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.L.; Vaziri, N.D. The Leaky Gut and Altered Microbiome in Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhou, X.; Li, X.; Zhu, Q.; Peng, J.; Zhu, B.; Zheng, X.; Lu, Y.; Yang, D.; Wang, B.; et al. Depletion of Gut Microbiota Impairs Gut Barrier Function and Antiviral Immune Defense in the Liver. Front. Immunol. 2021, 12, 636803. [Google Scholar] [CrossRef]
- Xu, Y.H.; Gao, C.L.; Guo, H.L.; Zhang, W.Q.; Huang, W.; Tang, S.S.; Gan, W.J.; Xu, Y.; Zhou, H.; Zhu, Q. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J. Endocrinol. 2018, 238, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Watane, A.; Cavuoto, K.M.; Rojas, M.; Dermer, H.; Day, J.O.; Banerjee, S.; Galor, A. Fecal Microbial Transplant in Individuals with Immune-Mediated Dry Eye. Am. J. Ophthalmol. 2022, 233, 90–100. [Google Scholar] [CrossRef]
- Sun, P.; Su, L.; Zhu, H.; Li, X.; Guo, Y.; Du, X.; Zhang, L.; Qin, C. Gut Microbiota Regulation and Their Implication in the Development of Neurodegenerative Disease. Microorganisms 2021, 9, 2281. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbo, S.; Porcari, S.; Settanni, C.R.; Giambo, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: Experience of a large-volume European FMT center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Hurtado, J.; Marchesi, J.R.; Mullish, B.H.; Chiang, A.; Thompson, C.C.; Cummings, B.P. Impact of fecal microbiota transplantation with capsules on the prevention of metabolic syndrome among patients with obesity. Hormones 2021, 20, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Koopen, A.M.; Almeida, E.L.; Attaye, I.; Witjes, J.J.; Rampanelli, E.; Majait, S.; Kemper, M.; Levels, J.H.M.; Schimmel, A.W.M.; Herrema, H.; et al. Effect of Fecal Microbiota Transplantation Combined With Mediterranean Diet on Insulin Sensitivity in Subjects with Metabolic Syndrome. Front. Microbiol. 2021, 12, 662159. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; de Vos, W.M.; Nieuwdorp, M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. 2021, 33, 1098–1110. [Google Scholar] [CrossRef]
- Danne, C.; Rolhion, N.; Sokol, H. Recipient factors in faecal microbiota transplantation: One stool does not fit all. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 503–513. [Google Scholar] [CrossRef]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal microbiota transplantation is highly effective in real-world practice: Initial results from the FMT National Registry. Gastroenterology 2021, 160, 183–192.e3. [Google Scholar] [CrossRef]
- Varga, A.; Kocsis, B.; Sipos, D.; Kasa, P.; Vigvari, S.; Pal, S.; Dembrovszky, F.; Farkas, K.; Peterfi, Z. How to Apply FMT More Effectively, Conveniently and Flexible—A Comparison of FMT Methods. Front. Cell Infect. Microbiol. 2021, 11, 657320. [Google Scholar] [CrossRef]
- Burrello, C.; Garavaglia, F.; Cribiu, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef]
- Burrello, C.; Giuffre, M.R.; Macandog, A.D.; Diaz-Basabe, A.; Cribiu, F.M.; Lopez, G.; Borgo, F.; Nezi, L.; Caprioli, F.; Vecchi, M.; et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells 2019, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Tang, W.; Zhou, C.; Sun, X.; Wei, Z.; Zhong, J.; Huang, Z. Fecal Microbiota Transplantation Is a Promising Method to Restore Gut Microbiota Dysbiosis and Relieve Neurological Deficits after Traumatic Brain Injury. Oxid. Med. Cell Longev. 2021, 2021, 5816837. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Papadopoulou, I.; Bantouna, D.; de Lastic, A.L.; Rodi, M.; Mouzaki, A.; Gogos, C.A.; Zolota, V.; Maroulis, I. Fecal Microbiota Transplantation and Hydrocortisone Ameliorate Intestinal Barrier Dysfunction and Improve Survival in a Rat Model of Cecal Ligation and Puncture-Induced Sepsis. Shock 2021, 55, 666–675. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Lenucci, M.S.; Fontana, S.; Forgia, F.M.; Minervini, F.; Scarano, A.; Santino, A.; Dalfino, G.; Gesualdo, L.; et al. In Vitro Selection of Probiotics, Prebiotics, and Antioxidants to Develop an Innovative Synbiotic (NatuREN G) and Testing Its Effect in Reducing Uremic Toxins in Fecal Batches from CKD Patients. Microorganisms 2021, 9, 1316. [Google Scholar] [CrossRef]
- De Mauri, A.; Carrera, D.; Bagnati, M.; Rolla, R.; Vidali, M.; Chiarinotti, D.; Pane, M.; Amoruso, A.; Del Piano, M. Probiotics-Supplemented Low-Protein Diet for Microbiota Modulation in Patients with Advanced Chronic Kidney Disease (ProLowCKD): Results from a Placebo-Controlled Randomized Trial. Nutrients 2022, 14, 1637. [Google Scholar] [CrossRef]
- Lau, W.L.; Chang, Y.; Vaziri, N.D. The consequences of altered microbiota in immune-related chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1791–1798. [Google Scholar] [CrossRef]
- Mosterd, C.M.; Kanbay, M.; van den Born, B.J.H.; van Raalte, D.H.; Rampanelli, E. Intestinal microbiota and diabetic kidney diseases: The Role of microbiota and derived metabolites inmodulation of renal inflammation and disease progression. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101484. [Google Scholar] [CrossRef]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Li, Y.; Su, X.; Gao, Y.; Lv, C.; Gao, Z.; Liu, Y.; Wang, Y.; Li, S.; Wang, Z. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165764. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jiang, Y.H.; Li, W.; Liu, Y. Astragalus membranaceus and Salvia miltiorrhiza ameliorates cyclosporin A-induced chronic nephrotoxicity through the "gut-kidney axis". J. Ethnopharmacol. 2021, 269, 113768. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Soulage, C.O.; Caggiano, G.; Glorieux, G.; Fouque, D.; Koppe, L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins 2020, 12, 741. [Google Scholar] [CrossRef]
- Hu, Z.B.; Lu, J.; Chen, P.P.; Lu, C.C.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; Ruan, X.Z.; Liu, B.C.; et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics 2020, 10, 2803–2816. [Google Scholar] [CrossRef]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKalpha activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef]
- Bastos, R.M.C.; Simplicio-Filho, A.; Savio-Silva, C.; Oliveira, L.F.V.; Cruz, G.N.F.; Sousa, E.H.; Noronha, I.L.; Mangueira, C.L.P.; Quaglierini-Ribeiro, H.; Josefi-Rocha, G.R.; et al. Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 3842. [Google Scholar] [CrossRef]
- Zheng, D.W.; Pan, P.; Chen, K.W.; Fan, J.X.; Li, C.X.; Cheng, H.; Zhang, X.Z. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure. Nat. Biomed. Eng. 2020, 4, 853–862. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Di, W.; Yang, Y. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren. Fail. 2021, 43, 928–933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).